Abstract
Background
Tumour cells show greater dependency on glycolysis so providing a sufficient and rapid energy supply for fast growth. In many breast cancers, estrogen, progesterone and epidermal growth factor receptor-positive cells proliferate in response to growth factors and growth factor antagonists are a mainstay of treatment. However, triple negative breast cancer (TNBC) cells lack receptor expression, are frequently more aggressive and are resistant to growth factor inhibition. Downstream of growth factor receptors, signal transduction proceeds via phosphatidylinositol 3-kinase (PI3k), Akt and FOXO3a inhibition, the latter being partly responsible for coordinated increases in glycolysis and apoptosis resistance. FOXO3a may be an attractive therapeutic target for TNBC. Therefore we have undertaken a systematic review of FOXO3a as a target for breast cancer therapeutics.
Methods
Articles from NCBI were retrieved systematically when reporting primary data about FOXO3a expression in breast cancer cells after cytotoxic drug treatment.
Results
Increased FOXO3a expression is common following cytotoxic drug treatment and is associated with apoptosis and cell cycle arrest. There is some evidence that metabolic enzyme expression is also altered and that this effect is also elicited in TNBC cells. FOXO3a expression serves as a positive prognostic marker, especially in estrogen (ER) receptor positive cells.
Discussion
FOXO3a is upregulated by a number of receptor-dependent and -independent anti-cancer drugs and associates with apoptosis. The identification of microRNA that regulate FOXO3a directly suggest that it offers a tangible therapeutic target that merits wider evaluation.
Keywords: Triple negative breast cancer, Phosphatidylinositol 3-kinase, Metabolism, Glycolysis, Oxidative stress, Apoptosis
Background
Breast cancer is the third most frequent cancer worldwide. Amongst women, it is the most common malignancy, making up 21% of all new cancer diagnoses. Survival rates have been steadily extending over the past 50 years, primarily due to improvements in diagnosis and treatment.
The drivers of proliferation in breast cancer are also the phenotypic drug targets; hormone (estrogen, progesterone and HER2) receptors are commonly overexpressed. This knowledge has been harnessed in development of targeted therapies for breast cancer patients which inhibit hormone receptor activity and can be co-administered with the conventional, but non-specific, radiation and chemotherapy. The earliest approved therapy for endocrine related breast cancers was tamoxifen, an anti-estrogen receptor (ER)-targeting compound. These agents are commonly used alongside general chemotherapy that induces DNA damage through agents such as cisplatin which act to reduce the DNA damage repair response or anti-angiogenic agents [1,2].
The highly aggressive triple negative breast cancer (TNBC), with a prevalence of 15% of breast cancer cases often presenting in younger patients, is characterised by tumours that lack expression of ER, progesterone receptor and HER2/neu and is associated with a poor clinical prognosis [3]. There is no targeted treatment regime for this type of breast cancer and many patients experience relapse to cytotoxic chemotherapy within 3 years of diagnosis, as well as a higher incidence and probability of metastasis [4,5].
The forkhead box O (FOXO) family is activated by and limits the negative consequences of oxidative stress, metabolic dysregulation, growth factor withdrawal and DNA damage. At a molecular level, FOXO3a can be activated by an increased AMP/ATP ratio through phosphorylation catalysed by 5′ AMP-activated protein kinase (AMPK) at six conserved sites, five of which are located within the transactivation domain [6]. In response to a reduced energy supply FOXO3a transcription factors act as important regulators of cellular proliferation, cell cycle arrest, apoptosis, autophagy and metabolism [7,8].
Cancer cells may be distinguished from healthy cells in part by their metabolic phenotype. Solid tumours are often hypoxic and so express hypoxia-inducible factor 1 (HIF1), which increases expression of glycolytic enzymes, glucose transporters and inhibitors of mitochondrial metabolism allowing cellular adaptation through reliance on glycolysis to produce ATP in low oxygen environments [9,10]. This is associated with an increased cellular uptake of glucose in order to maintain energy homeostasis and is largely mediated upstream through PI3k-regulated FOXO transcription factors [11]. Removal of growth factors increases nuclear localisation of FOXO3a. Nuclear FOXO3a binds to P300, which then detaches from its transcription factor complex with HIF1 so suppresses glycolytic enzyme expression.
Phosphatidylinositol 3-kinase (PI3k)-activated Akt inhibits the activity of FOXO3a by phosphorylation of key residues, Thr32, Ser253 and Ser315; Akt-phosphorylated FOXO3a is then chaperoned by 14-3-3 proteins so obscuring FOXO3a-DNA binding sites and further preventing its activity within the nucleus [12]. FOXO3a is subsequently exported into the cytoplasm, ubiquitinated and degraded via the proteasome. Stress-induced phosphorylation of 14-3-3 by c-Jun N-terminal kinase (JNK) reduces 14-3-3 protein-FOXO3a binding capacity and directly inhibits nuclear export of FOXO3a thereby increasing transcriptional activity of FOXO3a [13]. Further metabolic control of FOXO3a activity is achieved following nuclear shuttling by acetylation and NAD+-dependent deacetylation.
