Abstract
In order to investigate the potential of short antimicrobial peptides (AMPs) as alternative antibacterial agents during the treatment of peri-implantitis, the cytotoxic activity of three short AMPs, that is, Pac-525, KSL-W, and KSL, was determined using the MTT assay. The antimicrobial activity of these AMPs, ranging in concentration from 0.0039 mg/mL to 0.5 mg/mL, against the predominant planktonic pathogens, including Streptococcus sanguis, Fusobacterium nucleatum, and Porphyromonas gingivalis, involved in peri-implantitis was investigated. Furthermore, 2-day-old P. gingivalis biofilms cultured on titanium surfaces were treated with Pac-525 and subsequently observed and analysed using confocal laser scanning microscopy (CLSM). The average cell proliferation curve indicated that there was no cytotoxicity due to the three short AMPs. The minimum inhibitory concentration and minimum bactericidal concentration values of Pac-525 were 0.0625 mg/mL and 0.125 mg/mL, respectively, for P. gingivalis and 0.0078 mg/mL and 0.0156 mg/mL, respectively, for F. nucleatum. Using CLSM, we confirmed that compared to 0.1% chlorhexidine, 0.5 mg/mL of Pac-525 caused a significant decrease in biofilm thickness and a decline in the percentage of live bacteria. These data indicate that Pac-525 has unique properties that might make it suitable for the inhibition the growth of pathogenic bacteria around dental implants.
1. Introduction
Dental implants have revolutionised dental rehabilitation and prosthetic dentistry and have become a commonly used alternative to conventional removable and fixed partial dentures. The high survival rate reported in several 10-year follow-up studies has led to a widespread acceptance and use of dental implants [1–3]. Although the general impression of implant therapy is that the success rate is high, and inflammation or peri-implantitis is a common occurrence. Peri-implantitis is an inflammatory disease of infectious origin, which ultimately leads to the loss of the bone that supports the implant. It is a biofilm-mediated disease, and the attachment of planktonic pathogenic bacteria to the surface of the dental implant is the initial step in biofilm formation. Porphyromonas gingivalis is a late colonizer compared to Streptococcus sanguis and Fusobacterium nucleatum, which colonize earlier within the biofilm community in the subgingival crevice around dental implants; however, P. gingivalis is regarded as the predominant Gram-negative pathogen involved in the initiation and progression of severe forms of peri-implantitis [4, 5]. Thus, effective control of the attachment and proliferation of pathogens, especially P. gingivalis, around a dental implant, is key to preventing and/or treating peri-implantitis and improving the survival and success of dental implants.
Natural host defense antimicrobial peptides (AMPs) are small cationic peptides, which are produced by most living organisms, including bacteria, insects, plants, and vertebrates [6]. These peptides are low molecular weight proteins with broad-spectrum antimicrobial activity against many pathogens, including some multidrug-resistant bacteria. An updated database of AMPs is available online at http://aps.unmc.edu/AP/main.php. To date, more than 2,200 AMPs of different origins have been reported. Human saliva and gingival fluid contain at least 45 different AMPs that belong to several different functional classes, ranging from small cationic peptides to enzymes and protein macromolecule. This functional and structural diversity could be necessary to protect the oral epithelia from the large number of potentially harmful microbes, maintain the homeostasis of commensal and pathogenic oral bacteria, and prevent biofilm formation and subsequent gingival inflammation around natural teeth and dental implants. However, several drawbacks of biologically active natural AMPs have been recently described, including systemic and local toxicity; reduced activity based on salt, serum, and pH sensitivity; susceptibility to proteolysis and pharmacokinetic and pharmacodynamic factors; and high manufacturing cost [7, 8]. Appropriately designed peptidomimetics are a possible solution to these problems and have shown low toxicity towards mammalian cells, stability against proteolytic enzymes, and low cost of synthesis [9, 10].
Short AMPs are particularly attractive due to their lower synthesis cost and better structural stability. A novel short Trp-rich peptide, Ac-KWRRWVRWI-NH2, designated Pac-525, exhibited improved activity against both bacteria and fungi [11, 12]. Another short peptide, KKVVFKVKFK-NH2, designated KSL, was confirmed to have high efficiency against clinically important pathogens in the oral cavity, especially to oral streptococci [13]. Moreover, KSL was reported to inhibit Streptococcus mutans biofilm formation [14]. In addition, the KSL analogue, KKVVFWVKFK-NH2, designated KSL-W, exhibited excellent stability, adsorption, and sustained release performance and has been used in the chewing gum, as a potential antiplaque agent [15, 16]. As mentioned above, previous studies demonstrated that short AMPs mainly inhibited Gram-positive oral and nonoral bacteria, as well as fungi. However, data regarding the inhibition effects of these short AMPs on peri-implant pathogenic bacterial growth, especially of Gram-negative bacteria, is still unclear. Further, the effect of AMPs on biofilms is also unknown.
In the current study, we investigated the antimicrobial activity of three short AMPs against adherent pathogenic bacteria for planktonic state in vitro, which are indicative of the initial occurrence and development of peri-implantitis, including S. sanguis, F. nucleatum, and P. gingivalis. Pac-525 exhibited the highest antibacterial activity against all the tested bacteria. Furthermore, P. gingivalis biofilms cultured on titanium were treated with Pac-525 and compared to those treated with the conventional antibiotic, DXH, with the aim of providing information for further clinical use.
