Abstract
BACKGROUND
The effect of care setting on value of colon cancer care is unknown.
METHODS
A Surveillance, Epidemiology, and End Results (SEER)-Medicare cohort study of 6544 patients aged ≥66 years with stage IV colon cancer (based on the American Joint Committee on Cancer staging system) who were diagnosed between 1996 and 2005 was performed. All patients were followed through December 31, 2007. Using outpatient and carrier claims, patients were assigned to a treating hospital based on the hospital affiliation of the primary oncologist. Hospitals were classified academic or nonacademic using the SEER-Medicare National Cancer Institute Hospital File.
RESULTS
Of the 6544 patients, 1605 (25%) received care from providers affiliated with academic medical centers. The unadjusted median cancer-specific survival was 16.0 months at academic medical centers versus 13.9 months at nonacademic medical centers (P<.001). After adjustment, treatment at academic hospitals remained significantly associated with a reduced risk of death from cancer (hazard ratio, 0.87; 95% confidence interval [95% CI], 0.82–0.93 [P<.001]). Adjusted mean 12-month Medicare spending was $8571 higher at academic medical centers (95% CI, $2340–$14,802; P =.007). The adjusted median cost was $1559 higher at academic medical centers; this difference was not found to be statistically significant (95% CI, −$5239 to $2122; P =.41). A small percentage of patients who received very expensive care skewed the difference in mean cost; the only statistically significant difference in adjusted costs in quantile regressions was at the 99.9th percentile of costs (P<.001).
CONCLUSIONS
Among Medicare beneficiaries with stage IV colon cancer, treatment by a provider affiliated with an academic medical center was associated with a 2 month improvement in overall survival. Except for patients in the 99.9th percentile of the cost distribution, costs at academic medical centers were not found to be significantly different from those at nonacademic medical centers.
Keywords: colon cancer, academic medical centers, cost analysis, health policy, survival analysis, incremental cost-effectiveness ratio
INTRODUCTION
Nearly 20,000 individuals were expected to be diagnosed with metastatic colorectal cancer in the United States in 2013.1,2 As overall survival increases due to improved cancer treatment, costs of care rise exponentially. Colon cancer is not the only cancer for which this holds true. More effective treatments are now available than ever before for patients with metastatic solid tumors. Patients with stage IV breast, prostate, and lung cancer are living longer due to treatment advances.3–5 Although the costs of cancer care in general have grown dramatically in the United States over the last decades, the costs of patients with metastatic disease are particularly high.6 These patients tend to be older, have comorbid conditions, and receive costly therapies such as surgical resection of metastases and molecularly targeted therapy.7–10 This growing financial burden has adverse consequences for the US economy, governmental support of social programs, and individual patients.11
Given the cost and complexity of caring for older patients with metastatic colon cancer, the delivery of high-value cancer care to this population is critically important. High-value care is defined as care that maximizes patient outcomes while containing the cumulative costs of care.12,13 It is plausible that providers and the care setting substantially influence the value of care delivered. For example, academic medical centers offer specialized providers and a multidisciplinary approach to cancer care. However, the role of academic medical centers in providing high-value cancer care is uncertain. Care at an academic center has been associated with better outcomes in some settings, including surgery for colorectal cancer.14,15 However, academic medical centers have also been associated with higher costs of care and little difference in quality measures in other settings.16–21 In the current study, we evaluated overall survival and costs of care for elderly patients with stage IV colon cancer to determine whether hospital academic status was associated with differences in the value of the care delivered.
MATERIALS AND METHODS
Institutional Review
The current study was a retrospective cohort study of the outcomes and costs of cancer care delivered to patients with colon cancer diagnosed between January 1996 and December 2005. This study was exempted from review by the Institutional Review Board of the University of Pennsylvania.
Study Population
We identified 83,731 patients who were diagnosed with colon cancer between January 1, 1996 and December 31, 2005 from the Surveillance, Epidemiology, and End Results (SEER)-Medicare files.