Genomic analysis of TNBC has revealed frequent associations with mutations in class 1 PI3k which lies directly upstream of FOXO3a [14]. Mutations within the tumour suppressor PTEN, a negative regulator of Akt activation, can result in constitutively active Akt and inhibit FOXO3a, so promoting HIF1 expression, antioxidant gene expression and an increase membrane translocation of glucose transporters and rate-limiting enzymes such as phosphofructokinase-1 [15] (Figure 1).
Figure 1.
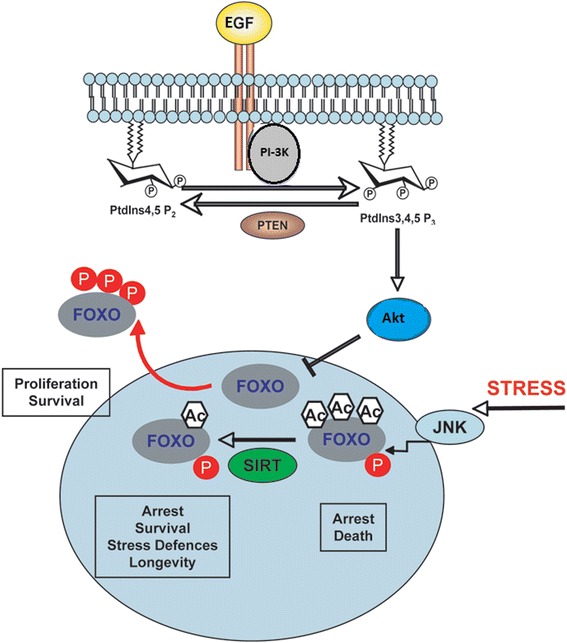
FOXO3a regulation by Akt. Growth factors/hormone stimulate PI3k phosphorylation of Akt, FOXO3a is phosphorylated by Akt, 14-3-3 also binds FOXO3a DNA binding sites, further preventing its activity in the nucleus. It is then tagged for degradation via ubiquitination and then degraded in a proteasome. Adapted from (Wilson, 2009 [49]).
Down-regulation of FOXO3a activity is often seen in cancers and ERK- or inhibitor κappa B kinase (IκKβ)-mediated inhibition of FOXO3a has been shown to promote tumorigenesis [16,17]. Despite evidence for FOXO3a down-regulation in breast cancer, the AMPK-FOXO3a pathway is still inducible, providing a potential therapeutic target for cancer chemotherapy which is independent of receptor status [6]. This evidence highlights FOXO3a as a potentially important target by cytotoxic drugs especially in receptor-negative cells. To investigate the validity of this hypothesis, we have undertaken a systematic approach to analysis of the published literature which describes the effects of breast cancer chemotherapeutic agents on FOXO3a. The goal is to determine whether FOXO3a represents a critical target of therapeutics used in the treatment of breast cancer, and therefore whether the evidence supports direct targeting of FOXO3a in chemoresistant-resistant disease notably in TNBC.
Results
The systematic search for relevant articles initially retrieved 148 articles. The 51 articles that met the inclusion criteria were then analysed and categorised according to the drug target (extracellular receptor; PI3k; AKT; FOXO3a), and assessed for dependency on FOXO3a activity. Twenty articles met these conditions and have been analysed here (Figure 2).
Figure 2.
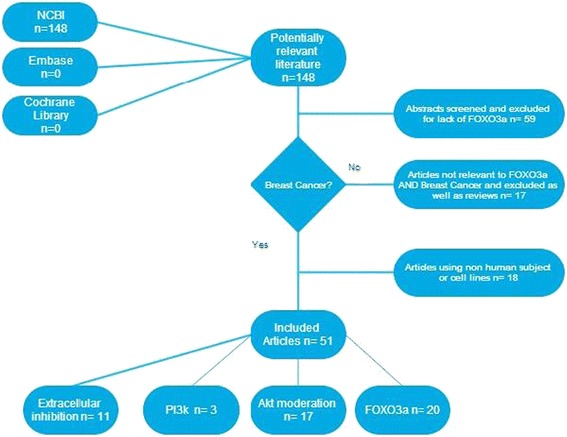
Flowchart showing the retrieval and review of literature according to systematic criteria.
Activation of growth factor receptors signals to promote tumour progression, cell survival and invasive characteristics. ER-positive cells are reported to have higher FOXO3a levels and increased apoptotic activity with less invasive characteristics than ER-negative cells. The ER status of breast tumours is an important indicator of prognosis, and although ER signaling plays a major role in tumour progression, ER-positive cancer is also associated with better prognosis than ER-negative breast cancers [18].
Following a systematic review of the literature, five articles were retrieved which described the effects of growth factor receptor inhibition on FOXO3a activity (Table 1). The mechanisms of action of anti-cancer agent classes upstream from FOXO3a are illustrated in Figure 3.
Table 1.