2. Materials and Methods
2.1. AMPs Synthesis
Pac-525 (Ac-KWRRWVRWI-NH2, molecular weight: 1,426.70 Da), KSL-W (KKVVFWVKFK-NH2, molecular weight: 1,307.69 Da), and KSL (KKVVFKVKFK-NH2, molecular weight: 1,249.65 Da) were synthesized and used in our study. A high-efficiency solid-phase peptide synthesis was performed by ChinaPeptides Co., Ltd. in Shanghai, China, by using an automatic peptide synthesizer (Symphony; Protein Technologies, Tucson, AZ). The purified peptide was characterised using analytical high performance liquid chromatography (HPLC; Shimadzu, Kyoto, Japan) and mass spectrometry (MS, Finnigan TSQ 7000; Thermo, Waltham, MA) with purity of over 99%. All the synthesized peptides were freeze-dried and stored at −20°C.
2.2. Cytotoxicity Assay
A MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was performed using MG-63 cells to assess the cytotoxicity of the AMPs. MG-63 cells were provided by Peking University and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, New York City, NY) supplemented with 10% fetal bovine serum (FBS; Gibco), penicillin (100 IU/mL; Gibco), and streptomycin (100 μg/mL; Gibco) at 37°C in a humidified 5% CO2 incubator. We cultured 5 × 103 MG-63 cells in 96-well plates and allowed them to grow to confluence. The cell culture medium was then replaced by twofold serial dilutions of AMPs, with final concentrations of Pac-525, KSL-W, and KSL peptide ranging from 0.0039 mg/mL to 0.5 mg/mL. DMEM without AMP served as a negative control. After 24 h of culture, 20 μL MTT dye (5 mg/mL in PBS) was added to each well and incubated at 37°C for 4 hours in the dark. During this process, metabolically active cells reduced the MTT to purple formazan. Following incubation, MTT was aspirated from each well, and 150 μL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals; the plate was then incubated for 20 min at room temperature in the dark. The optical density (OD) values for each well were measured at 490 nm by using a microplate reader (Thermo Fisher Scientific, Waltham, MA). A higher absorbance is related to a higher formazan concentration, which indicates higher metabolic activity of MG-63 cells in the wells.
2.3. Bacterial Strains and Growth Conditions
S. sanguis ATCC10556, F. nucleatum ATCC 10953, and P. gingivalis ATCC 33277 were used in the study. Strains were kindly provided by the School of Stomatology, Capital Medical University (Beijing, China). F. nucleatum was cultured in a liquid Bacto-tryptone-yeast extract-ascorbic acid-glucose 5 mg/mL NaCl solution; P. gingivalis and S. sanguis were cultured on brain-heart infusion (BHI, Difco; USA) media supplemented with hemin (5 μg/mL; Sigma) and vitamin K1 (1 μg/mL; Sigma). All cultures were incubated at 37°C under anaerobic conditions (80% N2, 10% CO2, and 10% H2). Bacterial cells were harvested by centrifugation at 10,000 × g for 10 min, washed with sterile phosphate-buffered saline (PBS), diluted to 1 × 108 CFU/mL by using a spectrophotometer-based standard curve calculation, and stored at −20°C.
2.4. Bacterial Susceptibility Assay
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were measured according to Clinical and Laboratory Standards Institute (CLSI) guidelines [17]. Briefly, for MIC determination, twofold serial dilutions of AMPs were prepared in BHI medium and the final concentration ranged from 0.0039 mg/mL to 0.5 mg/mL. Cultures of S. sanguis, F. nucleatum, and P. gingivalis were adjusted to 2 × 106 CFU/mL with BHI, and 100 μL of inoculum was added to each well of 96-well flat-bottom microtitre plates (Costar 3599; Corning Inc., Corning, NY) containing 100 μL of a series of dilutions of Pac-525, KSL-W, and KSL peptides. BHI with 1 × 106 CFU/mL bacterial suspension without AMP served as a negative control. Chlorhexidine (CHX) at 0.1% served as a positive control. The wells were examined for turbidity after incubation at 37°C under anaerobic conditions for 48 h, referenced by the negative and positive control wells. MIC was defined as the endpoint (the well with the lowest concentration of AMPs) at which no turbidity could be detected with respect to controls. The absorbance at 620 nm (A620) was measured using a microplate reader (Thermo, Waltham, MA) to assess bacterial cell growth. To measure the MBC, an aliquot of 20 μL cell suspension was taken from each clear well containing Pac-525, KSL-W, and KSL peptides, at a concentration above the MIC, and bacterial cells were enumerated after incubation at 37°C under anaerobic conditions for 48 h. The MBC was determined as the lowest AMP concentration that produced no visible colonies on the surface of an agar plate. The tests were performed in triplicate.
