Abstract
Background
The aim of this study was to identify factors predicting histologic chorioamnionitis (HCA) in women with preterm premature rupture of membranes (PPROM).
Material/Methods
We retrospectively enrolled 371 women diagnosed with PPROM at less than 34 weeks of gestation at the Second Affiliated Hospital of Wenzhou Medical University between January 2008 and December 2012. HCA was diagnosed by placental histopathology in 70% of participants. Binary logistic regression was used to identify factors associated with HCA and neonatal outcomes.
Results
Patient age, rate of parity, tocolysis, cesarean section, serum C reactive protein (CRP) level at admission, white blood cell count, and latency duration did not significantly differ between the 2 groups. Binary logistic regression revealed that oligohydramnios at admission, gestational age at PPROM, and serum CRP >8 mg/L before delivery were significantly associated with HCA. Gestational age at delivery and birth weight were significantly lower in HCA patients than control patients. The rate of 1-min Apgar score <7, abnormal neonatal intracranial ultrasound findings, neonatal pneumonia, bronchopulmonary dysplasia, early-onset neonatal sepsis, and mortality were higher in HCA patients, but no significant difference was observed in the incidence of neonatal respiratory distress syndrome, necrotizing enterocolitis, hyperbilirubinemia, or hypoglycemia.
Conclusions
Younger gestational age at time of PPROM, higher CRP level before delivery, and oligohydramnios at admission in women with PPROM are associated with HCA, and HCA is associated with some adverse neonatal outcomes.
MeSH Keywords: Chorioamnionitis, Gestational Age, Neonatal Abstinence Syndrome
Background
Pre-labor rupture of membranes before the 37th week of gestation, termed preterm premature rupture of membrane (PPROM), is a common obstetric complication which occurs in approximately 3–4.5% of all pregnancies [1]. PPROM is associated with 30% of neonatal morbidities and mortalities in preterm delivery [2], and remains a challenge for the obstetrician [3]. Over the past decade, studies have emerged associating maternal upper genital tract infection with PPROM and spontaneous preterm delivery [4,5].
Acute inflammation of the membranes and chorion of the placenta, chorioamnionitis (ChA), indicates a high risk of adverse neonatal outcomes [6–17]. ChA is typically the result of microbial invasion in patients with PPROM, but can also be caused by genital mycoplasmas, such as Ureaplasma and Mycoplasma hominis or systemic infection in spite of intact membranes [18]. Clinical ChA is diagnosed in patients presenting two or more of the following criteria: high temperature, maternal tachycardia, fetal tachycardia, uterine tenderness, foul-smelling amniotic fluid, maternal leukocytosis with bands, and positive C reactive protein (CRP) [19]. However, as symptoms are rarely recognized before birth, ChA is more frequently diagnosed by microscopic examination of the placenta after birth [18,20]. Infiltration of polymorphonuclear leukocytes and other immunocytes, such as macrophages and T-cells, inform the diagnosis of histologic chorioamnionitis (HCA), applied in 40–70% of pre-term births and 1–13% of term births [18,21].
Clinical measures that reliably predict HCA are readily sought [22–25]. Serologic or amniotic fluid tests have the potential to highlight activation of the host immune and inflammatory responses by microbial invasion of the amniotic cavity (e.g., cytokine or CRP and white blood cell count) [5,26,27], but none have been approved for clinical use. Currently, guidelines for the management of PPROM do not take prediction of HCA into account, and are usually based on the gestational age at which PPROM occurs [28].
An emerging theme in the literature concerns the association of HCA with neonatal outcomes [6–17]. Several studies have demonstrated that HCA was associated with increased risk of neonatal morbidity, including sepsis [7], pneumonia [7], intraventricular hemorrhage [8], cystic periventricular leukomalacia [9,10], cerebral palsy [10], bronchopulmonary dysplasia [11,12], respiratory distress syndrome, perinatal hypoxia and PDA [6], and mortality [29]. Chorionic vasculitis, reflecting placental infection of fetal origin and villous edema, has been reported to be a risk factor of brain damage in neonates born before the 34th week of gestation [30]. Other studies, however, have not shown an impact of HCA on adverse neonatal outcome [13–16].