Growth factor receptor antagonists consistently activated FOXO3a in sensitive breast cancer cells
| First author (Year) | Treatment | Effect on FOXO3a (Activates/Inactivates) | Cellular effects |
|---|---|---|---|
| Hegde (2007) [22] | Lapatinib | Activates in responsive cell lines; measured as Thr32 P-FOXO3a and by microarray, BT474 and SKBr3. No effect in resistant cell lines; MDA-MB-468 and T47D. | Decreased expression of glyceraldehyde-3-phosphate dehydrogenase, enolase 1, pyruvate kinase and fatty acid synthase expression in BT474 and SKBr3 |
| Growth rate reduced in BT474 and SKBr3 | |||
| Karadedou (2012) [23] | Lapatinib | Activates in BT474 or SKBR3 measured by FOXO3a nuclear translocation. | Decreased expression of VEGF. |
| Real PJ (2005) [20] | Trastuzumab | Activates in MB231 and SUM159, primary breast effusions; measured as nuclear translocation of FOXO3a. | Up-regulation of Bnip1. |
| Increased sensitivity to apoptosis. | |||
| Krol (2007) [19] | Gefitinib | Activates in BT474 and SKBR3, but no effect in gefitinib-resistant lines MCF-7, MDA-MB-231, and MDA-MB-453. Measured as Thr32 P-FOXO3a and nuclear translocation analysis. | Cell cycle arrest predominantly at the G(0)-G(1) phase and apoptosis. |
| McGovern (2009) [21] | Gefitinib | Activates in BT474 and SKBR3 but not the gefitinib-resistant lines MCF-7, MDA-MB-231, and MDA-MB-453. Measured as nuclear FOXO3a and microarray. | Increase in Bim, p27 kip1 |
Figure 3.
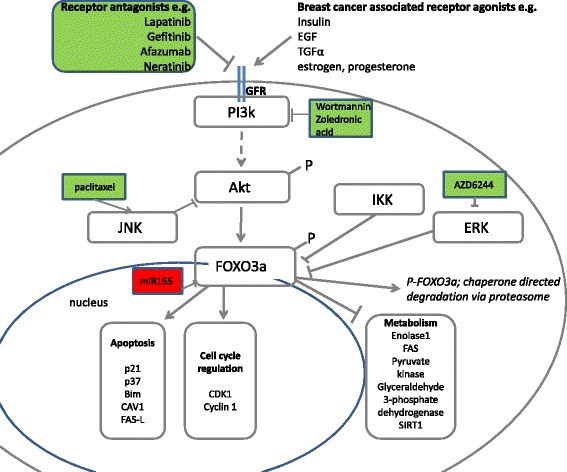
Schematic illustration of mechanisms of action of chemotherapeutic agents used in breast cancer therapy upstream of FOXO3a expression. Chemotherapeutic agents in green boxes increase FOXO3a expression/activity; those in red boxes decrease FOXO3a expression/activity. CAV1 = caveolin 1, CDK1 = cyclin dependent kinase 1, ERK = Extracellular signal-regulated kinases, FAS-L = FAS ligand, FAS = fatty acid synthase, FOXO3a – forkhead box O3a, GFR – growth factor receptor IκKβ – inhibitor kappa kinase beta, JNK - Jun N-terminal kinases, PI3k - phosphatidylinositol 3-kinase, SIRT1 = sirtuin 1.
Irrespective of the target receptor for each of the inhibitors, lapatinib and trastuzumab (epidermal growth factor receptor; EGFR and/or EGFR2; HER2, respectively), or whether it targeted receptor-associated tyrosine kinase (gefitinib), FOXO3a was consistently activated and translocated to the nucleus. Three studies confirmed an increase in apoptotic gene expression and extent of apoptosis [19-21] after anti-growth factor receptor antibody treatment. Only one study reported effects on metabolic gene expression with a switch away from anabolic metabolism [22] while Karadedou reported the inhibition of angiogenic VEGF expression [23].
Downstream from ligand-receptor binding, receptor-associated PI3k is activated and catalyses the production of phosphatidyl inositol (PtdIns) 4,5 bisphosphate and PtdIns 3,4,5 trisphosphate that act as second messengers, and via PDK1 activation results in Akt phosphorylation. The importance of PI3k for signal transduction is evidenced by the work of Reagan-Shaw et al. who showed activation of FOXO3a and induction of apoptosis when PI3k is knocked down [24]. The bisphosphonate, zoledronic acid, originally used for osteoporosis management, is now in clinical trials as a chemotherapeutic drug; it activates FOXO3a and inhibits expression of the proangiogenic factor CCN1 (Table 2) [25]. Zoledronic acid when used as an adjuvant to endocrine therapy in premenopausal women with hormone receptor-positive early breast cancer provides clinical benefit and is cost-effective [26].
Table 2.
