Abstract
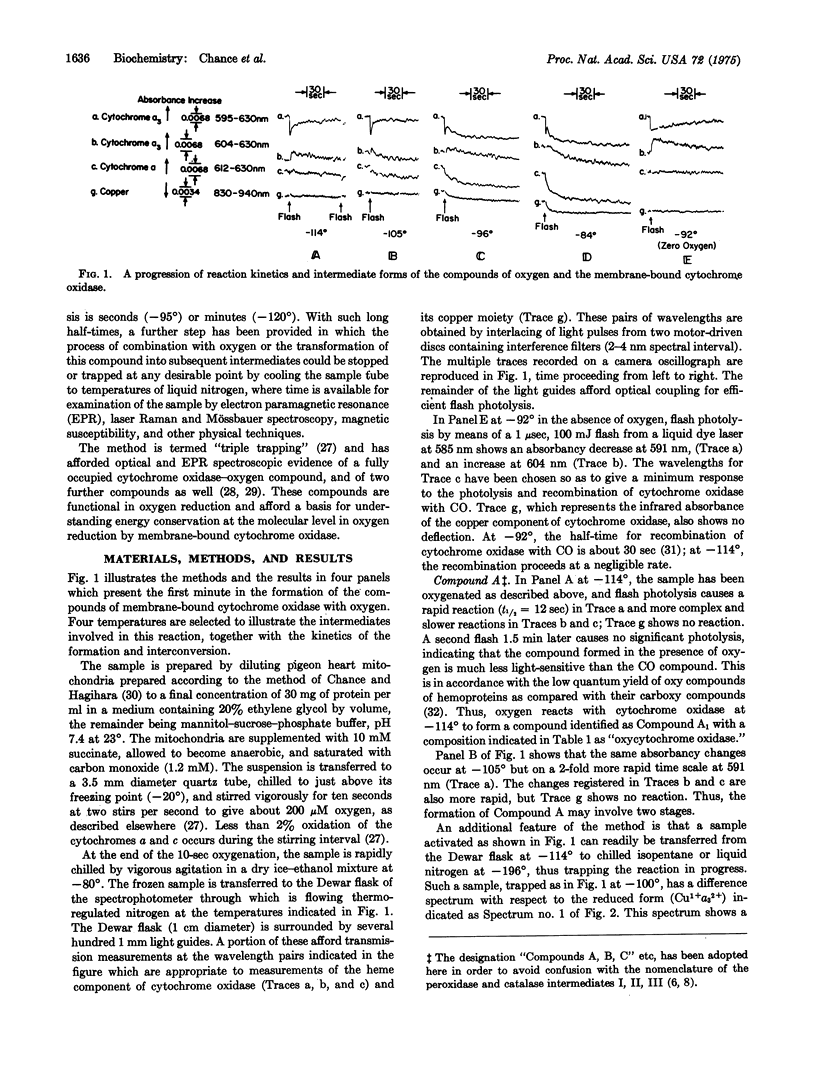
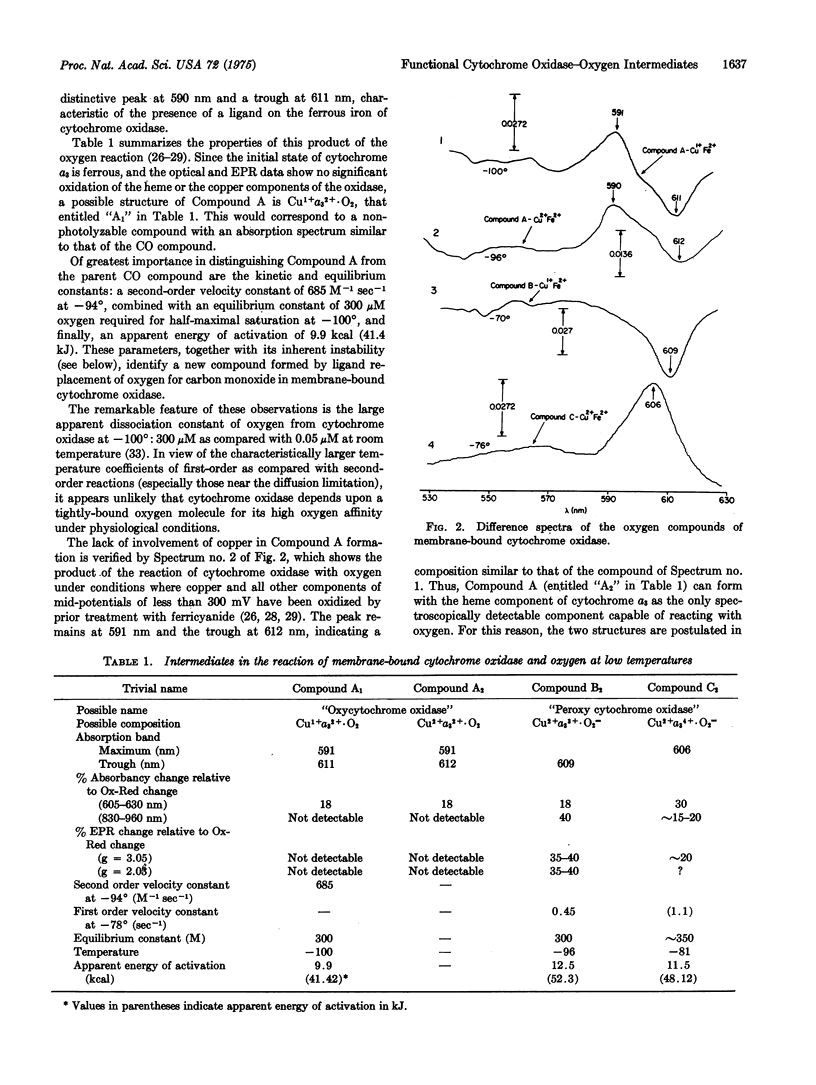
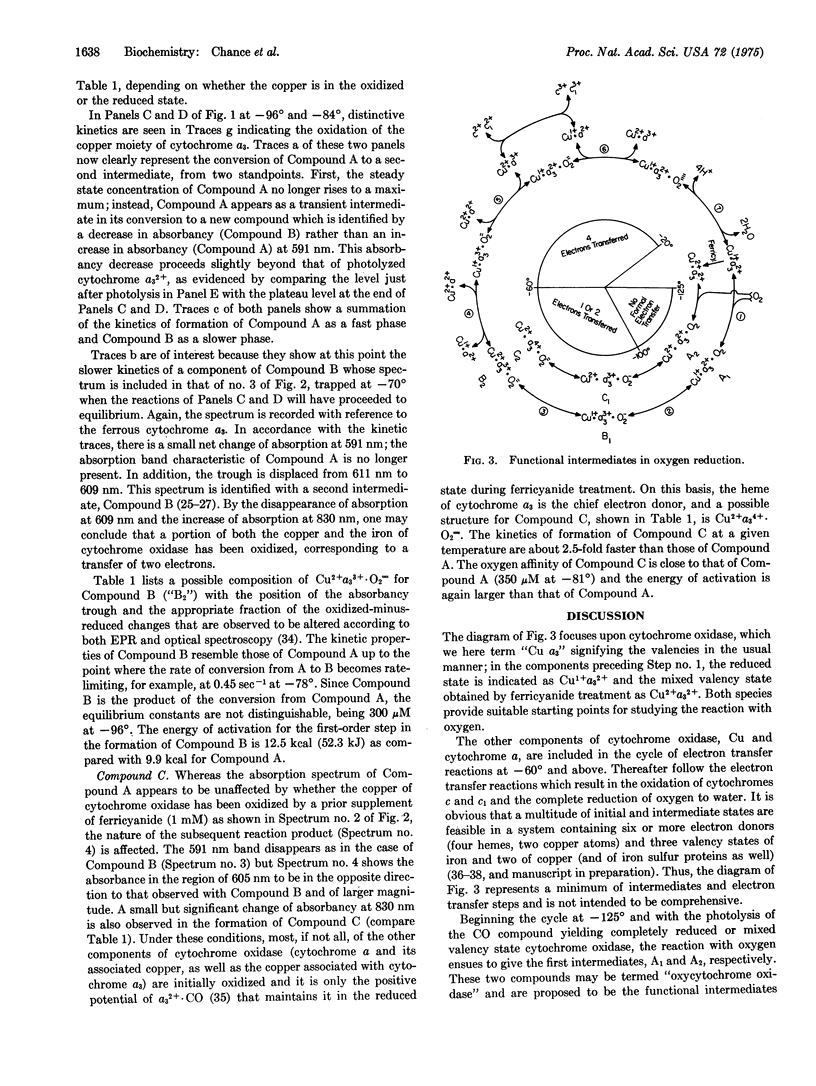
The development of a low temperature kinetic method for the flash photolysis of the compounds of membrane-bound cytochrome a3 with carbon monoxide in the presence of oxygen affords evidence for three categories of functional intermediate compounds of cytochrome a3 and oxygen. The three classes are identified as follows: Compounds of Type A are considered to be "oxy" compounds of the ferrous heme. They have the composition a3-2+. O2. Compounds of Type B are considered to be peroxide compounds (CU-2+A3-3+ O-2= or CU-2+A3-3+ O2H2) or the equivalent heme Fe-Cu peroxide bridge structures. Compounds of Type C are formed from the ferricyanide pretreated oxidase and may involve higher oxidation states of the heme iron such as quadrivalent iron, and peroxide. Kinetic and equilibrium studies show these compounds to be functional in oxygen reduction in the sequence A yields B yield cytochromes a, c, c1, etc.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B. Enzyme-substrate compounds. Adv Enzymol Relat Subj Biochem. 1951;12:153–190. doi: 10.1002/9780470122570.ch3. [DOI] [PubMed] [Google Scholar]
- Caughey W. S., Barlow C. H., Maxwell J. C., Volpe J. A., Wallace W. J. Reactions of oxygen with hemoglobin, cytochrome c oxidase and other hemeproteins. Ann N Y Acad Sci. 1975 Apr 15;244:1–9. doi: 10.1111/j.1749-6632.1975.tb41517.x. [DOI] [PubMed] [Google Scholar]