2.5. Effects of Pac-525 Peptide on P. gingivalis Biofilm Cultured on Titanium
2.5.1. Biofilm Formation of P. gingivalis on Titanium Discs
Commercially pure titanium discs (10 mm in diameter and 1 mm in thickness) were polished using waterproof silicon carbide paper until 2,400 c, and morphological images were captured using a Scanning Electron Microscope (SEM, QUANTA 450; SA) and an Atomic Force Microscope (AF, Nanoscope IIIA+; Veeco, USA) and shown in Figure 1. The titanium surface had a smooth and uniform physical structure with a roughness of 0.027 μm, which corresponds to the features of the smooth neck of the clinical dental implants [18]. To form an acquired salivary pellicle on titanium and to prepare for bacteria culture, we coated the discs with 300 μL of unstimulated human saliva at 37°C for 4 h, washed them twice with PBS, and transferred them into 24-well plates. P. gingivalis biofilms were developed using a modified version of the method described by Noiri et al. [19]. Briefly, 2 mL BHIS with 1 × 106 CFU/mL bacterial suspension of P. gingivalis was cultured on the surface of titanium discs and aerobically incubated at 37°C for 48 h. To observe the formation and morphology of P. gingivalis cells on the titanium surface, the titanium discs were gently rinsed twice with PBS, fixed with 2.5% glutaraldehyde in 0.1 M cacodylic acid buffer overnight, sequentially dehydrated in a series of ethanol (20%, 50%, 70%, 90%, and 100%) for 10 min each, immersed in isoamyl acetate for 1.5 min, sputter-coated with a thin layer of Au-Pd, and observed and imaged using SEM.
Figure 1.
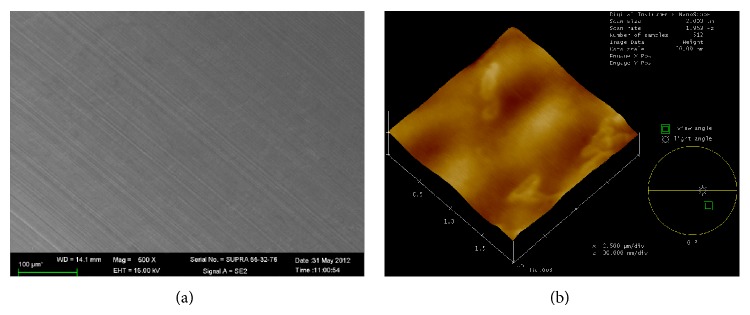
Physical morphology of titanium. (a) SEM image; (b) AF image.
2.5.2. Live/Dead Staining for Biofilms Treated with Pac-525
Following P. gingivalis biofilm formation, each titanium disc with 2-day-old biofilm was washed three times with PBS and transferred into a new 24-well plate. BHI containing Pac-525 peptide at concentrations of 0.125 mg/mL, 0.25 mg/mL, and 0.5 mg/mL (equal to the multiple proportions of MBC) was added into the wells and was further aerobically incubated at 37°C for 24 h. BHI without Pac-525 peptide served as a negative control, and 0.1% CHX served as a positive control. To ascertain the viability of P. gingivalis biofilms, cells were stained using a LIVE/DEAD BacLight Bacterial Viability Kit (L-13152; Molecular Probes Inc., Eugene, OR) for 20 min in the dark. Live bacteria were stained with SYTO 9 to emit a green fluorescence signal, and dead bacteria were stained with propidium iodide to produce red fluorescence. Confocal laser scanning microscopy (CLSM, Leica TCS SP2; Leica Microsystems, Germany) with beam path settings (488 nm and 543 nm) was used to observe the stained biofilm on the titanium discs. Three independent biofilm experiments were performed, and at least three image stacks per experiment were collected.
2.6. Statistical Analysis
Statistical analyses were performed using the SPSS 17.0 package. For the cell proliferation curve, OD490 values were compared using the t-test for independent samples. In the evaluation of biofilm formation, biofilm thickness and live bacteria percentage of P. gingivalis were compared using one-way analysis of variance (ANOVA). A P value of <0.05 was considered statistically significant.