Without placental examination, it is easy to overlook HCA, thus the condition remains under diagnosed. Pathologic examination of the placenta is a noninvasive and simple method with which to identify inflammation and determine whether the fetus developed a substantial systemic inflammatory response [31]. Therefore, the placental pathological examination is an important part of routine examination, and may contribute to early diagnosis of HCA, and thus highlight the potential for neonatal complications.
Our objective in this study was to determine factors predicting HCA in women with PPROM, and to evaluate the association between HCA and adverse neonatal outcomes.
Material and Methods
Study design
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study received ethics approval from the Second Affiliated Hospital of Wenzhou Medical University (L-20080012). All patients provided informed written consent.
We designed a retrospective study of 371 pregnant women admitted to Second Affiliated Hospital of Wenzhou Medical University for obstetric care between 18 and 38 years of age. Inclusion criteria included delivery between January 2008 and December 2012 following confirmed PPROM at less than 34 weeks of gestation. PPROM was diagnosed according to a standardized evaluation protocol consisting of assessment of vaginal fluid pH and ferning characteristics. Exclusion criteria included pregnancies complicated by multiple gestations, active labor at admission, and pregnancy-related complications such as gestational diabetes mellitus and/or hypertensive disorder. Patients diagnosed with clinical ChA at admission or during expectant management were also excluded.
In all patients, ultrasonographic examination was performed to evaluate oligohydramnios, characterized as an amniotic fluid index <5 cm [32], and fetal vitality at admission. Laboratory examinations such as white blood cell count and serum CRP level were performed at admission. Histologic examination of the placenta was performed after delivery.
Patients were monitored for 34 weeks after conception, at which point labor was induced. Prophylactic antibiotics such as cephalosporin and azithromycin were administrated. Ritodrine or magnesium sulfate was administrated intravenously to inhibit uterine contraction and steroids were given in 4 intramuscular doses of 6 mg to promote fetal lung maturation. Maternal and fetal status was closely monitored for development of ChA, labor, and/or fetal compromise. Delivery was induced in the case of clinical ChA, active labor, fetal compromise, placental abruption, progressive decrease of amniotic fluid, or when gestational age reached 34 weeks.
Based on histologic examination of the placenta, patients were divided by diagnosis of HCA into the “HCA group” (n=261) and “control group” (n=110). Maternal and neonate characteristics were recorded, including maternal age, multiparity, gestational age at time of PPROM, duration of latency, white blood cell count, CRP, body temperature, and the rate of cesarean section and oligohydramnios, neonate gestational age at delivery, birth weight, 1-min Apgar score of <7, abnormal neonatal intracranial ultrasound findings, neonatal respiratory distress syndrome, neonatal pneumonia, bronchopulmonary dysplasia, necrotizing enterocolitis, neonatal early-onset sepsis, hyperbilirubinemia, hypoglycemia, and neonatal mortality. Early-onset sepsis was defined as sepsis developing within 72 h after birth [33]. Abnormal neonatal intracranial ultrasound findings included abnormal cerebral ventricle echo, intraventricular hemorrhage, and cystic periventricular leukomalacia.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SSPS Inc.). The Kolmogorov-Smirnov test of normality was applied to patient data. Continuous variables are shown as mean ± standard deviation. The t test was used to compare the variables that exhibited normal Gaussian distribution, between 2 groups, and the Mann-Whitney U-test was used to compare patient group non-normally distributed data. The chi-square test or Fisher’s exact test was used to compare patient group categorical data, shown as incidence (%). Binary logistic regression analysis was performed to identify factors associated with HCA. A 2-sided P value of less than 0.05 was considered statistically significant.
