Abstract
Lung transplantation remains the gold standard for patients with end-stage lung disease. Nevertheless, the number of suitable donor lungs for the increasing number of patients on the waiting list necessitates alternative tools to expand the lung donor pool. Modern preservation and lung assessment techniques could contribute to improved function in previously rejected lungs.
Ex vivo lung perfusion (EVLP) already demonstrated its value in identification of transplantable grafts from the higher risk donor pool. Moreover, lungs from EVLP did not show significantly different postoperative results compared to standard criteria lungs. This could be explained by the reduction of the ischemia-reperfusion injury through EVLP application.
The aim of this article is to review technical characteristics and the growing clinical EVLP experience with special attention to EVLP application for donation after cardiac death (DCD) lungs.
MeSH Keywords: Donor Selection, Lung Transplantation, Organ Preservation
Background
Nowadays, the majority of donated lungs are deemed unsuitable. At the same time, the mortality rate on the lung waiting list is 12.8% per year in the USA and 21% per year in the UK. Against this background, only 17% of donated lungs are used in the USA and 15% in the UK [1,2]. Various novel approaches have been popularized to expand the pool of donor lungs: donation after cardiac death (DCD), cadaveric lung graft volume reduction or lobar transplantation, and living donor lobar transplantation.
Ex vivo lung perfusion (EVLP) is one of the most recent techniques in lung donor pool expansion. EVLP is a preservation/assessment technique to select “good” lung grafts from previously high-risk/rejected donors, which provides similar clinical outcomes to standard criteria lungs. The Lund group reported the use of EVLP for 6 recipients, with encouraging results and survival comparable to standard lung transplantation [3,4]. Keshavjee et al. [5] conducted a study on EVLP (HELP trial) in clinical lung transplantation and reported the successful transplantation of 20 high-risk lungs. The incidence of primary graft dysfunction (PGD) in this cohort was only 15% compared to 30% in the routine lung transplantation group.
In this review we will highlight and discuss the clinical value of EVLP in extended criteria donor lungs with a special focus on technical characteristics of EVLP.
Present Status of EVLP
Steen et al. [6] reported the first clinical experience with the application of EVLP. They retrieved lungs from a donor after 190 minutes of cardiopulmonary resuscitation. After that, EVLP was performed for 65 minutes, rendering in a right single lung transplant [6].
After these encouraging results, others [7–10] demonstrated that EVLP in high-risk donors contributes to similar clinical results when compared to standard criteria lungs. Our own group [11] and Wallinder et al. [12] stressed the profound role in organ donor pool expansion of EVLP by normothermic reassessment/reconditioning of previously rejected lungs rendering successful double lung transplants.
Donor Lung Preparation for EVLP
After standard donor lung harvesting, lungs are perfused ante-retrogradely with 5 liters of cold, low- molecular dextran (Perfadex, Medisan, Uppsala, Sweden) solution supplemented with CaCl2, 3.6% tromethamine (THAM, Hospira Inc, Lake Forest, IL), and epoprostenol sodium. In DCD donors, 3L of pneumoplegia are delivered antegradely and 2L retrogradely. For brain-dead donors (DBD), 4L antegradely and 1L retrogradely are perfused. Perfadex is used as a general transport medium.
Once the lungs are at the recipient center, sterile dissection of the main pulmonary artery and trachea is performed with a long left atrial cuff. The left atrial cuff is trimmed appropriately and anastomosed with 4-0 polypropylene to a cannula with an integrated transducer tool for pressure monitoring (Figure 1). Afterwards, the main pulmonary artery is cannulated with a customized cannula and an endotracheal tube (size 8) inserted into the trachea and secured with 2 heavy silk ties (Figure 2). After this important step, the inflated lungs are carefully transferred into the EVLP dome (Vitrolife, Gothenburg, Sweden) to provide humidity and temperature (Figure 3).
Figure 1.

Completed left atrial cuff-cannula anastomosis during preparation for EVLP.
Figure 2.
