Abstract
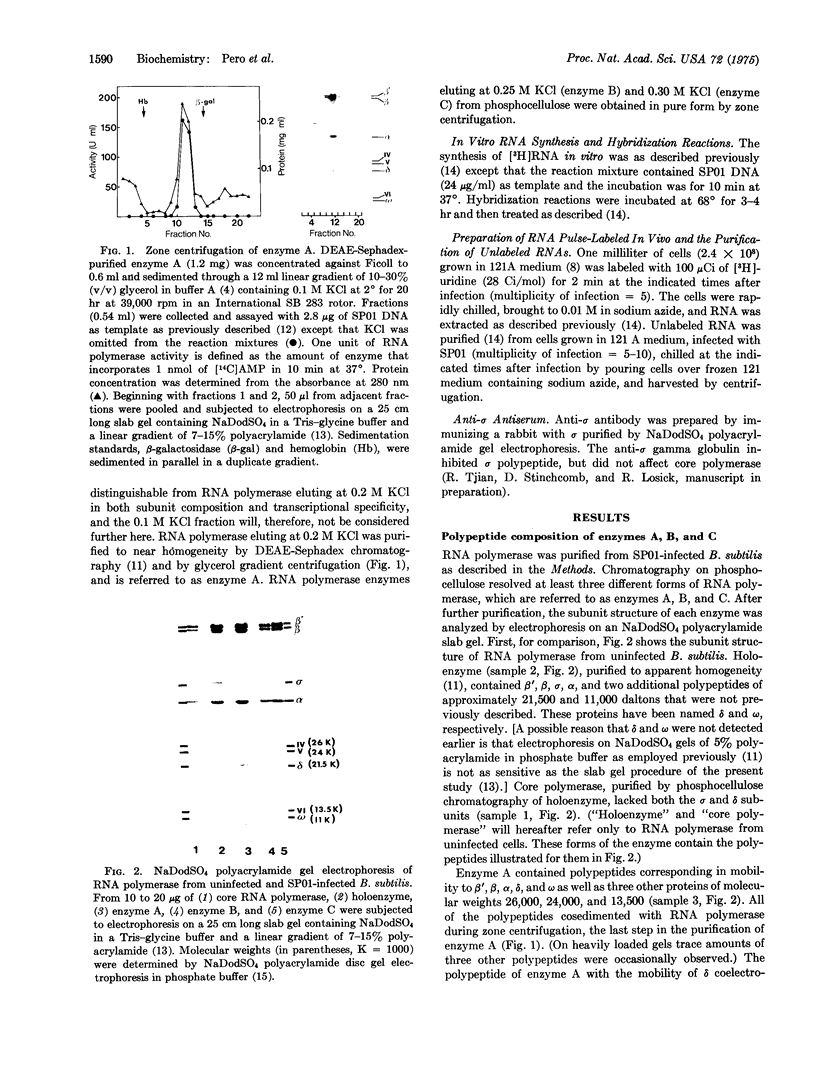
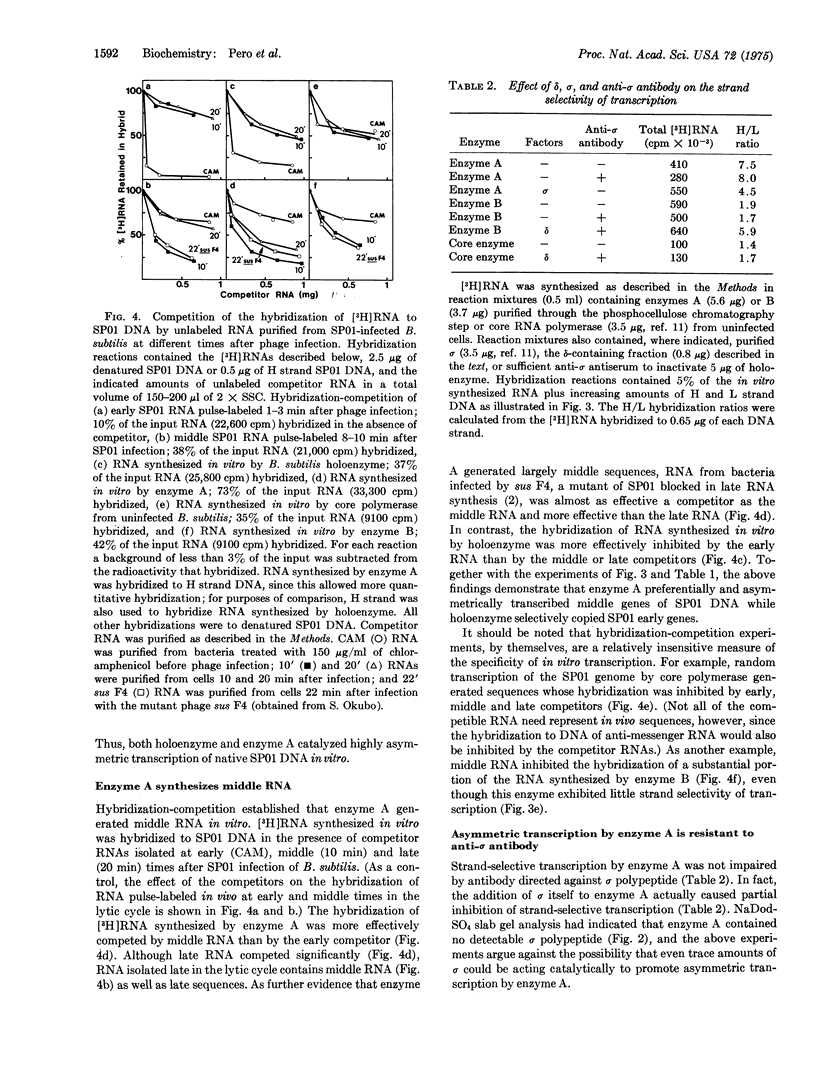
An RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) has been purified from phage-SP01-infected Bacillus subtilis that copies RNA almost exclusively from the heavy strand of native SP01 DNA, the DNA strand from which "middle" and "late" classes of RNA are copied in vivo. Hybridization-competition established that this RNA polymerase termed enzyme A, preferentially synthesizes middle RNA in vitro. Enzyme A contains beta',beta, alpha, and two newly identified host polypeptides, variation of (21,500 daltons) and omega (11,000 daltons). All of these polypeptides are associated with highly purified RNA polymerase from uninfected bacteria. In addition, enzyme A contains phage-induced subunits of 26,000, 24,000, and 13,500 daltons. Enzyme A lacks sigma polypeptide, and strand-selective transcription by this enzyme is resistant to anti-sigma antibody. A reconstitution experiment strongly suggests that the host variation of protein is required in addition to a phage-induced subunit(s) (or an unidentified phage-induced modification) for strand-selective transcription of SP01 middle genes in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chessin H., Summers W. C. Initiation by RNA polymerase on UV or x-ray damaged T7 DNA. Biochem Biophys Res Commun. 1970 Jan 6;38(1):40–45. doi: 10.1016/0006-291x(70)91080-6. [DOI] [PubMed] [Google Scholar]
- Duffy J. J., Geiduschek E. P. Transcription specificity of an RNA polymerase fraction from bacteriophage SP01-infected B. subtilis. FEBS Lett. 1973 Aug 15;34(2):172–174. doi: 10.1016/0014-5793(73)80786-0. [DOI] [PubMed] [Google Scholar]