PI3k inhibition causes FOXO3a activation in breast cancer cells
| First author (Year) | Treatment | Effect on FOXO3a (Activates/Inactivates) | Cellular effects |
|---|---|---|---|
| Espinoza (2011) [25] | Zoledronic acid (ZOL) | Activates in MDA-MB-231 and MCF-7 measured by nuclear translocation of FOXO3a. | Inhibited proangiogenic factor, CCN1 in TNBC |
| Guo (2004) [34] | Wortmannin, EGCG | Activates in MCF-7 and ZR-75 cells; and Hs578T and MDA-MB-231 cells, measured as FOXO3a expression and nuclear translocation. | Increased ER expression |
The phosphorylation of Akt indirectly via PDK1 activation by PI3k increases Akt activity which, in turn, phosphorylates FOXO3a. A number of small molecule inhibitors of Akt were identified in the review as consistently activating FOXO3a with subsequent arrest of the cell cycle (via p21 cip1 and p27 kip1 expression, [27,28]) and induction of apoptosis (Bim, [29-33]); Table 3. The majority of the small molecules did not target AKT directly but mediated AKT activation via unknown targets and other kinases such as JNK and P38. Effects on expression of ER differed between inhibitors treatments [27,34].
Table 3.
AKT inhibition activates FOXO3a in breast cancer cells
| First author (Year) | Treatment | Effect on FOXO3a (Activates/Inactivates) | Cellular effects |
|---|---|---|---|
| Brandi (2013) [27] | Indole-3-carbinol cyclic tri- and tetrameric derivatives, specific target unknown but inhibits AKT directly or indirectly. | Activates in MCF-7 and MDA-MB-231 breast cancer cell lines) and in vivo in a tumour xenograft measured as nuclear translocation of FOXO3a. | Increased expression of p21 cip1, p27 kip1 and decreased ER expression. |
| Li (2007) [29] | Selenium and Doxorubicin via p38 mediated inhibition of AKT. | Activates in MCF7 measured by P-FOXO3a and reporter assay. | Increased Bim expression and apoptosis. |
| Sharma (2012) [33] | 18β-glycyrrhetinic acid (GRA) specific target unknown but inhibits AKT directly or indirectly. | Activates in MCF7 but not normal breast cell line MCF-10 measured as increased expression and nuclear translocation. | Increased Bim expression and caspase-dependent apoptosis. |
| Sunters (2006) [32] | Paclitaxel inhibits AKT via JNK | Activates in MCF7 measured as nuclear localisation of FOXO3a. | JNK1 activation and apoptosis in MCF7 and also in a panel of other cells lines MT 3522, 734 B, ZR-75-1, T47-D, CAL-51, CAMA-1, MDA-MB-231, and SKBR-7. |
| Xie (2010) [31] | SZ-685C (marine anthraquinone) specific target unknown. Inhibits AKT directly or indirectly. | Activates in MCF-7 and MDA-MB-435. | AKT inhibition. |
| Increased Bim. | |||
| Increased apoptosis. | |||
| Increased caspase activity. | |||
| Zhao (2013) [30] | 5,7-dihydroxy-8-nitrochrysin (NOC)-specific target unknown. Inhibits AKT directly or indirectly. | Activates in MDA-MB-453. | Increased Bim expression |
| Increased apoptosis. | |||
| Lin (2011) [28] | FLOT1 silencing associated with suppression of Akt activity | Activates in MCF7 and MDA-231 measured as expression level and P-FOXO3a. | Up-regulation of p21 cip1 and p27 kip1 |
A number of regulatory (patho)-physiological microRNA (miR) and small molecule activators have been shown to target FOXO3a directly and regulate its nuclear localisation and transcriptional activity (Table 4; [35-40]).
Table 4.
FOXO3a activation in breast cancer cells increases apoptosis
| First author (Year) | Treatment | Effect on FOXO3a (Activates/Inactivates) | Cellular effects |
|---|---|---|---|
| Kong (2010) [35] | miR-155 | Inhibits in BT-474 measured by protein expression. | Decreased Bim and p27 expression decreased apoptosis. |
| Kong (2012) [36] | AZD6244, indirectly as an ATP-uncompetitive inhibitor of MEK ½ | Activates FOXO3a in MTDH knock-down, AZD6244 resistant lines. | Increased apoptosis. |
| Lam (2012) [37] | Aqueous extract of Fagonia | Activates FOXO3a measured by Western blot in MCF7 and MDA231. | Cell cycle arrest and apoptosis. |
| Lin (2010) [38] | miR-96 | Inhibits in BT549, ZR-75-30, Bcap37, MDA-MB231, MDA-MB435, MCF-7, SKBR3 measured as FOXO3a expression and by reporter assay. | Down-regulation of p21 cip1, p27 kip1, CDK and cyclin 1. |
| Liu (2012) [39] | Arsenic trioxide | Activates in MCF7 measured as nuclear translocation and expression. | Decreased IKKB. |
| Increased apoptosis. | |||
| Stan (2008) [40] | Withaferin. | Activates in MCF-7 (estrogen-responsive) and MDA-MB-231. | Increased Bim expression. |
| Increased apoptosis. |
miR155, which is up-regulated in cancer, is a negative regulator of FOXO3a and subsequently Bim and p27 kip1 expression, but inhibition of miR155 restores sensitivity to apoptosis [35]. Similarly, miR96 suppressed expression through the 3′-UTR on the FOXO3a gene, results in down-regulation of p21cip1, p27kip1 and cyclin dependent kinases [38]. Modulation of FOXO3a by anti-miR may prove useful to promote apoptosis. Other small molecules appear to modulate FOXO3a activity by regulating the activity of FOX3a-regulating kinases such as IκKβ and MEK so preventing FOXO3a phosphorylation, increasing its nuclear half-life and transcriptional activity.