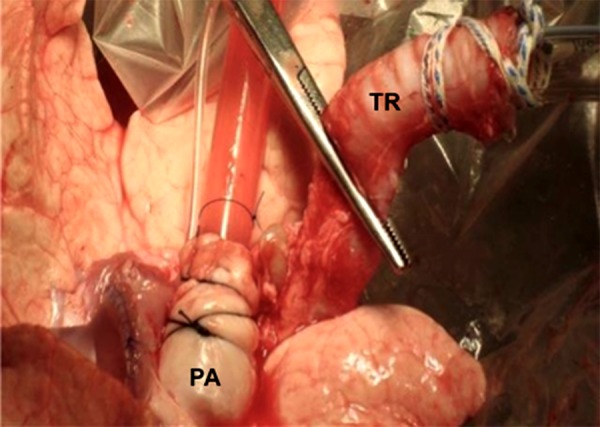
Representative image showing the cannulated pulmonary artery (PA) and intubated trachea (TR).
Figure 3.
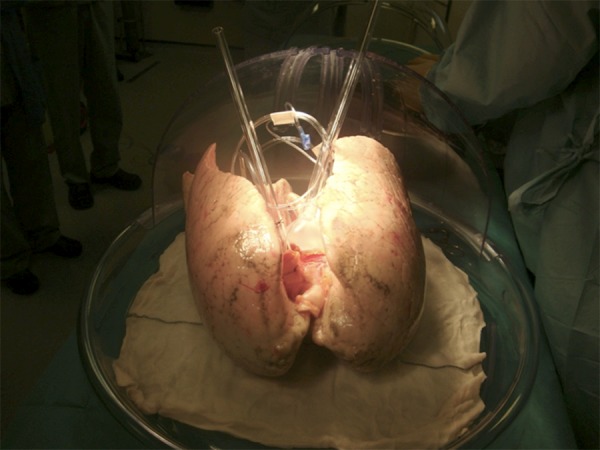
EVLP dome for lung assessment (Vitrolife, Göteborg, Sweden).
Thereafter, the lungs are retrogradely perfused with 1L Perfadex, augmented with CaCl2 and 3.6% THAM buffer. This maneuver warrants adequate deairing and visualization of any spilled clots.
EVLP Circuit and Operating Mode
After the aforementioned steps, the pulmonary cannula is connected to the EVLP system and antegrade perfusion with supplemented Steen Solution (Vitrolife, Gothenburg, Sweden) comprising THAM buffer, heparin, methylprednisolone, and antibiotics. The antibiotic regimen is carefully matched to the donor/recipient culture. After meticulous deairing and connection of the EVLP with the left atrial cuff cannula, perfusion is commenced.
A schematic illustration of the EVLP circuit is shown in Figure 4. The makeup of a dedicated circuit consists of a centrifugal pump, a leukocyte filter, a hollow-fiber oxygenator heat exchanger, and a hardshell reservoir. Perfusate flowing from the left atrium runs into the reservoir, then to the centrifugal pump in order to arrive at the gas exchanger that is linked with the heat and gas cylinder containing a mixture of 6% O2 and 8% CO2 balanced with N2. Here, a deoxygenation and warming process takes place until the fluid reaches the partial gas pressure and temperature of normal venous blood. It is subsequently returned to the pulmonary artery via the leukocyte filter. By gradually augmenting the initial flow of 150 ml/min the target flow of 40% donor calculated cardiac index of 3.0 liters/min/m2 is achieved. Pulmonary artery pressure is maintained ≤15 mm Hg and left atrial pressure ≤5 mm Hg.
Figure 4.
Schematic illustration of the EVLP circuit. LA, left atrium; LAP, left atrial pressure; PA, pulmonary artery; PAP, pulmonary artery pressure; CDI, Terumo Cardiovascular Systems Corp, Ann Arbor, MI.
The lungs are warmed up until the target temperature of 37°C is reached. Cautious recruitment maneuvers and mechanical ventilation are commenced when the graft has achieved 32°C of temperature. The following protective lung ventilation strategies were deployed: tidal volume, 6 to 8 ml/kg; respiratory rate, 7 breaths/min; duration of inspiration/expiration (I/E), 1:2; and positive end-expiratory pressure, 5 to 10 cm H2O. The perfusion is kept for up to 4 hours. In case one of the lungs is estimated as being too damaged for clinical EVLP (e.g., due to pneumonia), a perfusion of the contralateral lung by itself is possible. Attention is required at the moment of division regarding adequate arterial and atrial cuffs as well as a long trachea/bronchus. An extensive overview of the procedure has previously been detailed by the Toronto and Harefield group [8,11].