3. Results
3.1. MTT Analysis of MG-63 Cells
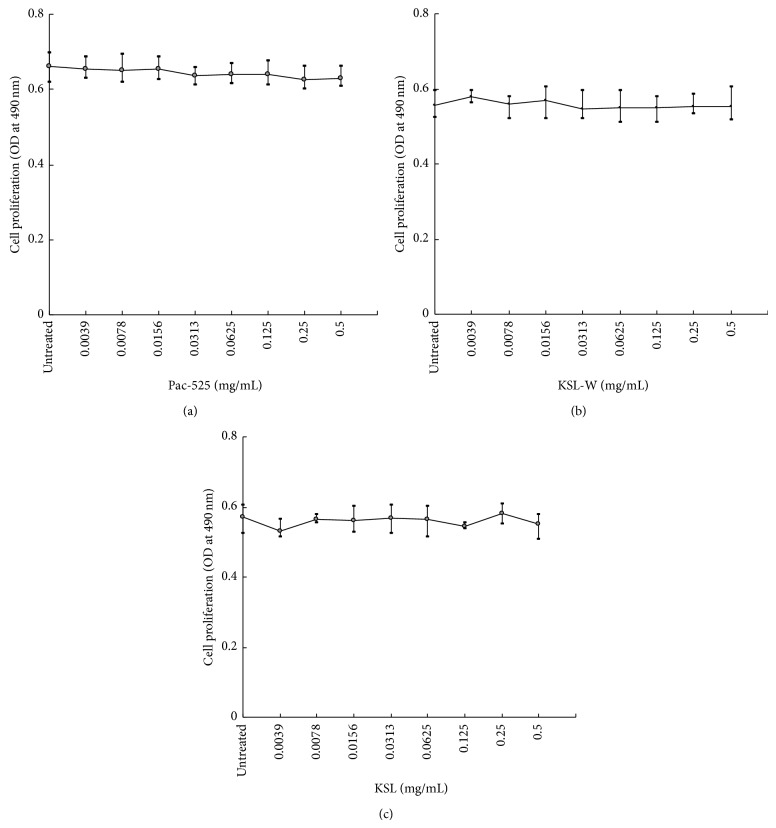
Figure 2 shows the average cell proliferation curve, with the concentration of AMPs on the X-axis and OD values on the Y-axis. Compared with the untreated group, the absorbance values for each well (treated with Pac-525, KSL-W, or KSL) were similar for each concentration. There was no statistical difference between the AMP-treated group and the untreated group and no statistical difference among the eight concentrations of AMPs.
Figure 2.
Proliferation curves for MG-63 cells exposed to (a) Pac-525, (b) KSL-W, or (c) KSL antimicrobial peptide solutions at concentrations ranging from 0 to 0.5 mg/mL.
3.2. Minimum Inhibitory Concentrations and Minimum Bactericidal Concentrations of AMPs against S. sanguis, F. nucleatum, and P. gingivalis
MIC and MBC recorded for the three antibacterial peptides against planktonic bacteria of S. sanguis, F. nucleatum, and P. gingivalis are summarized in Table 1. Amongst the tested bacterial strains, S. sanguis was susceptible to Pac-525, with a MIC value of 0.125 mg/mL and a MBC value of 0.5 mg/mL, whereas it was insensitive to KSL-W and KSL within the range of tested concentrations. The MIC value of Pac-525 was the lowest: 0.0625 mg/mL for P. gingivalis and 0.0078 mg/mL for F. nucleatum. Similarly, the MBC value of Pac-525 was the lowest, with 0.125 mg/mL for P. gingivalis, and 0.0156 mg/mL for F. nucleatum.
Table 1.
Susceptibility of S. sanguis, F. nucleatum, and P. gingivalis to AMPs in vitro.
| Bacterial strain | AMPs | |||||
|---|---|---|---|---|---|---|
| AMPs concentration (mg/mL)a | ||||||
| Pac-525 | KSL-W | KSL | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| S. sanguis 10556 | 0.125 | 0.5 | — | — | — | — |
| F. nucleatum 10953 | 0.0078 | 0.0156 | 0.0156 | 0.0313 | 0.0156 | 0.0313 |
| P. gingivalis 33277 | 0.0625 | 0.125 | 0.125 | 0.5 | 0.125 | 0.5 |
aValues represent the geometric mean of three individual experiments.
3.3. The Effects of Pac-525 Peptide on P. gingivalis Biofilm
P. gingivalis suspension formed an intact mature biofilm on the titanium surfaces after anaerobic incubation for 48 h. The bacterial cells in the biofilm were uniformly distributed, closely packed, and had channels connecting each other (Figure 3).
Figure 3.
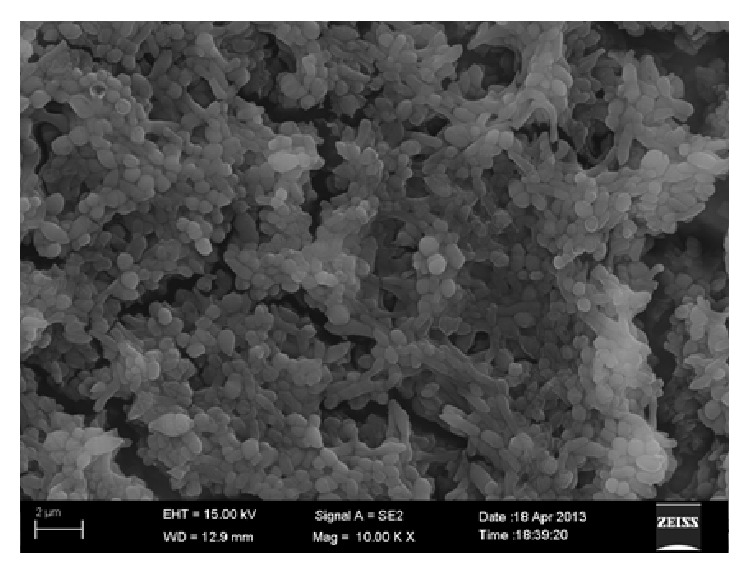
SEM image of a P. gingivalis biofilm on titanium.