Results
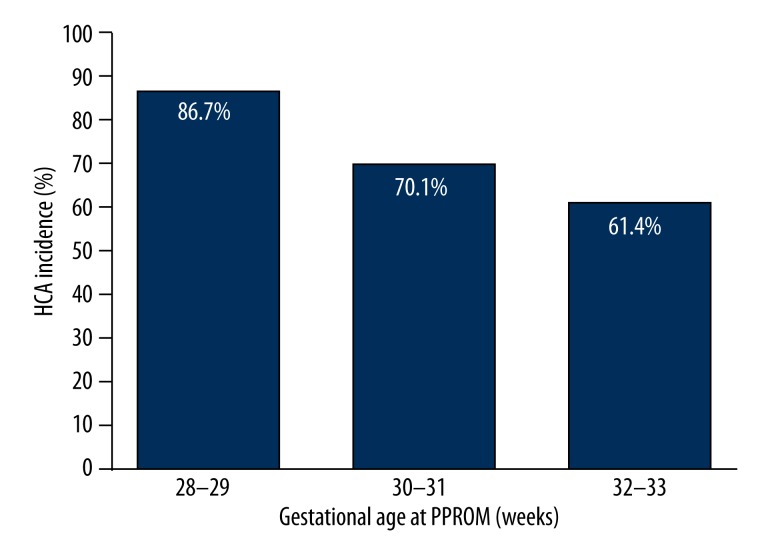
Seventy percent (261/371) of participants with PPROM were diagnosed with HCA. The mean gestational age at PROM was 31.1±1.6 weeks, and the incidence of HCA increased significantly with decreasing gestational age (Figure 1). Mean gestational age at time of PPROM was significantly lower in the HCA group (30.9±1.6 weeks) than the control group (31.7±1.3 weeks) (Table 1).
Figure 1.
Incidence of HCA after PPROM at various gestational ages.
Table 1.
Clinical characteristics of patients with or without HCA.
| HCA (n=261) | Without HCA (n=110) | P value | |
|---|---|---|---|
| Maternal age (y) | 27.6±4.8 | 28.2±4.8 | 0.276 |
| Multiparity (%) | 128 (49.0%) | 42 (38.2%) | 0.055 |
| Gestational age at PPROM (weeks) | 30.9±1.6 | 31.7±1.3 | <0.001 |
| Duration of latency (h) | 136.9±117.9 | 133.4±137.6 | 0.802 |
| White blood cell count (109/L) | |||
| At admission | 11.9±3.3 | 11.3±2.7 | 0.081 |
| Before delivery | 13.5±4.0 | 12.6±3.8 | 0.061 |
| C reactive protein (mg/L) | |||
| At admission | 9.2±7.1 | 7.7±6.9 | 0.058 |
| Before delivery | 18.6±21.5 | 10.7±11.4 | <0.001 |
| Oligohydramnios (%) | 135 (51.7%) | 30 (27.3%) | <0.001 |
| Cesarean section (%) | 94 (36.0%) | 45 (40.9%) | 0.374 |
| Tocolysis (%) | 183 (70.1%) | 68 (61.8%) | 0.119 |
Data is presented as mean ± standard deviation or frequency (percentage). A two-sided P value of less than 0.05 was considered statistically significant.
As shown in Table 1, mean CRP before delivery and rate of oligohydramnios were also significantly higher in the HCA group (18.6±21.5 mg/L and 51.7%, respectively) than that in the control group (10.7±11.4 mg/L and 27.3%, respectively).
A binary logistic regression analysis of predictive factors associated with HCA found that oligohydramnios (OR=2.47), gestational age at time of PPROM (OR=0.755), and serum CRP level >8 mg/L before delivery (OR=2.586) were significantly associated with HCA (P<0.05). However, duration of latency, serum CRP level >8 mg/L at admission, and white blood cell count >15×109/L at admission or before delivery were not associated with HCA (P>0.05) (Table 2).