Assessment During EVLP
Gas exchange is evaluated after full flow has been obtained. Therefore, for 5 minutes the FiO2 supply on the ventilator is enhanced to 100%, by reporting arterial blood gases (ABGs) from perfusate of the left atrial cannula and separately from each pulmonary vein. At the start of ventilation, a flexible bronchoscopy with bronchoalveolar lavage for urgent Gram stain and culture is sampled. Both ABGs and flexible bronchoscopy are repeated every hour. At the beginning as well as the end of EVLP a chest x-ray (CRX) is performed (Figure 5). In difficult cases a CT scan can also be implemented (Figure 6). Condition of the lungs is assessed at arrival, after that every hour by visual and manual evaluation for atelectasis, consolidation, and pulmonary edema. After each evaluation a pulmonary recruitment is conducted every 30 minutes by raising the tidal volume with ensuing inspiratory hold maneuvers up to 25 cm H2O for ten seconds. At the start of ventilation a deflation test for compliance is conducted, which is repeated hourly. In due consideration of all parameters observed the decision to implant the lungs is made.
Figure 5.
Chest x-ray at the beginning of EVLP (A) and at the end of assessment (B). It is clearly visible that EVLP ameliorates congestion of the lungs.
Figure 6.

CT scan during EVLP assessment.
EVLP Experience
A small amount of centers worldwide have applied EVLP clinically and have reported promising outcomes. Steen et al. [6] were the first to report about clinical experience utilizing the method of EVLP as a means to reassess lungs from a DCD donor prior to implantation. Subsequently, the first account on the usage of short-term EVLP for the reconditioning of initially rejected lungs due to their low oxygenation was published [13]. The 30-day survival rate of this group was at 100%; in comparison to contemporaneous lung transplants applying standard criteria organs, no statistical dissimilarities with regards to time in mechanical ventilation, time in intensive care unit (ICU), and overall hospital stay were determined [4].
The reported first promising experience by Steen [6] resulted in a number of reports accounting efforts trying to determine the mechanism by which EVLP facilitates lung reconditioning. By comparing the results of prolonged cold ischemia to ELVP, Cypel et al. [7] were able to show that EVLP preserves lung quality by discontinuing prolonged cold storage injury and re-establishing a physiological metabolic cell status. While Yamada et al. [14] reported early resolution of hydrostatic pulmonary edema during EVLP, Karamanou et al. [15] reported a decrease in the donor bacterial load throughout EVLP, proposing that it may have the ability to protect the immunosuppressed recipient from donor-acquired infections.
Moreover, the possible negative effects of EVLP have been subject of examination. Cypel et al. [8], for instance, reported exceptional post-transplant lung function, no case of edema, and normal histology after prolonged EVLP of non-injured swine lungs. More recently, Otani et al. [16] showed that no differences occurred after transplantation when contrasting swine DCD lungs after 2 hours of cold ischemia to 2 hours of EVLP. These reports validated that EVLP could be applied without injuring the lung.
The outcomes of the first clinical trial (HELP trial) performed by the Toronto group were published in 2011. Out of 23 high-risk donors who underwent EVLP, 20 were deemed appropriate for transplantation. Criteria regarding the termination of perfusion and non-transplantability consisted of a PVR, dynamic compliance, peak inspiratory pressure decline by more than 15%, and ΔpO2/FiO2 less than 350 mmHg. By comparing the ELVP group to contemporary transplants utilizing standard criteria organs, no noteworthy dissimilarities could be found with respect to PGD, day in mechanical ventilation after transplant, ICU stay, hospital stay, or 30-day mortality [5].
The Viennese group published their experience with EVLP in 2012, describing the results of 9 double-lung transplants [17]. Early results, for example, days on mechanical ventilation, ICU stay, hospital stay and 30-day mortality proved to be similar to 119 standard preservation transplants.
At the ISHLT meeting in 2013, the groups from Toronto, Vienna and Paris introduced their EVLP experience [18]. Overall, 125 EVLPs had been conducted with a transplantation rate of 82.5%. Comparable to earlier reports, the occurrence of PGD at 72 hours was at 5%, the 12-month mortality at 12%.