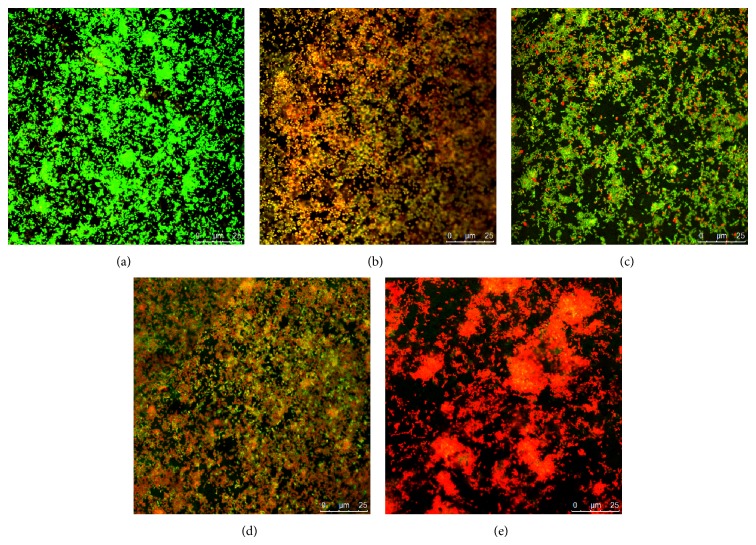
P. gingivalis biofilms on the surface of titanium discs were imaged by CLSM and are shown in Figure 4. In the images, the live bacteria were stained and emitted a green fluorescence signal, while the dead one was stained and produced red fluorescence. The nontreated control biofilm emitted green fluorescence (a), and the CHX-treated biofilm emitted red (b). Compared to the controls, Pac-525 had a significant impact on the viability of biofilm cells. The biofilm showed extensive green fluorescence and scattered red fluorescence after treatment with 0.125 mg/mL Pac-525 (c), obvious red fluorescence and inconspicuous green fluorescence with 0.25 mg/mL Pac-525 (d), and almost completely red fluorescence with 0.5 mg/mL Pac-525 (e). The average thickness of P. gingivalis biofilms was 55.89 μm and 41.64 μm at Pac-525 concentrations of 0.25 mg/mL and 0.5 mg/mL, respectively, which were significantly lower than those in the nontreated control group (P < 0.05). Furthermore, the thickness of biofilms treated with 0.5 mg/mL Pac-525 was significantly lower than that in the CHX-treated control group (P < 0.05, Figure 5). Similar to the results for biofilm thickness, live bacteria percentages were 0.39 ± 0.02 and 0.30 ± 0.02 after treatment with Pac-525 concentrations of 0.25 mg/mL and 0.5 mg/mL, respectively. These values were significantly lower than those of the nontreated control group (P < 0.05), whereas the live bacteria percentage of the 0.5 mg/mL Pac-525-treated group was significantly lower than that of the CHX-treated control group (P < 0.05, Figure 6), suggesting that Pac-525, especially at a concentration of 0.5 mg/mL, had the favourable ability to decrease biofilm thickness, diffuse cell distribution, and reduce P. gingivalis viability. These results indicated that Pac-525 was not only able to kill P. gingivalis within the biofilm on the titanium but was also able to cause the mature biofilm to disintegrate.
Figure 4.
Confocal laser scanning microscopy images of P. gingivalis biofilms on titanium discs (oil, ×63). (a) Untreated P. gingivalis biofilm (control); (b) P. gingivalis biofilm treated with 0.1% chlorhexidine (CHX); (c) P. gingivalis biofilm treated with 0.125 mg/mL Pac-525; (d) P. gingivalis biofilm treated with 0.25 mg/mL Pac-525; and (e) P. gingivalis biofilm treated with 0.5 mg/mL Pac-525.
Figure 5.
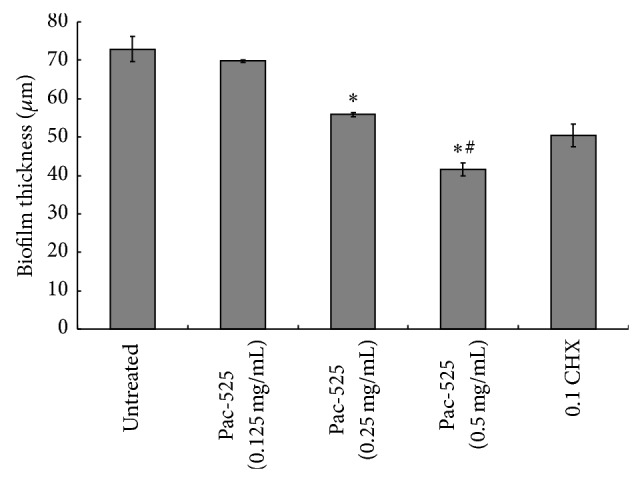
Average thickness of P. gingivalis biofilms (on titanium discs) treated with Pac-525. *Significantly different from untreated control. #Significantly different from CHX-treated control (P < 0.05).
Figure 6.