Table 2.
Binary logistic regression analysis of factors contributing to HCA.
| OR (95% CI) | P-value | |
|---|---|---|
| Gestational age at PPROM (weeks) | 0.755 (0.637–0.894) | 0.001 |
| Oligohydramnios (%) | 2.476 (1.459–4.202) | 0.001 |
| Duration of latency (h) | 1.001 (0.999–1.003) | 0.552 |
| White blood cell count (>15×109/L) | ||
| At admission | 1.784 (0.788–4.037) | 0.165 |
| Before delivery | 0.868 (0.481–1.567) | 0.639 |
| C reactive protein (>8 mg/L) | ||
| At admission | 1.474 (0.817–2.659) | 0.197 |
| Before delivery | 2.586 (1.525–4.386) | <0.001 |
OR – Odds Ratio; 95% CI – 95% confidence interval. A two-sided P value of less than 0.05 was considered statistically significant.
The mean gestational age at delivery (32.5±1.4 weeks) and birth weight (1903.0±338.3 g) in the HCA group were significantly lower than those in the control group (31.7±1.6 weeks and 1684.5±406.0 g, respectively) (P<0.05, Table 3). The incidence of 1-min Apgar score of <7, abnormal neonatal intracranial ultrasound findings, neonatal pneumonia, bronchopulmonary dysplasia, early-onset neonatal sepsis, and mortality in the HCA group were significantly higher than those in the control group (P<0.05). There were no significant differences between the 2 groups in the incidence of necrotizing enterocolitis, hyperbilirubinemia, hypoglycemia, or neonatal respiratory distress syndrome (P>0.05, Table 3).
Table 3.
Neonatal outcome of pregnancies with and without HCA.
| HCA (n=261) | No HCA (n=110) | P value | |
|---|---|---|---|
| Gestational age at delivery (w) | 32.5±1.4 | 31.7±1.6 | <0.001 |
| Birth weight (g) | 1903.0±338.3 | 1684.5±406.0 | <0.001 |
| 1-min Apgar score of <7 | 48 (18.4%) | 10 (9.1%) | 0.024 |
| Abnormal neonatal intracranial ultrasound findings | 38 (17.0%) | 7 (6.8%) | 0.027 |
| Neonatal respiratory distress syndrome | 68 (26.1%) | 30 (27.3%) | 0.808 |
| Neonatal pneumonia | 34 (13.0%) | 6 (5.5%) | 0.032 |
| Bronchopulmonary dysplasia | 15 (5.9%) | 1 (0.92%) | 0.036 |
| Necrotizing enterocolitis | 10 (3.8%) | 1 (0.9%) | 0.130 |
| Early-onset neonatal sepsis | 17 (6.5%) | 1 (0.92%) | 0.022 |
| Hypoglycemia | 24 (9.2%) | 11 (10.0%) | 0.809 |
| Hyperbilirubinemia | 20 (7.7%) | 4 (3.6%) | 0.150 |
| Prenatal mortality | 30 (11.5%) | 5 (4.5%) | 0.037 |
Data is presented as mean ± standard deviation or frequency (percentage). A two-sided P value of less than 0.05 was considered statistically significant.
Discussion
Recently, numerous studies have failed to identify predictive factors for HCA [22–25]. We sought to identify factors predicting HCA in women with PPROM. In our sample, 70% of women with PPROM were diagnosed with HCA. Patients diagnosed with HCA had significantly lower gestational ages at PPROM, higher CRP before delivery, and a higher rate of oligohydramnios.
We also sought to evaluate the association between HCA and adverse neonatal outcomes. The gestational age at delivery and birth weight of HCA group infants were significantly lower than those of control group infants, and 1-min Apgar score of <7, abnormal neonatal intracranial ultrasound findings, neonatal pneumonia, bronchopulmonary dysplasia, early-onset neonatal sepsis, and mortality were higher in the HCA group than in the control group.