- Chance B., Nishimura M. ON THE MECHANISM OF CHLOROPHYLL-CYTOCHROME INTERACTION: THE TEMPERATURE INSENSITIVITY OF LIGHT-INDUCED CYTOCHROME OXIDATION IN CHROMATIUM. Proc Natl Acad Sci U S A. 1960 Jan;46(1):19–24. doi: 10.1073/pnas.46.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B. On the mechanism of the reaction of cytochrome oxidase with oxygen. Ann N Y Acad Sci. 1975 Apr 15;244:163–173. doi: 10.1111/j.1749-6632.1975.tb41529.x. [DOI] [PubMed] [Google Scholar]
- DeVault D., Chance B. Studies of photosynthesis using a pulsed laser. I. Temperature dependence of cytochrome oxidation rate in chromatium. Evidence for tunneling. Biophys J. 1966 Nov;6(6):825–847. doi: 10.1016/s0006-3495(66)86698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douzou P., Sireix R., Travers F. Temporal resolution of individual steps in an enzymic reaction at low temperature. Proc Natl Acad Sci U S A. 1970 Jul;66(3):787–792. doi: 10.1073/pnas.66.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M., Chance B. Studies on the electron transport chain at subzero temperatures: electron transport at site 3. Arch Biochem Biophys. 1972 Jul;151(1):304–315. doi: 10.1016/0003-9861(72)90501-2. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., AINSWORTH S. Photosensitivity of haem compounds. Nature. 1957 Dec 21;180(4599):1416–1417. doi: 10.1038/1801416b0. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. KINETIC OBSERVATIONS ON THE NEAR INFRARED BAND OF CYTOCHROME C OXIDASE. J Biol Chem. 1965 Jun;240:2694–2698. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilgenfritz G., Schuster T. M. Kinetics of oxygen binding to human hemoglobin. Temperature jump relaxation studies. J Biol Chem. 1974 May 10;249(9):2959–2973. [PubMed] [Google Scholar]