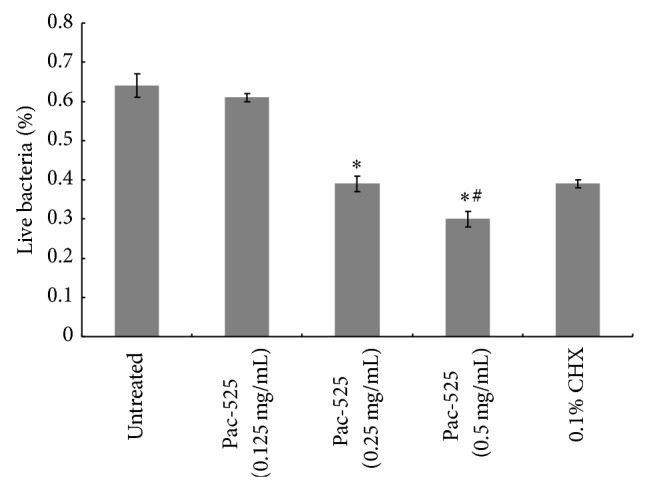
Percentage of live bacteria in P. gingivalis biofilms (on titanium discs) treated with Pac-525. *Significantly different from untreated control. #Significantly different from CHX-treated control (P < 0.05).
4. Discussion
CHX, a membrane active bisbiguanide, is regarded as the “gold standard” antibacterial agent in oral care for reducing plaque and biofilm formation and thus preventing caries, gingivitis, periodontitis, and peri-implantitis. However, long term use of CHX in clinical application can be contraindicated due to oral mucosa irritation, the risk of tooth staining, and dental calculus formation [20]. Thus, it is important to develop alternative antimicrobial agents with fewer side effects. AMPs, such as Pac-525, KSL-W, and KSL, have been reported to efficiently protect the host from bacterial or fungal pathogens and to kill or inhibit cells from planktonic and biofilm cultures; thus, they have been proposed as promising alternative antimicrobial agents in the treatment of oral disease. In this study, we tested the antibacterial activity of these AMPs against the predominant pathogenic bacteria involved in peri-implantitis to determine the most effective AMPs for use as antibacterial agents for dental implants.
One of the most desired properties of AMPs is low toxicity to eukaryotic cells. In this study, an MTT assay was conducted with MG-63 cells to assess cytotoxicity. The three short AMPs did not exhibit cytotoxic effects, which was consistent with previous results that examined the proliferation of keratinocyte cells and human gingival fibroblasts [13, 21, 22]. Our findings confirmed that all the tested short AMPs had high biosafety and were suitable for use as antimicrobial agents in the oral cavity.
Amongst the bacterial strains tested under clinical conditions of dental implants, S. sanguis is the early colonizer and P. gingivalis is the late one. F. nucleatum is the major coaggregation bridge organism that links early and late colonizers. In this study, S. sanguis appeared insensitive to KSL-W and KSL within the range of tested concentrations, which differed from the results of Concannon et al. [13] and Liu et al. [14]. This variation may be due to differences in AMPs purity, bacterial strains, and concentrations of bacterial suspension and AMPs solution used in each experiment. However, S. sanguis was susceptible to Pac-525, with a MIC value of 0.125 mg/mL and a MBC value of 0.5 mg/mL. For P. gingivalis and F. nucleatum, the MIC values of Pac-525 were 0.0625 mg/mL and 0.0078 mg/mL, respectively, which were half of the values of KSL and KSL-W. Therefore, among the three short AMPs, Pac-525 was the most efficient in inhibiting the tested planktonic bacteria. To our knowledge, this is the first report describing the inhibitory effects of Pac-525 against planktonic S. sanguis, F. nucleatum, and P. gingivalis. Our findings suggest that Pac-525 has the ability to prevent the adherence of bacteria to the surface of dental implants and therefore decrease biofilm formation, thus decreasing the occurrence to peri-implantitis.
Biofilms have been shown to be many times more resistant to antibiotics and more toxicity to host than planktonic or free-swimming cells so as to improve the possibility of metabolization and survival in the antibacterial conditions [23, 24]. Bacteria that live inside a biofilm are strongly resistant to antimicrobials and exhibit little sensitivity to host defense systems [25]. Because of P. gingivalis being the predominant pathogenic bacteria involved in peri-implantitis, we studied the effects of the Pac-525 peptide on P. gingivalis biofilms, based on MIC and MBC results suggesting that Pac-525 inhibits planktonic bacteria. Furthermore, commercially pure titanium, having a roughness similar to that of clinical implants, was selected as the carrier material for P. gingivalis biofilm formation, in order to imitate clinical conditions and interaction between P. gingivalis biofilms and dental implants. The results showed that when treated with 0.5 mg/mL Pac-525, the biofilm thickness, and the percentage of live bacteria were the lowest among the tested groups and were even significantly lower than those of the groups treated with CHX. This demonstrated that Pac-525 has the ability to kill bacteria within P. gingivalis biofilms cultured on a titanium surface. Data from our investigation indicated that the effective inhibitory concentration of Pac-525 against P. gingivalis biofilm was eight times higher than the MIC for planktonic cells, whereas previous studies showed that inhibitory concentrations against biofilms are often 10 to 1,000 times higher than MICs for planktonic cells [26–28]. Hence, compared to a conventional antibacterial agent, Pac-525 had the remarkable advantage of inhibiting P. gingivalis biofilm formation. The inhibitory effect might be related to the structure of Pac-525, which is a novel Trp-rich peptide containing nine amino acids. Trp has a high potential for insertion into membranes and likely endows the Trp-rich Pac-525 peptide with the ability to enter membranes and to affect the lipopolysaccharides of Gram-negative bacteria [29]. Furthermore, Li et al. [30] reported that a derivative of D-Nal-Pac-525 was designed by replacing the tryptophans of Pac-525 with D-β-naphthyalanines and was proved to have superior antibacterial activities against the planktonic S. Mutans and its biofilm. This report suggests that both Pac-525 and its derivative are potential candidates to prevent oral diseases in terms of dental caries, periodontitis, and peri-implantitis.