The incidence of HCA is reported to range from 33% to 71%, varying according to the diagnostic criteria used [11]. The incidence of HCA in our sample of PPROM patients was 68.0%, which is likely higher than the incidence in the general population because we enrolled women with PPROM diagnosed before 34 weeks of gestation.
We found HCA to be significantly correlated with lower gestational age, as previously reported [22,25,34]. Although other studies reported contradictory results, a meta-analysis of 6 trials including 466 patients has suggested that maternal CRP is not a good predictor for ChA [35]. Nowak et al. also found CRP to be a more accurate and earlier predictor of HCA than white blood cell count and erythrocyte sedimentation rate in a group of 80 pregnant women with PPROM [34]. Consistent with the findings of Nowak et al., we found plasma CRP levels to be elevated before delivery in the HCA group, and serum CRP levels above 8 mg/L indicated significantly increased risk of HCA. The concentration of CRP at admission appears to be the most accurate marker for the prediction of early-onset neonatal infection in routine use, with a sensitivity >90%.
We also observed that HCA was associated with oligohydramnios. Yoon et al. previously reported that oligohydramnios was associated with inflammatory responses in fetal, amniotic, and maternal compartments, such as in HCA [32]. Conversely, a secondary analysis of 290 women participating in a trial of antibiotic therapy for PPROM at 24–32 weeks did not confirm an association between low amniotic fluid index and subsequent histologic amnionitis [36]. This observation may be explained by the fact that oligohydramnios appears to be associated with HCA in some women, but antibiotic treatment reduces the impact of this association.
Aziz et al. found that the latency duration was not related to higher rates of perinatal infection, including ChA and neonatal sepsis, in patients experiencing latency lasting longer than 1 week [24]. Similarly, we also found the duration of latency in the HCA group to be longer than in the control group, and the gestational age at which PPROM occurred was inversely associated with HCA. Hence, strict monitoring of patients in which PPROM occurred before the 34th week may guide clinical intervention and improve outcomes.
Previous studies have identified adverse neonatal outcomes such as neonatal pneumonia, bronchopulmonary dysplasia, cystic periventricular leukomalacia, intraventricular hemorrhage, cerebral palsy, and neonatal early-onset sepsis to be associated with HCA [6–8,11,12,16]. We found that infants in the HCA group were more likely to suffer morbidity than those in the control group. Adverse neonatal outcomes such as bronchopulmonary dysplasia, neonatal pneumonia, bronchopulmonary dysplasia, early-onset neonatal sepsis, and neonatal mortality were significantly associated with HCA. Although neonatal respiratory distress syndrome was not associated with HCA in this study, HCA was associated with increased risk of bronchopulmonary dysplasia. This may be explained by a maturational effect accelerated by adrenal stimulation in HCA, which in turn may influence lung susceptibility to further injury, fostering bronchopulmonary dysplasia [17]. The decreased proliferation of epithelial, endothelial, and smooth muscle cells and increased apoptosis observed in the distal airways of fetal lungs exposed to ChA might be responsible for the development of bronchopulmonary dysplasia [37].
