Abstract
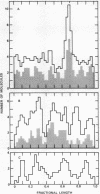
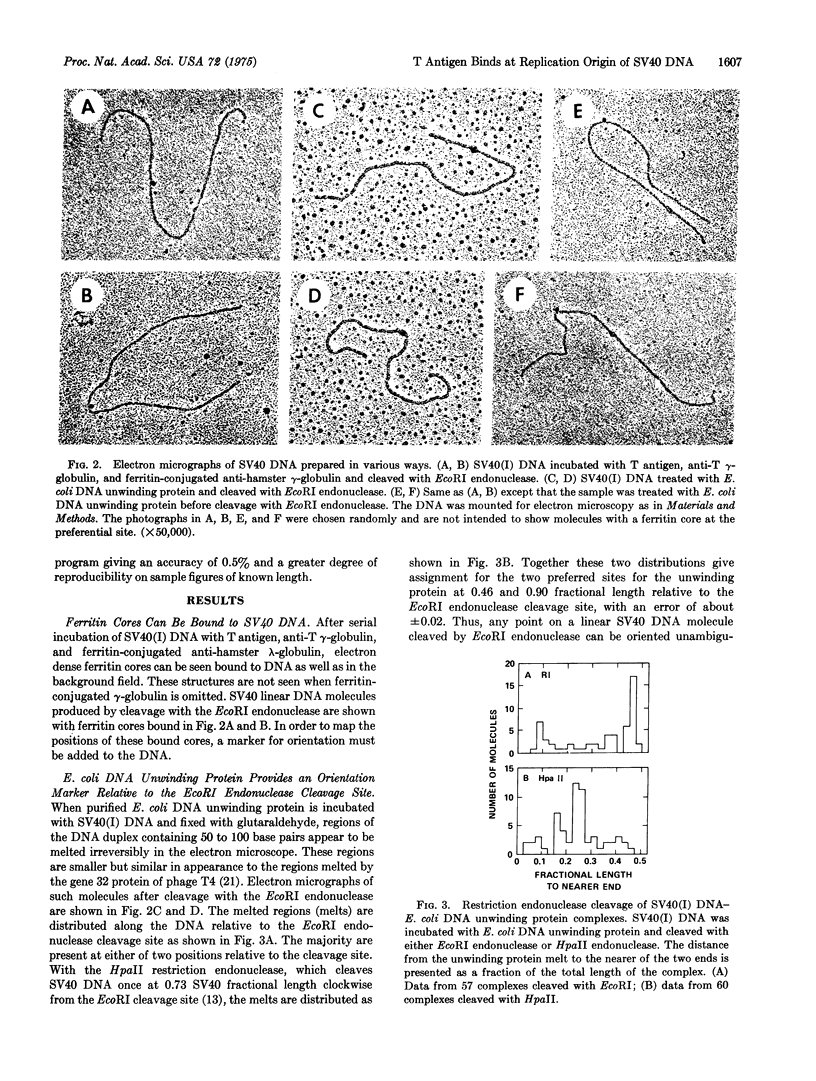
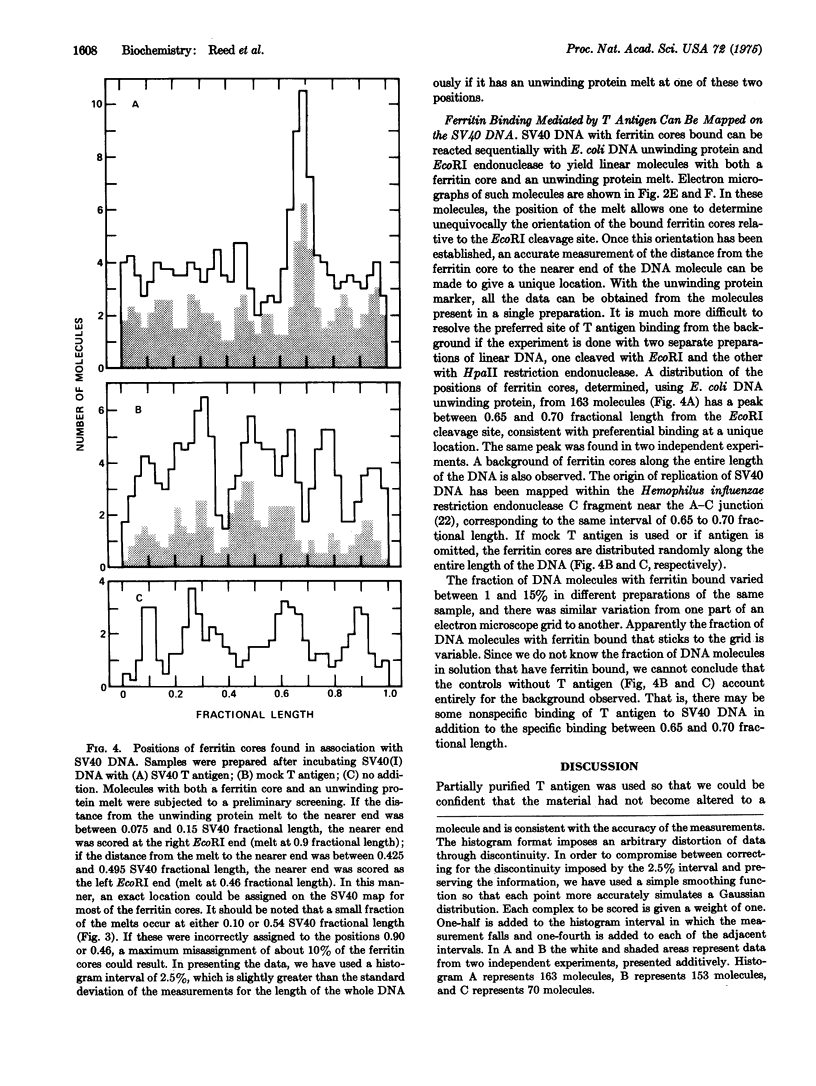
A technique employing ferritin-conjugated antibody has been developed to visualize specific protein-DNA complexes in the electron microscope and has been used to demonstrate the preferential binding of simian virus 40 (SV40) T antigen at or near the origin of replication of SV40 DNA, 0.67 fractional length clockwise from the EcoRI restriction endonuclease cleavage site. urified covalently closed supercoiled circles of SV40DNA were treated with partially purified T antigen and the complex was stabilized by crosslinking with glutaraldehyde. Hamster antiT antigen gamma-globulin, ferritin-labeled goat anti-hamster gamma-globulin, and glutaraldehyde were then added sequentially. The location of the bound ferritin cores was measured with respect to the EcoRI cleavage site and the orientation of the cores relative to the ends of the DNA was determined with respect to the locations of Escherichia coli DNA unwinding protein, which binds to covalently closed supercoiled SV40 DNA at either of two preferred sites, 0.46 or 0.90 fractional length clockwide from the EcoRI cleavage site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S., Ternynck T. Biologically active water-insoluble protein polymers. I. Their use for isolation of antigens and antibodies. J Biol Chem. 1967 Apr 10;242(7):1651–1659. [PubMed] [Google Scholar]
- Carroll R. B., Hager L., Dulbecco R. Simian virus 40 T antigen binds to DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3754–3757. doi: 10.1073/pnas.71.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Villano B. C., Defendi V. Characterization of the SV40 T antigen. Virology. 1973 Jan;51(1):34–46. doi: 10.1016/0042-6822(73)90363-2. [DOI] [PubMed] [Google Scholar]
- Delius H., Mantell N. J., Alberts B. Characterization by electron microscopy of the complex formed between T4 bacteriophage gene 32-protein and DNA. J Mol Biol. 1972 Jun 28;67(3):341–350. doi: 10.1016/0022-2836(72)90454-8. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Koller T., Sogo J. M., Bujard H. An electron microscopic method for studying nucleic acid-protein complexes. Visualization of RNA polymerase bound to the DNA of bacteriophages T7 and T3. Biopolymers. 1974 May;13(5):995–1009. doi: 10.1002/bip.1974.360130514. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Henderson I. C., Hudson J. SV40 T antigen: partial purification and properties. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):283–289. doi: 10.1101/sqb.1974.039.01.037. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Viable deletion mutants of simian virus 40: selective isolation by means of a restriction endonuclease from Hemophilus parainfluenzae. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4879–4883. doi: 10.1073/pnas.71.12.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Location of the T4 gene 32 protein binding site on simian virus 40 DNA. J Virol. 1973 Dec;12(6):1631–1632. doi: 10.1128/jvi.12.6.1631-1632.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. R., Prockop D. J. Ferritin-conjugated antibodies used for labeling of organelles involved in the cellular synthesis and transport of procollagen. Proc Natl Acad Sci U S A. 1974 May;71(5):2033–2037. doi: 10.1073/pnas.71.5.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. SV40: T antigen, the A function and transformation. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):267–276. doi: 10.1101/sqb.1974.039.01.035. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Hopkins N., Davis R. W., Delius H., Mulder C. Action of Escherichia coli P1 restriction endonuclease on simian virus 40 DNA. J Mol Biol. 1974 Nov 15;89(4):517–544. doi: 10.1016/0022-2836(74)90034-5. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Tegtmeyer P., Ishikawa A., Ozer H. L. Antigenic phenotypes and complementation groups of temperature-sensitive mutants of simian virus 40. J Virol. 1974 Mar;13(3):662–665. doi: 10.1128/jvi.13.3.662-665.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVER J. L. Application of a microtechnique to viral serological investigations. J Immunol. 1962 Mar;88:320–329. [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Spira G., Popescu M., Cymbalista S., Biezunski N., Goldblum N. Isolation of the SV40 induced tumor ("T") antigen from transformed hamster kidney cells. Arch Gesamte Virusforsch. 1972;37(2):236–242. doi: 10.1007/BF01268006. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Kirschstein R. L., Habel K. Mutants of simian virus 40 differing in plaque size, oncogenicity, and heat sensitivity. J Bacteriol. 1966 Oct;92(4):990–994. doi: 10.1128/jb.92.4.990-994.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Altered patterns of protein synthesis in infection by SV40 mutants. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):9–15. doi: 10.1101/sqb.1974.039.01.004. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J. H., Bertsch L. L., Kornberg A. The deoxyribonucleic acid unwinding protein of Escherichia coli. Properties and functions in replication. J Biol Chem. 1975 Mar 25;250(6):1972–1980. [PubMed] [Google Scholar]
- Wiblin C. N., MacPherson I. A. The transformation of BHK 21 hamster cells by simian virus 40. Int J Cancer. 1972 Sep 15;10(2):296–309. doi: 10.1002/ijc.2910100210. [DOI] [PubMed] [Google Scholar]