We excluded patients without a histologic finding of adenocarcinoma (3168 patients), those in whom the diagnosis had been made at autopsy or on a death certificate (2 patients), or patients who were aged <66 years (to enable inclusion of 1 year of claims for comorbidity assessment) or >99 years at the time of diagnosis (11,278 patients). Patients who did not have Medicare Part A or Medicare Part B coverage (3754 patients) at any time during the study period or patients who were enrolled in a health maintenance organization in the 12 months before or the 12 months after diagnosis (13,097 patients) were excluded because of incomplete claims data. Patients with missing data regarding stage of disease or stage 0 disease were excluded (908 patients). Of the remaining 51,524 patients, 40,862 were assigned to a treating hospital as described below. Among these, 6549 patients had stage IV cancer at diagnosis, based on the American Joint Committee on Cancer overall cancer stage provided in the SEER files for each patient. Five patients with incomplete or missing payment data were omitted, resulting in a final cohort of 6544 patients who were followed through December 31, 2007.
Hospital Assignment
Because the majority of colon cancer care is delivered in the outpatient setting, assigning a patient to a treating hospital using inpatient claims can be challenging. We assigned each patient in the current study cohort to a treating hospital by applying a method developed by Bynum et al22 in which patients are assigned to a hospital based on their predominant ambulatory physicians’ hospital affiliation. This method was previously validated in a cohort of Medicare fee-for-service patients for whom both generalists and medical specialists, including medical oncologists, were identified as the predominant ambulatory physician.22 The patients’ primary medical oncologists were defined as the medical oncologist billing for the greatest number of evaluation and management visits in the 6 months before and 12 months after diagnosis. Medical oncologists were assigned to the one hospital in which they provided the most inpatient care. Finally, patients were assigned to a treating hospital based on the hospital affiliation of their primary medical oncologist. Further details regarding the hospital assignment method can be found in the online supporting information.
Outcome Measures
We examined 2 distinct outcomes: overall survival and cost of care. Overall survival was calculated as the number of months from the date of the initial colon cancer diagnosis to the date of death or to December 31, 2007 if the patient was still alive at the end of the follow-up period.
Cost of care was defined as the total payment made by Medicare for each patient’s inpatient and outpatient care, determined by summing payments obtained from the Medicare Provider Analysis and Review file, the National Claims History file, and the Outpatient Standard Analytic File. Costs were summed and analyzed over the time interval extending to 12 months beyond the date of the initial cancer diagnosis.
Predictor Variable
The primary predictor of interest was the academic status of the treating hospital for each patient. Academic status was obtained from the National Cancer Institute Hospital File included in SEER-Medicare data. The National Cancer Institute obtains hospital information from the annual Center for Medicare and Medicaid Services Health Care Cost Report and Provider of Service survey. Hospitals classified in the Hospital File as having a major medical school affiliation were considered academic hospitals in the current analyses. All other hospitals were classified as nonacademic hospitals.
Covariates
Before data analysis, we identified potential confounders and effect modifiers of the association between hospital academic status and the outcome variables. These included patient age, race, sex, comorbidity score according to Elixhauser et al23 excluding cancer-related diagnoses, and ZIP code-based socioeconomic status.
Statistical Analysis
To assess univariate associations between the type of hospital (academic vs nonacademic) and clinical and demographic factors, we used chi-square tests for categorical variables and Student t tests for continuous variables. We constructed Kaplan-Meier curves to assess unadjusted overall survival. We estimated a Cox proportional hazards model to assess the association between hospital academic status and overall survival while adjusting for important patient characteristics. We modeled time to death from any cause and time to colon cancer-related death.
To examine the association between hospital academic status and the mean cost of care while adjusting for important patient characteristics, we estimated a generalized linear model with a log-link and a gamma family. Standard errors were adjusted to account for clustering of patients within hospitals. We also estimated quantile regression models to examine associations between hospital teaching status and cost of care at various quantiles (25th, 50th, 75th, 90th, 95th, 99th, 99.5th, and 99.9th) along the cost distribution, while adjusting for important patient characteristics. In all models, standard errors were adjusted to account for clustering of patients within hospitals.
We assessed two-way interactions between hospital academic status and patient age, race, and number of comorbid conditions using likelihood ratio and Wald tests to assess the joint significance of interaction terms; no interactions were found to be statistically significant.
Statistical significance was set at a P value <.05. All statistical analyses were performed using SAS statistical software (version 9.1; SAS Institute Inc, Cary, NC) or STATA software (version 12.1; STATA Corporation, College Station, Tex).