Conclusions
HCA is associated with the gestational age of PPROM, amniotic fluid index, duration of latency, and serum CRP level before delivery, resulting in poor outcomes such as bronchopulmonary dysplasia, early-onset sepsis, and neonatal morality in preterm infants. Detection of factors predicting HCA and evaluation of the risk of HCA for the patients with PPROM before the 34th week of gestation may facilitate preemptive treatment and improvement in neonatal outcomes. Further prospective studies are required to validate our observations and explore more sensitive and specific predictive factors for HCA and the relationship between HCA and adverse neonatal outcomes.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Source of support: This work was supported by Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents
References
- 1.Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clin Perinatol. 2001;28:721–34. doi: 10.1016/s0095-5108(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 2.Gopalani S, Krohn M, Meyn L, et al. Contemporary management of preterm premature rupture of membranes: determinants of latency and neonatal outcome. Am J Perinatol. 2004;21:183–90. doi: 10.1055/s-2004-828609. [DOI] [PubMed] [Google Scholar]
- 3.Garite TJ. Management of premature rupture of membranes. Clin Perinatol. 2001;28:837–47. doi: 10.1016/s0095-5108(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 5.Asrat T. Intra-amniotic infection in patients with preterm prelabor rupture of membranes. Pathophysiology, detection, and management. Clin Perinatol. 2001;28:735–51. doi: 10.1016/s0095-5108(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 6.Ecevit A, Anuk-Ince D, Yapakci E, et al. Association of respiratory distress syndrome and perinatal hypoxia with histologic chorioamnionitis in preterm infants. Turk J Pediatr. 2014;56:56–61. [PubMed] [Google Scholar]
- 7.Dempsey E, Chen MF, Kokottis T, et al. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. Am J Perinatol. 2005;22:155–59. doi: 10.1055/s-2005-865020. [DOI] [PubMed] [Google Scholar]
- 8.De Felice C, Toti P, Laurini RN, et al. Early neonatal brain injury in histologic chorioamnionitis. J Pediatr. 2001;138:101–4. doi: 10.1067/mpd.2001.109605. [DOI] [PubMed] [Google Scholar]
- 9.Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–29. doi: 10.1002/mrdd.10003. [DOI] [PubMed] [Google Scholar]
- 10.Nelson KB, Grether JK, Dambrosia JM, et al. Neonatal cytokines and cerebral palsy in very preterm infants. Pediatr Res. 2003;53:600–7. doi: 10.1203/01.PDR.0000056802.22454.AB. [DOI] [PubMed] [Google Scholar]
- 11.Been JV, Rours IG, Kornelisse RF, et al. Chorioamnionitis alters the response to surfactant in preterm infants. J Pediatr. 2010;156:10–5 e1. doi: 10.1016/j.jpeds.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–15. [PubMed] [Google Scholar]
- 13.Tauscher MK, Berg D, Brockmann M, et al. Association of histologic chorioamnionitis, increased levels of cord blood cytokines, and intracerebral hemorrhage in preterm neonates. Biol Neonate. 2003;83:166–70. doi: 10.1159/000068924. [DOI] [PubMed] [Google Scholar]
- 14.Dexter SC, Pinar H, Malee MP, et al. Outcome of very low birth weight infants with histopathologic chorioamnionitis. Obstet Gynecol. 2000;96:172–77. doi: 10.1016/s0029-7844(00)00886-3. [DOI] [PubMed] [Google Scholar]
- 15.Lahra MM, Beeby PJ, Jeffery HE. Intrauterine inflammation, neonatal sepsis, and chronic lung disease: a 13-year hospital cohort study. Pediatrics. 2009;123:1314–19. doi: 10.1542/peds.2008-0656. [DOI] [PubMed] [Google Scholar]
- 16.Hendson L, Russell L, Robertson CM, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr. 2011;158:397–402. doi: 10.1016/j.jpeds.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Thomas W, Speer CP. Chorioamnionitis: important risk factor or innocent bystander for neonatal outcome? Neonatology. 2011;99:177–87. doi: 10.1159/000320170. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RK. Chorioamnionitis and labor. Obstet Gynecol Clin North Am. 2005;32:287–96. x. doi: 10.1016/j.ogc.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol. 2004;190:147–51. doi: 10.1016/j.ajog.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Martinelli P, Sarno L, Maruotti GM, Paludetto R. Chorioamnionitis and prematurity: a critical review. J Matern Fetal Neonatal Med. 2012;25(Suppl 4):29–31. doi: 10.3109/14767058.2012.714981. [DOI] [PubMed] [Google Scholar]