In conclusion, the present study demonstrated that Pac-525, KSL-W, and KSL were not cytotoxic and showed high biosafety. Meanwhile, the AMPs showed favourable inhibitory effects against the predominant planktonic pathogenic bacteria involved in peri-implantitis, including S. sanguis, F. nucleatum, and P. gingivalis, although S. sanguis appeared insensitive to KSL-W and KSL within the range of tested concentrations. Among the three short AMPs tested, Pac-525 was the most efficient inhibitor of planktonic bacteria. Furthermore, Pac-525 was confirmed to have the ability to kill bacteria within the P. gingivalis biofilm cultured on the titanium surface. Thus, the short AMPs tested in our study had unique properties and could have a potential clinical application in the prevention and/or treatment of peri-implantitis by the inhibition of pathogenic bacteria around dental implants.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (no. 81000669), the Liaoning Province Programs for Science and Technology (no. 201102045 and no. 2008225017), and the Fund of Dalian Programs for Science and Technology (no. 2012E12SF075).
Disclosure
Ji-yin Li and Xue-jin Wang are co-first authors. Li Xu and Guo-wu Ma are the co-corresponding authors.
Conflict of Interests
The authors have declared no conflict of interests.
References
- 1.Pjetursson B. E., Thoma D., Jung R., Zwahlen M., Zembic A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clinical Oral Implants Research. 2012;23(6):22–38. doi: 10.1111/j.1600-0501.2012.02546.x. [DOI] [PubMed] [Google Scholar]
- 2.Romeo E., Storelli S. Systematic review of the survival rate and the biological, technical, and aesthetic complications of fixed dental prostheses with cantilevers on implants reported in longitudinal studies with a mean of 5 years follow-up. Clinical Oral Implants Research. 2012;23(6):39–49. doi: 10.1111/j.1600-0501.2012.02551.x. [DOI] [PubMed] [Google Scholar]
- 3.Simonis P., Dufour T., Tenenbaum H. Long-term implant survival and success: a 10–16-year follow-up of non-submerged dental implants. Clinical Oral Implants Research. 2010;21(7):772–777. doi: 10.1111/j.1600-0501.2010.01912.x. [DOI] [PubMed] [Google Scholar]
- 4.Charalampakis G., Leonhardt Å., Rabe P., Dahlén G. Clinical and microbiological characteristics of peri-implantitis cases: a retrospective multicentre study. Clinical Oral Implants Research. 2012;23(9):1045–1054. doi: 10.1111/j.1600-0501.2011.02258.x. [DOI] [PubMed] [Google Scholar]
- 5.Pye A. D., Lockhart D. E. A., Dawson M. P., Murray C. A., Smith A. J. A review of dental implants and infection. Journal of Hospital Infection. 2009;72(2):104–110. doi: 10.1016/j.jhin.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Wang G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies. Oxfordshire, UK: CAB International; 2010. [Google Scholar]
- 7.Zhang L., Falla T. J. Host defense peptides for use as potential therapeutics. Current Opinion in Investigational Drugs. 2009;10(2):164–171. [PubMed] [Google Scholar]
- 8.McDermott A. M. Cationic antimicrobial peptides. A future therapeutic option? Archivos de la Sociedad Espanola de Oftalmologia. 2007;82(8):467–470. [PubMed] [Google Scholar]
- 9.Fonseca S. B., Pereira M. P., Kelley S. O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Advanced Drug Delivery Reviews. 2009;61(11):953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani A., Pirri G., Bozzi A., Di Giulio A., Aschi M., Rinaldi A. C. Antimicrobial peptides: natural templates for synthetic membrane-active compounds. Cellular and Molecular Life Sciences. 2008;65(16):2450–2460. doi: 10.1007/s00018-008-8188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei S.-Y., Wu J.-M., Kuo Y.-Y., et al. Solution structure of a novel tryptophan-rich peptide with bidirectional antimicrobial activity. Journal of Bacteriology. 2006;188(1):328–334. doi: 10.1128/JB.188.1.328-334.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J.-M., Wei S.-Y., Chen H.-L., Weng K.-Y., Cheng H.-T., Cheng J.-W. Solution structure of a novel D-β-naphthylalanine substituted peptide with potential antibacterial and antifungal activities. Peptide Science. 2007;88(5):738–745. doi: 10.1002/bip.20736. [DOI] [PubMed] [Google Scholar]