RESULTS
Baseline Characteristics and Univariate Analysis
The final cohort consisted of 6544 patients with stage IV colon cancer at the time of diagnosis. A total of 1605 patients (25%) received cancer care from providers affiliated with an academic hospital. Table 1 shows the baseline characteristics of the cohort and univariate analyses of associations between these characteristics and hospital academic status. Patients whose medical oncologists were assigned to academic hospitals were more likely to be black and in a higher income bracket (P<.001) than patients whose medical oncologists were assigned to nonacademic hospitals. There were no significant differences concerning age, sex, or comorbid conditions observed with regard to hospital academic status.
TABLE 1.
Univariate Analysis of Patient Characteristics and Hospital Academic Status*
| Characteristic | Academic Hospital | Nonacademic Hospital | P |
|---|---|---|---|
| No. (%) | 1605 | 4939 | |
| Vital status (as of 12/31/2007) | |||
| Alive | 137 (9) | 351 (7) | |
| Dead | 1468 (91) | 4588 (93) | .058 |
| Age, y | |||
| 65–69 | 321 (20) | 982 (20) | |
| 70–74 | 444 (28) | 1263 (26) | |
| 75–79 | 411 (26) | 1309 (27) | |
| 80–84 | 278 (17) | 874 (18) | |
| ≥85 | 151 (9) | 511 (10) | .469 |
| Race | |||
| White | 1262 (79) | 4262 (86) | |
| Black | 212 (13) | 396 (8) | |
| Other | 131 (8) | 281 (6) | <.001 |
| Sex | |||
| Female | 763 (48) | 2310 (47) | |
| Male | 843 (52) | 2633 (53) | .588 |
| No. of comorbid conditions | |||
| 0 | 46 (3) | 111 (2) | |
| 1 | 116 (7) | 294 (6) | |
| ≥2 | 1443 (90) | 4531 (92) | .063 |
| Median household income | |||
| 0% to <25% | 374 (23) | 1363 (28) | |
| ≥25% to 50% | 373 (23) | 1287 (26) | |
| ≥50% to 75% | 383 (24) | 1256 (25) | |
| ≥75% to 100% | 475 (30) | 1033 (21) | <.001 |
| Y of diagnosis | |||
| 1995 | 107 (7) | 286 (6) | |
| 1996 | 111 (7) | 274 (6) | |
| 1998 | 91 (6) | 286 (6) | |
| 1999 | 109 (7) | 252 (5) | |
| 2000 | 168 (10) | 553 (11) | |
| 2001 | 176 (11) | 609 (12) | |
| 2002 | 193 (12) | 633 (13) | |
| 2003 | 195 (12) | 572 (12) | |
| 2004 | 181 (11) | 616 (13) | |
| 2005 | 146 (9) | 551 (11) | .003 |
Data shown are N (%). P values are derived from chi-square tests. Proportions may not add to 100% because of rounding.
Survival Analysis
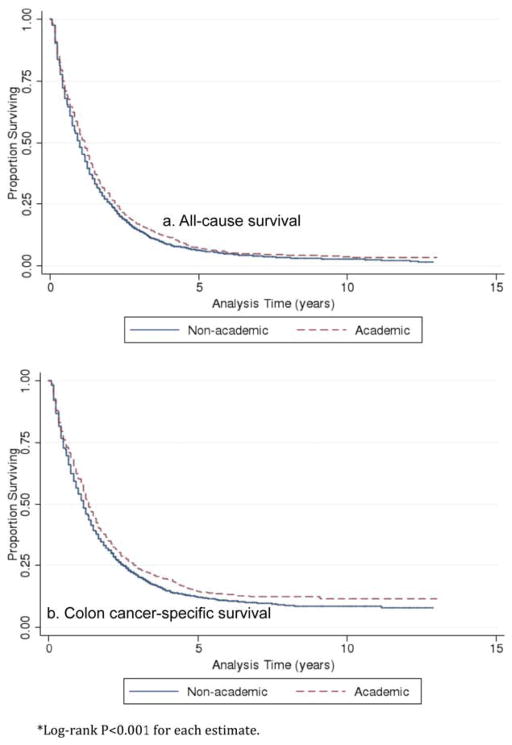
Unadjusted Kaplan-Meier curves for all-cause and colon cancer-specific mortality are shown in Figure 1. Univariate analysis using log-rank testing indicated that academic status was significantly associated with improved survival; the median survival (all-cause mortality) for patients with stage IV colorectal cancer who were treated at academic hospitals was 13.9 months compared with 11.9 months for patients treated at nonacademic hospitals (P<.001). The difference persisted for colon cancer-specific survival; the median survival was 16.0 months for patients treated at academic medical centers versus 13.9 months for patients treated at nonacademic medical centers (P<.001).