- Fox T. D., Pero J. New phage-SPO1-induced polypeptides associated with Bacillus subtilis RNA polymerase. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2761–2765. doi: 10.1073/pnas.71.7.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita D. J., Ohlsson-Wilhelm B. M., Geiduschek E. P. Transcription during bacteriophage SPO1 development: mutations affecting the program of viral transcription. J Mol Biol. 1971 Apr 28;57(2):301–317. doi: 10.1016/0022-2836(71)90348-2. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriophage SPO1 development: six classes of SPO1 RNA. J Mol Biol. 1971 Apr 28;57(2):279–297. doi: 10.1016/0022-2836(71)90346-9. [DOI] [PubMed] [Google Scholar]
- Gage L. P., Geiduschek E. P. RNA synthesis during bacteriphage SPO1 development. II. Some modulations and prerequisites of the transcription program. Virology. 1971 Apr;44(1):200–210. doi: 10.1016/0042-6822(71)90165-6. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T., Fujita D. J., Olsson-Wilhelm B. M. The genetics of bacteriophage SPO1. Biken J. 1972 Jun;15(2):81–97. [PubMed] [Google Scholar]
- Shorenstein R. G., Losick R. Purification and properties of the sigma subunit of ribonucleic acid polymerase from vegetative Bacillus subtilis. J Biol Chem. 1973 Sep 10;248(17):6163–6169. [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. In vivo and in vitro transcription by ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1483–1489. [PubMed] [Google Scholar]
- Spiegelman G. B., Whiteley H. R. Purification of ribonucleic acid polymerase from SP82-infected Bacillus subtilis. J Biol Chem. 1974 Mar 10;249(5):1476–1482. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]