Discussion
There is a growing need for new drug targets in cancer treatment. These should efficiently combine fewer side-effects with lower likelihood of resistance development. The multi-step growth factor, PI3k/PTEN, Akt and FOXO3a pathway provides a potentially important target network. Dysregulation of its components, such as PTEN, PIK3CA, and AKT (PKB), are common in solid tumours but despite evidence for FOXO3a down-regulation in breast cancer, the AMPK-FOXO3a pathway is still inducible.
This systematic review has highlighted that several different classes of drugs can increase FOXO3a activation and promote apoptosis by targeting many of the key steps in the pathway downstream of the receptor in breast cancer cell lines irrespective of hormone receptor status. There is some evidence that metabolic pathways are also affected when FOXO3a is targeted, favouring oxidative phosphorylation rather than glycolysis. Our systematic literature approach has largely retrieved papers published around FOXO3a effects on cell cycle control and induction of apoptosis. The importance of metabolic switching as a mechanism for FOXO3a up-regulation in slowing growth of breast cancer cells has been reported in one study only, probably because the published studies have limited their focus to analysis of death and death pathways.
Recently, a dual network strategy for therapeutic intervention has been proposed to target various diseases [41]; such an approach inhibits central nodes in disease and also their influencing networks based on the premise that during disease progression the importance of any given target may change and so may be best approached using sequential multi-target therapy [41]. If FOXO3a expression changes occur early in the lifecycle of breast cancer, the metabolic switch through a FOXO3a node may prove to be an important route to delaying disease progression.
FOXO3a can inhibit expression of glycolytic enzymes through HIF-1 inhibition and can regulate mitochondrial gene expression, ACO2, LARS2, MRPLI2, OXNAP1 and ATP5G1, which are typically down-regulated in hypoxia [42]. The combined effect is to starve hypoxic tumours and prevent further growth ultimately leading to autophagy and death.
FOXO3a expression is a positive prognostic marker for breast cancer [43]. The activity of FOXO3a, which regulates ERα expression, is likely to be responsible. The expression of ERα is associated with a higher degree of differentiation of tumours and lower speed of tumour cell proliferation [44], it can activate the cell cycle progression through either genomic or non-genomic pathways [45], and estrogen-inducible genes can suppress tumour progression [46]. However, ER knockdown results in a switch towards increased invasiveness in the presence of increased FOXO3a expression suggesting that the nuclear receptor represents a crucial switch in FOXO3a control of breast cancer cell aggressiveness [18]. Stratification of patients according to receptor positivity may be critical to improve the benefit:risk ratio of targeting FOXO3a.
Low FOXO3a expression is associated with poor prognosis in a number of other cancers including neuroblastoma, gastric adrenocarcinoma, hepatocarcinoma and poor metastasis-free survival in renal cell carcinoma [47-50]. In common with observations in breast cancer cells, treatment of colon cancer cells with selenite induced ROS-dependent, FOXO3a-mediated apoptosis [51]. Enhancing FOXO3a expression appears to be a relevant to treatment for a number of cancers. One emerging means of modulating FOXO3a expression therapeutically is post-transcriptionally via specifically targeted miR. In this review, we have identified two papers that report the effects of specific miR which affect FOXO3a expression in breast cancer cells. It has been estimated that there may be thousands of target genes for miR-155, although significant overlap has been observed between miR-155 targets and the molecular profile of mutant p53-expressing breast tumours suggesting that this may prove to be a particularly useful target with fewer side-effects [52]. Far fewer genes have been has been described as targets for miR-96 although existing works share the common conclusion that interference with miR-96 using antisense miRNA (antagomiR) molecules increase apoptosis in breast cancer cells. More work is needed before to understand potential off-target effects before their potential as therapeutic targets for breast cancer can be appreciated [53].
Chemotherapeutic drug resistance can limit the application of many anti-cancer therapies. Acquired resistance to lapatinib and trastuzumab frequently occurs in breast cancer patients, possibly as a consequence of FOXO3a de-repression and increased ER signaling [54], however, this does present an opportunity for adjuvant therapy with drugs such as tamoxifen that target ER. Similarly, upregulation of HER3 is induced in a FOXO3a dependent way which attenuates the beneficial effects of PI3k inhibitors unless used in combination with HER2/3 antagonists [55]. Indeed preclinical data support the suggestion that targeting of the PI3k/mTOR pathway in combination with trastuzumab is beneficial in trastuzumab-resistant breast cancer [56]. The studies highlighted here show the potential for targeting the upregulation of FOXO3a to overcome resistance to AZD6244 [35].
The clinical trials register lists two studies currently underway which will assess FOXO3a status (albeit not as primary endpoints) one using an ER antagonist with a pan-class I PI3k inhibitor and the other using reparaxin, an IL-8 receptor antagonist.