Figure 1.
Kaplan-Meier survival estimates of unadjusted overall survival are shown by hospital academic status. Dashed lines represent academic medical centers and solid lines represent nonacademic medical centers.
Hospital academic status was included in a Cox proportional hazards model that adjusted for patient characteristics. After adjustment, hospital academic status remained significantly associated with improved survival (all-cause mortality: hazard ratio [HR], 0.90; 95% confidence interval [95% CI], 0.85–0.96 [P =.001]; and colon cancer-specific mortality: HR, 0.87; 95% CI, 0.82–0.93 [P<.001]). Table 2 shows results of the Cox proportional hazards model.
TABLE 2.
Effect of Hospital Academic Status on Adjusted Overall Survival: Cox Proportional Hazards Modela
| Characteristic | All-Cause Mortality
|
Colon Cancer-Specific Mortality
|
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Hospital academic status (vs nonacademic) | ||||
| Academic | 0.90 (0.85–0.96) | .001 | 0.87 (0.82–0.93) | <.001 |
| Age (vs 65–69), y | ||||
| 70–74 | 1.08 (1.00–1.17) | .041 | 1.07 (0.98–1.16) | .12 |
| 75–79 | 1.20 (1.12–1.30) | <.001 | 1.19 (1.10–1.30) | <.001 |
| 80–84 | 1.41 (1.30–1.53) | <.001 | 1.36 (1.24–1.50) | <.001 |
| ≥85 | 1.81 (1.64–2.00) | <.001 | 1.72 (1.55–1.92) | <.001 |
| Race (vs white) | ||||
| Black | 1.09 (0.87–1.23) | .062 | 1.04 (0.94–1.15) | .499 |
| Other | 0.93 (0.84–1.04) | .208 | 0.91 (0.80–1.02) | .105 |
| Sex (vs female) | ||||
| Male | 1.04 (0.99–10.9) | .133 | 1.09 (1.03–1.15) | .004 |
| No. of comorbid conditions (vs 0 conditions) | ||||
| 1 | 1.06 (0.87–1.23) | .591 | 1.01 (0.81–1.25) | .499 |
| ≥ 2 | 1.40 (1.18–1.67) | <.001 | 1.34 (1.11–1.62) | .002 |
| Median household income (vs. 0% to <25%) | ||||
| ≥25% to 50% | 0.95 (0.89–1.02) | .163 | 0.92 (0.85–1.00) | .052 |
| ≥50% to 75% | 0.98 (0.92–1.06) | .653 | 0.98 (0.91–1.06) | .681 |
| ≥75% to 100% | 0.94 (0.87–1.01) | .103 | 0.94 (0.87–1.02) | .144 |
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio.
Year of diagnosis was also included in the model; output not shown here.
Cost of Care
Twelve-month unadjusted and adjusted costs of care are shown in Table 3. The unadjusted mean cost of care was $6370 higher for patients treated at academic medical centers (95% CI, $2123–$10,616; P =.003), whereas the unadjusted median cost of care was $490 higher for patients treated at academic medical centers (95% CI, −$3785 to $4765; P =.822). Nominal differences in the unadjusted median costs of care increased across the 25th, 50th, 75th, 90th, 95th, 99th, 99.5th and 99.9th percentiles, with the greatest unadjusted difference in cost ($283,363) observed for those patients whose 12-month costs fell within the 99.9th percentile. Only the unadjusted differences in median cost at the 99.5th (P =.009) and 99.9th (P<.001) percentiles were found to be statistically significant.
TABLE 3.