- 13.Concannon S. P., Crowe T. D., Abercrombie J. J., et al. Susceptibility of oral bacteria to an antimicrobial decapeptide. Journal of Medical Microbiology. 2003;52(12):1083–1093. doi: 10.1099/jmm.0.05286-0. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y., Wang L. N., Zhou X. D., Hu S., Zhang S. Y., Wu H. K. Effect of the antimicrobial decapeptide KSL on the growth of oral pathogens and Streptococcus mutans biofilm. International Journal of Antimicrobial Agents. 2011;37(1):33–38. doi: 10.1016/j.ijantimicag.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Na D. H., Faraj J., Capan Y., Leung K. P., DeLuca P. P. Chewing gum of antimicrobial decapeptide (KSL) as a sustained antiplaque agent: preformulation study. Journal of Controlled Release. 2005;107(1):122–130. doi: 10.1016/j.jconrel.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Na D. H., Faraj J., Capan Y., Leung K. P., DeLuca P. P. Stability of antimicrobial decapeptide (KSL) and its analogues for delivery in the oral cavity. Pharmaceutical Research. 2007;24(8):1544–1550. doi: 10.1007/s11095-007-9274-8. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Document. M100-S20. Wayne, Pa, USA: CLSI; 2010. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 18.Löberg J., Mattisson I., Hansson S., Ahlberg E. Characterisation of titanium dental implants I: critical assessment of surface roughness parameters. The Open Biomaterials Journal. 2010;2:18–35. doi: 10.2174/1876502501002010018. [DOI] [Google Scholar]
- 19.Noiri Y., Okami Y., Narimatsu M., Takahashi Y., Kawahara T., Ebisu S. Effects of chlorhexidine, minocycline, and metronidazole on Porphyromonas gingivalis strain 381 in biofilms. Journal of Periodontology. 2003;74(11):1647–1651. doi: 10.1902/jop.2003.74.11.1647. [DOI] [PubMed] [Google Scholar]
- 20.van Strydonck D. A. C., Slot D. E., van Der Velden U., van Der Weijden F. Effect of a chlorhexidine mouthrinse on plaque, gingival inflammation and staining in gingivitis patients: a systematic review. Journal of Clinical Periodontology. 2012;39(11):1042–1055. doi: 10.1111/j.1600-051x.2012.01883.x. [DOI] [PubMed] [Google Scholar]
- 21.Qi X., Zhou C., Li P., et al. Novel short antibacterial and antifungal peptides with low cytotoxicity: efficacy and action mechanisms. Biochemical and Biophysical Research Communications. 2010;398(3):594–600. doi: 10.1016/j.bbrc.2010.06.131. [DOI] [PubMed] [Google Scholar]
- 22.Semlali A., Leung K. P., Curt S., Rouabhia M. Antimicrobial decapeptide KSL-W attenuates Candida albicans virulence by modulating its effects on Toll-like receptor, human β-defensin, and cytokine expression by engineered human oral mucosa. Peptides. 2011;32(5):859–867. doi: 10.1016/j.peptides.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Fricks-Lima J., Hendrickson C. M., Allgaier M., et al. Differences in biofilm formation and antimicrobial resistance of Pseudomonas aeruginosa isolated from airways of mechanically ventilated patients and cystic fibrosis patients. International Journal of Antimicrobial Agents. 2011;37(4):309–315. doi: 10.1016/j.ijantimicag.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elias S., Banin E. Multi-species biofilms: living with friendly neighbors. FEMS Microbiology Reviews. 2012;36(5):990–1004. doi: 10.1111/j.1574-6976.2012.00325.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith A. W. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Advanced Drug Delivery Reviews. 2005;57(10):1539–1550. doi: 10.1016/j.addr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Wright T. L., Ellen R. P., Lacroix J. M., Sinnadurai S., Mittelman M. W. Effects of metronidazole on Porphyromonas gingivalis biofilms. Journal of Periodontal Research. 1997;32(5):473–477. doi: 10.1111/j.1600-0765.1997.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 27.Larsen T. Susceptibility of Porphyromonas gingivalis in biofilms to amoxicillin, doxycycline and metronidazole. Oral Microbiology and Immunology. 2002;17(5):267–271. doi: 10.1034/j.1399-302x.2002.170501.x. [DOI] [PubMed] [Google Scholar]
- 28.Miyake Y., Fujiwara S., Usui T., Suginaka H. Simple method for measuring the antibiotic concentration required to kill adherent bacteria. Chemotherapy. 1992;38(5):286–290. doi: 10.1159/000239015. [DOI] [PubMed] [Google Scholar]
- 29.Yu H. Y., Huang K. C., Yip B. S., et al. Rational design of tryptophan-rich antimicrobial peptides with enhanced antimicrobial activities and specificities. ChemBioChem. 2010;11(16):2273–2282. doi: 10.1002/cbic.201000372. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Cheng J.-W., Yu H.-Y., Xin Y., Tang L., Ma Y. Effect of the antimicrobial peptide D-Nal-Pac-525 on the growth of Streptococcus mutans and its biofilm formation. Journal of Microbiology and Biotechnology. 2013;23(8):1070–1075. doi: 10.4014/jmb.1212.12035. [DOI] [PubMed] [Google Scholar]