- 22.Popowski T, Goffinet F, Maillard F, et al. Maternal markers for detecting early-onset neonatal infection and chorioamnionitis in cases of premature rupture of membranes at or after 34 weeks of gestation: a two-center prospective study. BMC Pregnancy Childbirth. 2011;11:26. doi: 10.1186/1471-2393-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidokoro K, Furuhashi M, Kuno N, Ishikawa K. Amniotic fluid neutrophil elastase and lactate dehydrogenase: association with histologic chorioamnionitis. Acta Obstet Gynecol Scand. 2006;85:669–74. doi: 10.1080/01443610600604432. [DOI] [PubMed] [Google Scholar]
- 24.Aziz N, Cheng YW, Caughey AB. Factors and outcomes associated with longer latency in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2008;21:821–25. doi: 10.1080/14767050802251255. [DOI] [PubMed] [Google Scholar]
- 25.Wu HC, Shen CM, Wu YY, et al. Subclinical histologic chorioamnionitis and related clinical and laboratory parameters in preterm deliveries. Pediatr Neonatol. 2009;50:217–21. doi: 10.1016/S1875-9572(09)60066-8. [DOI] [PubMed] [Google Scholar]
- 26.Kacerovsky M, Musilova I, Jacobsson B, et al. Cervical fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014 doi: 10.3109/14767058.2014.908179. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Kacerovsky M, Musilova I, Andrys C, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325.e1–e10. doi: 10.1016/j.ajog.2013.10.882. [DOI] [PubMed] [Google Scholar]
- 28.RCOG. Preterm labour rupture of membranes. Guideline. Royal college of Obstetricians and gynaecologists; 2006. [Google Scholar]
- 29.Elimian A, Verma U, Beneck D, et al. Histologic chorioamnionitis, antenatal steroids, and perinatal outcomes. Obstet Gynecol. 2000;96:333–36. doi: 10.1016/s0029-7844(00)00928-5. [DOI] [PubMed] [Google Scholar]
- 30.Mehta R, Nanjundaswamy S, Shen-Schwarz S, Petrova A. Neonatal morbidity and placental pathology. Indian J Pediatr. 2006;73:25–28. doi: 10.1007/BF02758255. [DOI] [PubMed] [Google Scholar]
- 31.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 32.Yoon BH, Kim YA, Romero R, et al. Association of oligohydramnios in women with preterm premature rupture of membranes with an inflammatory response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1999;181:784–88. doi: 10.1016/s0002-9378(99)70301-7. [DOI] [PubMed] [Google Scholar]
- 33.Bersani I, Thomas W, Speer CP. Chorioamnionitis – the good or the evil for neonatal outcome? J Matern Fetal Neonatal Med. 2012;25(Suppl 1):12–16. doi: 10.3109/14767058.2012.663161. [DOI] [PubMed] [Google Scholar]
- 34.Nowak M, Oszukowski P, Szpakowski M, et al. [Intrauterine infections. I. The role of C-reactive protein, white blood cell count and erythrocyte sedimentation rate in pregnant women in the detection of intrauterine infection after preliminary rupture of membranes]. Ginekol Pol. 1998;69:615–22. [in Polish] [PubMed] [Google Scholar]
- 35.Wiwanitkit V. Maternal C-reactive protein for detection of chorioamnionitis: an appraisal. Infect Dis Obstet Gynecol. 2005;13:179–81. doi: 10.1080/10647440500068321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer BM, Rabello YA, Thurnau GR, et al. The NICHD-MFMU antibiotic treatment of preterm PROM study: impact of initial amniotic fluid volume on pregnancy outcome. Am J Obstet Gynecol. 2006;194:438–45. doi: 10.1016/j.ajog.2005.07.097. [DOI] [PubMed] [Google Scholar]
- 37.May M, Marx A, Seidenspinner S, Speer CP. Apoptosis and proliferation in lungs of human fetuses exposed to chorioamnionitis. Histopathology. 2004;45:283–90. doi: 10.1111/j.1365-2559.2004.01936.x. [DOI] [PubMed] [Google Scholar]