Twelve-Month Costs by Hospital Academic Statusa
| Outcome | Academic Hospital | Nonacademic Hospital | Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) |
|---|---|---|---|---|
| Mean cost | $56,797 | $50,427 | $6370 ($2123 to $10,616)b | $8571 ($2340 to $14,802)c |
| Median cost | $31,896 | $31,406 | $490 (−$3785 to $4765) | $1559 (−$2133 to $25,251) |
| 25th percentile | $12,115 | $11,769 | $346 (−$1742 to $2434) | $930 (−1057 to $2917) |
| 75th percentile | $66,499 | $63,546 | $2953 (−$6,984 to $12,890) | $1448 (−$4855 to $7751) |
| 90th percentile | $115,581 | $111,456 | $4125 (−$14,525 to $22,775) | $7502 (−$4544 to $19,548) |
| 95th percentile | $188,545 | $154,665 | $33,880 (−$5129 to $72,890) | $17,293 (−$12,121 to $46,707) |
| 99th percentile | $434,434 | $324,740 | $109,694 (−$61,050 to $280,438) | $20,917 (−$35,686 to $77,521) |
| 99.5th percentile | $618,693 | $465,032 | $153,661 ($38,912 to $268,409)d | $26,708 ($18,179 to $71,594) |
| 99.9th percentile | $1,005,610 | $722,247 | $283,363 ($156,534 to $410,191)e | $71,457 ($48,330 to $94,584)f |
Abbreviations: 95% CI, 95% confidence interval.
The mean and median costs displayed in the first 2 columns are unadjusted costs. Unadjusted mean costs were obtained via a 2-sample Student t test, and the adjusted difference in mean costs was obtained via a generalized linear model. Unadjusted and adjusted median and percentile-based costs were obtained using quantile regression. Only significant P values are listed below; all other P values were insignificant.
P=.003.
P=.007.
P=.009.
P<.001.
P<.001.
Hospital academic status was included in a generalized linear model that adjusted for patient characteristics. After adjustment, hospital academic status remained significantly associated with a difference in the mean cost. The adjusted mean cost of care was $8571 higher for patients treated at academic medical centers (95% CI, $2340–$14,802; P =.007). The adjusted median cost of care was $1559 higher for patients treated at academic medical centers, and this was not statistically significant (95% CI, −$5239 to $2122; P =.410). Differences in adjusted median costs increased across the 25th, 50th, 75th, 90th, 95th, 99th, 99.5th, and 99.9th percentiles, with the greatest difference in adjusted median cost ($71,457) noted for those patients whose 12-month cost of care fell within the 99.9th percentile. The only statistically significant adjusted difference in cost from the quantile regressions was that observed at the 99.9th percentile (P<.001).
DISCUSSION
The results of the current study indicate that for patients with metastatic colon cancer at the time of diagnosis, receiving colon cancer care from a provider affiliated with an academic medical center was associated with a statistically significant increase in all-cause and colon cancer-specific overall survival of approximately 2 months. Furthermore, although the adjusted mean 12-month cost of care was $8571 higher for patients treated at academic medical centers and the difference was statistically significant, the mean cost of care was skewed by a small percentage of patients who received very expensive care. In fact, the adjusted median 12-month cost was a modest $1559 higher for patients treated at academic medical centers, and this difference was not statistically significant. Moreover, our quantile regression indicated that the adjusted difference in cost was significant only for those patients whose costs fell within the 99.9th percentile.
To quantify the value of treatment at an academic medical center, we estimated the incremental cost-effectiveness ratio by dividing the incremental cost by the incremental benefit. Using the higher estimate of incremental cost based on the difference in means ($8571) and the incremental benefit based on unadjusted median survival (2.1 months) implies that the “price” of an extra year of survival from treatment at an academic medical center is $48,977. The estimate drops to $8909 when measuring the incremental cost at the median. This figure is only a rough estimate and is not directly comparable to quality-adjusted life year acceptability thresholds, such as the common but arbitrary $50,000 per quality-adjusted life year, because it does not account for quality of life. Nevertheless, these estimates compare favorably with a standard therapy for metastatic colorectal cancer, bevacizumab, which improved the median survival by approximately 4.5 months at an annual cost of up to $100,000, suggesting an incremental cost-effectiveness ratio of up to $266,667 per year of life.24,25
The unadjusted, colon cancer-related, median overall survival of patients treated at academic (16.0 months) and nonacademic (13.9 months) medical centers is lower than the literature-based median overall survival of 24 months for patients with stage IV colorectal cancer.7 This discrepancy is likely due to the finding that the current study data were population-based and collected over the course of 10 years, whereas survival rates currently reported from studies reflect the increasing use of resection of liver metastases and combination chemotherapy regimens.26,27 In fact, the hazard ratio for colon cancer-specific death in the current study decreased steadily over time (see online supporting information).