In 2012, our group made public their experience with 6 double-lung transplants obtained from 13 EVLP assessments [11]. Even though the average requirement of mechanical ventilation post-transplant was more than 7 days, each of the patients treated was discharged from the hospital and alive at three months of follow-up.
The Newcastle group reported 6 lung transplants from 18 EVLPs, with all patients surviving until release from the hospital [19]. Moreover, their experience suggested a potential advantage of EVLP: the numbers of bacterial loads in bronchoalveolar lavages were considerably lower at the end of EVLP than on samples obtained at its beginning. The authors also described that, in spite of the reduction of bacterial loads, a growth of Candida sp. load was detected in 2 cases. With the knowledge of this finding, Amphotericin B was now routinely added to the perfusate. Prospective studies need to further clarify the function of anti-fungal therapy in EVLP.
As an FDA-mandated multicenter clinical trial, the NOVEL Lung Trial is conducting research on EVLP for marginal donors. Their first report described 31 patients who received EVLP lungs. Early results turned out to be comparable to 31 non-EVLP controls [20]. In 2014, an update on the trial results was given, describing 76 EVLPs resulting in 42 lung transplants [21]. Compared to 42 controls, early results and 1-year survival were the same.
The Gothenburg group has published their results describing 11 EVLPs over the course of an 18-month period [12]. Overall, 8 double and 3 single lung transplants were conducted and, even though hospital stays were comparable, mechanical ventilation and ICU stay turned out to be longer in the EVLP cohort in comparison to standard transplants. However, hospital survival in the EVLP cohort was at 100%.
Moreover, the utilization of EVLP as a platform to provide different medications was examined. Nakajima et al. [22] added nitroglycerin and dibutyryl cyclic adenosine monophosphate to Steen solution during EVLP of lungs subjected to 4 hours of warm ischemia. Subsequent to single LTx, EVLP lungs showed improved function, lower histological signs of acute lung injury as well as better microvascular patency in comparison to standard preservation lungs. Mulloy et al. [23], on the other hand, in a model of 1 hour of warm ischemia in pigs added a selective adenosine 2A agonist to the perfusate. Following procurement, lungs subjected to additional 4 hours of cold ischemia and subsequently 4 hours of EVLP functioned considerably better than lungs subjected to 4 hours of cold ischemia alone, while showing lower rates of histological lung injury as well as lower levels of inflammatory cytokines after lung transplantation.
Finally, several groups have advanced further clinical utilization of uncontrolled DCDs. An important source of organs for transplantation after ex vivo assessment are DCD category I and II uncontrolled donor lungs, as described in the pioneering work of Egan et al. [24] in 1991. Further research on behalf of the Hospital Universitario de Hierro has initially exposed high incidence of PGD, with 17% hospital mortality as well as 57% 1-year survival from 29 uncontrolled DCD lung transplants [25]. Recently, Tom Egan has demonstrated the realizability of a comparable approach in a clinical trial in the USA by having procured and perfused uncontrolled DCD lungs [26]. Advancing the development of this technique might result in extending the safe ex vivo perfusion time and treatment of different pathologies besides pulmonary edema.
Conclusions
The usage of ELVP resulted in raising the amount of lung transplants by determining grafts from the suboptimal donor pool whose performance is considered equal to those regarded standard. Since EVLP is being utilized more extensively and new ways of therapy are being adapted for the clinical environment, its application promises a new era in lung transplantation.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
Source of support: Self financing
References
- 1.The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. OPTN/SRTR annual report. 2009. http://optn.transplant.hrsa.gov/ar2009/
- 2.NHS Blood and Transplant. Transplant activity in the UK. Activity Report 2009/2010. http://www.nhsbt.nhs.uk/downloads/pdfs/temp/report.pdf.