Conclusion
The identification of new classes of FOXO3a inhibitors offers a promising strategy for future anti-cancer drug design by targeting a downstream node of the PI3k pathway that is not commonly mutated in cancers. In the age of personalised medicine and following the identification of regulatory miR that target FOXO3a directly, their inhibition as an adjunct therapy alongside conventional cytotoxic and/or receptor antagonists in stratified patient groups merits evaluation.
Methods
We have designed a systematic literature review to identify published studies describing the effects of anti-cancer drugs on FOXO3a in: human cell lines or primary cells from breast cancer patients and from immunohistochemical analyses of tissue.
PubMed/Medline, Cochrane and Embase databases were searched from 1 January 2014 to 1 April 2014. Neither the Cochrane library nor Embase yielded relevant results using specific search criteria. Therefore PubMed/Medline was the database used for information, the Boolean search terms are listed below: ((FOXO3a[All Fields] OR (Forkhead[All Fields] AND Box[All Fields] AND O3[All Fields])) OR (Forkhead[All Fields] AND Box[All Fields] AND (“proteins”[MeSH Terms] OR “proteins”[All Fields] OR “protein”[All Fields]) AND 3[All Fields])) AND (“breast neoplasms”[MeSH Terms] OR (“breast”[All Fields] AND “neoplasms”[All Fields]) OR “breast neoplasms”[All Fields] OR (“breast”[All Fields] AND “cancer”[All Fields]) OR “breast cancer”[All Fields]).
Results were limited to peer-reviewed, English language articles only. Reviews, meta-analyses, case reports, editorials and letters lacking primary data were excluded.
Acknowledgements
CP was funded by the Hewlett charitable bequest to Aston University. There was no involvement from the funder in design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Abbreviations
- AMPK
AMP-activated protein kinase
- ER
Estrogen receptor
- EGFR
Epidermal growth factor receptor
- HER2
Human epidermal growth factor like 2
- FOXO3a
Forkhead box O3a
- HIF1α
Hypoxia inducible factor 1alpha
- IκKβ
Inhibitor kappa kinase beta
- JNK
Jun N-terminal kinases
- PI3k
Phosphatidylinositol 3-kinase
- MiR
microRNA
- TNBC
Triple negative breast cancer
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ST was responsible for acquisition of data, analysis and interpretation of data; and was involved in drafting the manuscript. ML was involved in study design and drafting the manuscript. CP was involved in interpretation of the data and drafting the manuscript. JEPB participated in the design of the study and drafting the manuscript. ARC was contributed to interpretation of the results and drafting the manuscript. HRG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors’ read and approved the final manuscript.
Contributor Information
Simon Taylor, Email: Simon.Taylor@gilead.com.
Matthew Lam, Email: matt.lam@hotmail.co.uk.
Chathyan Pararasa, Email: pararasc@aston.ac.uk.
James EP Brown, Email: j.e.p.brown@aston.ac.uk.
Amtul R Carmichael, Email: Amtul.Carmichael@dgh.nhs.uk.
Helen R Griffiths, Email: h.r.griffiths@aston.ac.uk.
References
- 1.Andre F, Zielinski CC. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol. 2012;23 Suppl 6:vi46–51. doi: 10.1093/annonc/mds195. [DOI] [PubMed] [Google Scholar]
- 2.Berrada N, Delaloge S, Andre F. Treatment of triple-negative metastatic breast cancer: toward individualized targeted treatments or chemosensitization? Ann Oncol. 2010;21 Suppl 7:vii30–5. doi: 10.1093/annonc/mdq279. [DOI] [PubMed] [Google Scholar]
- 3.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8(3):235–44. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 4.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 5.Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–81. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 6.Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9(6):1091–6. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 7.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117(4):421–6. doi: 10.1016/S0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40(2):280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–82. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Lu W, Garcia-Prieto C, Huang P. The Warburg effect and its cancer therapeutic implications. J Bioenerg Biomembr. 2007;39(3):267–74. doi: 10.1007/s10863-007-9086-x. [DOI] [PubMed] [Google Scholar]