- Lemberg R., Mansley G. E. Cytochrome oxidase and its derivatives. V. The reaction of ferrous cytochrome c oxidase with oxygen and hydrogen peroxide in the presence of sodium dithionite. Biochim Biophys Acta. 1966 Apr 12;118(1):19–35. doi: 10.1016/s0926-6593(66)80141-8. [DOI] [PubMed] [Google Scholar]
- ORII Y., OKINUKI K. Studies on cytochrome a. IX. Significance of oxygenated cytochrome a in the cytochrome oxidase mechanism. J Biochem. 1963 Jun;53:489–499. doi: 10.1093/oxfordjournals.jbchem.a127728. [DOI] [PubMed] [Google Scholar]
- Oshino N., Sugano T., Oshino R., Chance B. Mitochondrial function under hypoxic conditions: the steady states of cytochrome alpha+alpha3 and their relation to mitochondrial energy states. Biochim Biophys Acta. 1974 Dec 19;368(3):298–310. doi: 10.1016/0005-2728(74)90176-5. [DOI] [PubMed] [Google Scholar]
- Rieske J. S. Changes in oxidation-reduction potential of cytochrome b observed in the presence of antimycin A. Arch Biochem Biophys. 1971 Jul;145(1):179–193. doi: 10.1016/0003-9861(71)90025-7. [DOI] [PubMed] [Google Scholar]
- Tyler D. D., Estabrook R. W. The influence of deuterium oxide and organic solvents on the interaction of respiratory chain components. J Biol Chem. 1966 Apr 25;241(8):1672–1680. [PubMed] [Google Scholar]
- Wilson D. F., Leigh J. S., Jr Heme-heme interaction in cytochrome c oxidase in situ as measured by EPR spectroscopy. Arch Biochem Biophys. 1972 May;150(1):154–163. doi: 10.1016/0003-9861(72)90022-7. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Chance B., Kajiwara S. Crystalline cytochrome c peroxidase and complex ES. J Biol Chem. 1966 Jun 25;241(12):2981–2982. [PubMed] [Google Scholar]