- 3.Ingemansson R, Eyolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009;87:255–60. doi: 10.1016/j.athoracsur.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 4.Lindstedt S, Hlebowicz J, Kolu B, et al. Comparative outcome of double lung transplantation using conventional donor lungs and non- acceptable donor lungs reconditioned ex-vivo. Interact CardioVasc Thorac Surg. 2011;12:162–65. doi: 10.1510/icvts.2010.244830. [DOI] [PubMed] [Google Scholar]
- 5.Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med. 2011;364:1431–40. doi: 10.1056/NEJMoa1014597. [DOI] [PubMed] [Google Scholar]
- 6.Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet. 2001;357:825–29. doi: 10.1016/S0140-6736(00)04195-7. [DOI] [PubMed] [Google Scholar]
- 7.Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:2262–69. doi: 10.1111/j.1600-6143.2009.02775.x. [DOI] [PubMed] [Google Scholar]
- 8.Cypel M, Yeung JC, Hirayama S, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27:1319–25. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200–6. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Ingemansson R, Eyjolfsson A, Mared L, et al. Clinical transplantation of initially rejected donor lungs after reconditioning ex vivo. Ann Thorac Surg. 2009;87:255–60. doi: 10.1016/j.athoracsur.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 11.Zych B, Popov AF, Stavri G, et al. Early outcomes of bilateral sequential single lung transplantation after ex vivo lung evaluation and reconditioning. J Heart Lung Transplant. 2012;31:274–81. doi: 10.1016/j.healun.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Wallinder A, Ricksten SE, Silverborn M, et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg. 2014;45:40–44. doi: 10.1093/ejcts/ezt250. discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 13.Steen S, Ingemansson R, Eriksson L, et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg. 2007;83:2191–94. doi: 10.1016/j.athoracsur.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Yamada T, Nakajima D, Sakamoto J, et al. Reconditioning of lungs with pulmonary edema in ex vivo lung perfusion circuit. J Heart Lung Transplant. 2011;30(4 Suppl):S144. [Google Scholar]
- 15.Karamanou DM, Perry J, Walden HR, et al. The effect of ex-vivo perfusion on the microbiological profile of the donor lung. J Heart Lung Transplant. 2010;29(2 Suppl):S94. [Google Scholar]
- 16.Otani S, Oto T, Kakishita T, et al. Early effects of the ex vivo evaluation system on graft function after swine lung transplantation. Eur J Cardiothorac Surg. 2011;40:956–61. doi: 10.1016/j.ejcts.2010.12.071. [DOI] [PubMed] [Google Scholar]
- 17.Aigner C, Slama A, Hötzenecker K, et al. Clinical ex vivo lung perfusion – pushing the limits. Am J Transplant. 2012;12:1839–47. doi: 10.1111/j.1600-6143.2012.04027.x. [DOI] [PubMed] [Google Scholar]
- 18.Cypel M, Aigner C, Sage E, et al. Three center experience with clinical normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2013;32:S16. [Google Scholar]
- 19.Andreasson A, Karamanou DM, Perry JD, et al. The effect of ex vivo lung perfusion on microbial load in human donor lungs. J Heart Lung Transplant. 2014;33(9):910–16. doi: 10.1016/j.healun.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez PG, Davis RD, D’Ovidio F, et al. Normothermic ex vivo. lung perfusion as an assessment of marginal donor lungs – the NOVEL lung trial. J Heart Lung Transplant. 2013;32:S16–17. [Google Scholar]
- 21.Sanchez PG, Davis RD, D’Ovidio F, et al. The NOVEL Lung Trial One-Year Outcomes. J Heart Lung Transplant. 2014;33:S71–72. [Google Scholar]
- 22.Nakajima D, Chen F, Yamada T, et al. Reconditioning of lungs donated after circulatory death with normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2012;31:187–93. doi: 10.1016/j.healun.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Mulloy DP, Stone ML, Crosby IK, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results insuperior lung function. J Thorac Cardiovasc Surg. 2012;144:1208–15. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egan TM, Lambert CJ, Jr, Reddick R, et al. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg. 1991;52:1113–20. doi: 10.1016/0003-4975(91)91290-c. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non- heart-beating donors revisited. J Heart Lung Transplant. 2012;31:349–53. doi: 10.1016/j.healun.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Egan T, Blackwell J, Forrest L, et al. Evaluation of Human Lungs From Category 1 Non-Heart-Beating Donors (NHBDs) With Ex-Vivo Lung Perfusion (EVLP) in the U.S. Chest. 2014;145:635A. [Google Scholar]