- 12.Dobson M, Ramakrishnan G, Ma S, Kaplun L, Balan V, Fridman R, et al. Bimodal regulation of FoxO3 by AKT and 14-3-3. Biochim Biophys Acta. 2011;1813(8):1453–64. doi: 10.1016/j.bbamcr.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170(2):295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon V, Banerji S. Molecular pathways: PI3K pathway targets in triple-negative breast cancers. Clin Cancer Res. 2013;19(14):3738–44. doi: 10.1158/1078-0432.CCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 15.Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci U S A. 2008;105(7):2622–7. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JY, Zong CS, Xia W, Yamaguchi H, Ding Q, Xie X, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat Cell Biol. 2008;10(2):138–48. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117(2):225–37. doi: 10.1016/S0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 18.Sisci D, Maris P, Cesario MG, Anselmo W, Coroniti R, Trombino GE, et al. The estrogen receptor alpha is the key regulator of the bifunctional role of FoxO3a transcription factor in breast cancer motility and invasiveness. Cell Cycle. 2013;12(21):3405–20. doi: 10.4161/cc.26421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krol J, Francis RE, Albergaria A, Sunters A, Polychronis A, Coombes RC, et al. The transcription factor FOXO3a is a crucial cellular target of gefitinib (Iressa) in breast cancer cells. Mol Cancer Ther. 2007;6(12 Pt 1):3169–79. doi: 10.1158/1535-7163.MCT-07-0507. [DOI] [PubMed] [Google Scholar]
- 20.Real PJ, Benito A, Cuevas J, Berciano MT, de Juan A, Coffer P, et al. Blockade of epidermal growth factor receptors chemosensitizes breast cancer cells through up-regulation of Bnip3L. Cancer Res. 2005;65(18):8151–7. doi: 10.1158/0008-5472.CAN-05-1134. [DOI] [PubMed] [Google Scholar]
- 21.McGovern UB, Francis RE, Peck B, Guest SK, Wang J, Myatt SS, et al. Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol Cancer Ther. 2009;8(3):582–91. doi: 10.1158/1535-7163.MCT-08-0805. [DOI] [PubMed] [Google Scholar]
- 22.Hegde PS, Rusnak D, Bertiaux M, Alligood K, Strum J, Gagnon R, et al. Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther. 2007;6(5):1629–40. doi: 10.1158/1535-7163.MCT-05-0399. [DOI] [PubMed] [Google Scholar]
- 23.Karadedou CT, Gomes AR, Chen J, Petkovic M, Ho KK, Zwolinska AK, et al. FOXO3a represses VEGF expression through FOXM1-dependent and -independent mechanisms in breast cancer. Oncogene. 2012;31(14):1845–58. doi: 10.1038/onc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reagan-Shaw S, Ahmad N. RNA interference-mediated depletion of phosphoinositide 3-kinase activates forkhead box class O transcription factors and induces cell cycle arrest and apoptosis in breast carcinoma cells. Cancer Res. 2006;66(2):1062–9. doi: 10.1158/0008-5472.CAN-05-1018. [DOI] [PubMed] [Google Scholar]
- 25.Espinoza I, Liu H, Busby R, Lupu R. CCN1, a candidate target for zoledronic acid treatment in breast cancer. Mol Cancer Ther. 2011;10(5):732–41. doi: 10.1158/1535-7163.MCT-10-0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delea TE, Taneja C, Sofrygin O, Kaura S, Gnant M. Cost-effectiveness of zoledronic acid plus endocrine therapy in premenopausal women with hormone-responsive early breast cancer. Clin Breast Cancer. 2010;10(4):267–74. doi: 10.3816/CBC.2010.n.034. [DOI] [PubMed] [Google Scholar]
- 27.Brandi G, Fraternale A, Lucarini S, Paiardini M, De Santi M, Cervasi B, et al. Antitumoral activity of indole-3-carbinol cyclic tri- and tetrameric derivatives mixture in human breast cancer cells: in vitro and in vivo studies. Anticancer Agents Med Chem. 2013;13(4):654–62. doi: 10.2174/1871520611313040014. [DOI] [PubMed] [Google Scholar]
- 28.Lin C, Wu Z, Lin X, Yu C, Shi T, Zeng Y, et al. Knockdown of FLOT1 impairs cell proliferation and tumorigenicity in breast cancer through upregulation of FOXO3a. Clin Cancer Res. 2011;17(10):3089–99. doi: 10.1158/1078-0432.CCR-10-3068. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Zhou Y, Wang R, Zhang H, Dong Y, Ip C. Selenium sensitizes MCF-7 breast cancer cells to doxorubicin-induced apoptosis through modulation of phospho-Akt and its downstream substrates. Mol Cancer Ther. 2007;6(3):1031–8. doi: 10.1158/1535-7163.MCT-06-0643. [DOI] [PubMed] [Google Scholar]
- 30.Zhao XC, Cao XC, Liu F, Quan MF, Ren KQ, Cao JG. Regulation of the FOXO3a/Bim signaling pathway by 5,7-dihydroxy-8-nitrochrysin in MDA-MB-453 breast cancer cells. Oncol Lett. 2013;5(3):929–34. doi: 10.3892/ol.2012.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie G, Zhu X, Li Q, Gu M, He Z, Wu J, et al. SZ-685C, a marine anthraquinone, is a potent inducer of apoptosis with anticancer activity by suppression of the Akt/FOXO pathway. Br J Pharmacol. 2010;159(3):689–97. doi: 10.1111/j.1476-5381.2009.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, et al. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66(1):212–20. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 33.Sharma G, Kar S, Palit S, Das PK. 18beta-glycyrrhetinic acid induces apoptosis through modulation of Akt/FOXO3a/Bim pathway in human breast cancer MCF-7 cells. J Cell Physiol. 2012;227(5):1923–31. doi: 10.1002/jcp.22920. [DOI] [PubMed] [Google Scholar]
- 34.Guo S, Sonenshein GE. Forkhead box transcription factor FOXO3a regulates estrogen receptor alpha expression and is repressed by the Her-2/neu/phosphatidylinositol 3-kinase/Akt signaling pathway. Mol Cell Biol. 2004;24(19):8681–90. doi: 10.1128/MCB.24.19.8681-8690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kong W, He L, Coppola M, Guo J, Esposito NN, Coppola D, et al. MicroRNA-155 regulates cell survival, growth, and chemosensitivity by targeting FOXO3a in breast cancer. J Biol Chem. 2010;285(23):17869–79. doi: 10.1074/jbc.M110.101055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Kong X, Moran MS, Zhao Y, Yang Q. Inhibition of metadherin sensitizes breast cancer cells to AZD6244. Cancer Biol Ther. 2012;13(1):43–9. doi: 10.4161/cbt.13.1.18868. [DOI] [PubMed] [Google Scholar]
- 37.Lam M, Carmichael AR, Griffiths HR. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS ONE. 2012;7(6):e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu C, et al. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS One. 2010;5(12):e15797. doi: 10.1371/journal.pone.0015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Gong Y, Li H, Jiang G, Zhan S, Liu H, et al. Arsenic trioxide-induced growth arrest of breast cancer MCF-7 cells involving FOXO3a and IkappaB kinase beta expression and localization. Cancer Biother Radiopharm. 2012;27(8):504–12. doi: 10.1089/cbr.2012.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68(18):7661–9. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gyurkó DM, Veres DV, Módos D, Lenti K, Korcsmáros T, Csermely P. Adaptation and learning of molecular networks as a description of cancer development at the systems-level: Potential use in anti-cancer therapies. Semin Cancer Biol. 2013;23(4):262–9. doi: 10.1016/j.semcancer.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Ferber EC, Peck B, Delpuech O, Bell GP, East P, Schulze A. FOXO3a regulates reactive oxygen metabolism by inhibiting mitochondrial gene expression. Cell Death Differ. 2012;19(6):968–79. doi: 10.1038/cdd.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Y, Zou L, Lu WQ, Zhang Y, Shen AG. Foxo3a expression is a prognostic marker in breast cancer. PLoS One. 2013;8(8):e70746. doi: 10.1371/journal.pone.0070746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madeira M, Mattar A, Logullo AF, Soares FA, Gebrim LH. Estrogen receptor alpha/beta ratio and estrogen receptor beta as predictors of endocrine therapy responsiveness-a randomized neoadjuvant trial comparison between anastrozole and tamoxifen for the treatment of postmenopausal breast cancer. BMC Cancer. 2013;13:425. doi: 10.1186/1471-2407-13-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong C-W, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci. 2002;99(23):14783–8. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Finlin BS, Gau C-L, Murphy GA, Shao H, Kimel T, Seitz RS, et al. RERG is a novel ras-related, estrogen-regulated and growth-inhibitory gene in breast cancer. J Biol Chem. 2001;276(45):42259–67. doi: 10.1074/jbc.M105888200. [DOI] [PubMed] [Google Scholar]
- 47.Santo EE, Stroeken P, Sluis PV, Koster J, Versteeg R, Westerhout EM. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013;73(7):2189–98. doi: 10.1158/0008-5472.CAN-12-3767. [DOI] [PubMed] [Google Scholar]
- 48.Yang XB, Zhao JJ, Huang CY, Wang QJ, Pan K, Wang DD, et al. Decreased expression of the FOXO3a gene is associated with poor prognosis in primary gastric adenocarcinoma patients. PLoS One. 2013;8(10):e78158. doi: 10.1371/journal.pone.0078158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie C, Song LB, Wu JH, Li J, Yun JP, Lai JM, et al. Upregulator of cell proliferation predicts poor prognosis in hepatocellular carcinoma and contributes to hepatocarcinogenesis by downregulating FOXO3a. PLoS One. 2012;7(7):e40607. doi: 10.1371/journal.pone.0040607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni D, Ma X, Li HZ, Gao Y, Li XT, Zhang Y, et al. Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma. Clin Cancer Res. 2014;20(7):1779–90. doi: 10.1158/1078-0432.CCR-13-1687. [DOI] [PubMed] [Google Scholar]
- 51.Yang JY, Chang CJ, Xia W, Wang Y, Wong KK, Engelman JA, et al. Activation of FOXO3a is sufficient to reverse mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor chemoresistance in human cancer. Cancer Res. 2010;70(11):4709–18. doi: 10.1158/0008-5472.CAN-09-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neilsen PM, Noll JE, Mattiske S, Bracken CP, Gregory PA, Schulz RB, et al. Mutant p53 drives invasion in breast tumors through up-regulation of miR-155. Oncogene. 2013;32(24):2992–3000. doi: 10.1038/onc.2012.305. [DOI] [PubMed] [Google Scholar]
- 53.Gyparaki MT, Basdra EK, Papavassiliou AG. MicroRNAs as regulatory elements in triple negative breast cancer. Cancer Lett. 2014;354(1):1–4. doi: 10.1016/j.canlet.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 54.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14(4):320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109(8):2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Brien NA, McDonald K, Tong L, von Euw E, Kalous O, Conklin D, et al. Targeting PI3K/mTOR overcomes resistance to HER2-targeted therapy independent of feedback activation of AKT. Clin Cancer Res. 2014;20(13):3507–20. doi: 10.1158/1078-0432.CCR-13-2769. [DOI] [PubMed] [Google Scholar]