Although the absolute difference in survival was modest, the relative effect of hospital academic status on overall survival was substantial, with a 13% decreased risk of colon cancer mortality and a 10% decreased risk of overall mortality associated with treatment at an academic medical center. There are several possible reasons for this difference. First, the management of metastatic colon cancer has become increasingly complex. Providers associated with academic medical centers are highly specialized, often with advanced training in surgical oncology, colorectal and liver surgery, and chemotherapy specific to colon cancer. To our knowledge, at least one study to date has shown an association between receipt of treatment by a specialized surgeon and improved overall survival in patients with colorectal cancer.28 Treatment by a specialized medical oncologist with expertise in colon cancer and the ability to appropriately select those treatment options most likely to confer clinical benefit could also contribute to improved outcomes.
The multidisciplinary nature of academic settings may also contribute to the difference in outcomes noted in the current study. Academic medical centers provide a range of cancer care specialists, and are more likely to provide primary, preventive, and supportive care services to patients with cancer.29 This is particularly important in the care of elderly patients with cancer, who often have multiple comorbid conditions in addition to cancer.30 The availability of consulting physicians in an academic setting may help to meet the complete health needs of patients with colon cancer and may contribute to the reduced risk of all-cause mortality observed in the current study.
In addition, the undertreatment of elderly patients with colorectal cancer has been well described in the literature.31,32 Providers at academic medical centers may have more experience treating elderly patients with colon cancer and a greater level of comfort offering and managing therapies that prolong overall survival. Although elderly patients are significantly underrepresented in clinical trial accrual in general,33,34 those treated at academic medical centers have greater access to clinical trials than those patients treated at nonacademic medical centers, and this may also contribute, in small part, to the difference in overall survival.
There are several limitations to the current study. First, patients were assigned to the hospital at which they were likely to have received their cancer care using the primary medical oncologist as an intermediary. It was not possible to assign all patients to a treating hospital using this method, and some patients may have been incorrectly assigned. Second, there may be unmeasured differences between patients with colon cancer who receive care at academic medical centers and those who receive care at nonacademic medical centers. Perhaps these patient differences, rather than hospital academic status, are associated with the observed effects on outcomes. However, when we adjusted for extensive patient-level factors, the differences associated with hospital academic status persisted. Nevertheless, the current analysis did not reveal the causal mechanisms underlying this difference; hospital academic status could be a proxy for some other causative factor. Third, we excluded out-of-pocket costs to patients in our calculation of overall cost to evaluate costs from the payer’s (ie, Medicare) perspective. Because all patients in the current study cohort were covered under Medicare fee-for-service, we do not expect that the inclusion of those costs would have significantly changed the results of the current study. Fourth, our cost analyses do not account for patient censoring. Patients who live longer receive more care and thus incur more costs; given the improved overall survival of patients treated at academic medical centers, costs at these centers may appear greater than those at nonacademic centers. Fifth, consistent with prior studies,35,36 we used a Cox proportional hazards approach to model survival, which treats deaths due to other causes as independent from deaths due to colon cancer. Sixth, SEER-Medicare does not collect data regarding quality of life and therefore we could not include this important patient outcome in our value estimates. Finally, because the population of the current study was restricted to patients aged >65 years, these findings cannot be generalized to younger patients with colon cancer. However, the average age of an individual at the time of a colon cancer diagnosis in the United States is 69 years, with the majority of patients diagnosed after age 65 years. Thus, it is reasonable to study colon cancer in the Medicare population.
Metastatic colon cancer is an ideal disease setting in which to study site effects on the value of cancer care delivered and should serve as the model for further studies. Unlike early-stage colon cancer, for which consensus regarding the standard treatment approach and clear guidelines for care exist, the treatment of metastatic colon cancer is highly individualized with the potential for substantial variation depending on the care setting. Using robust population-based data, the results of the current study indicated that overall survival was significantly extended when elderly patients with metastatic colon cancer received cancer care from providers affiliated with an academic medical center. Although the adjusted mean cost of care was significantly greater for patients treated at academic medical centers, it was only a small percentage of patients who received very expensive care who contributed to this difference. Further research is needed to identify the drivers behind the differences in outcomes for patients with metastatic colon cancer based on care setting. Faced with high and rising health care costs, the delivery of high-value health care that maximizes patient outcomes while containing overall costs should be a national priority.
Supplementary Material
Acknowledgments
We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services Inc; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
FUNDING SUPPORT
Supported by the National Cancer Institute at the National Institutes of Health (grant T32 CA009615-21 to Dr. Veenstra).
Footnotes
Additional Supporting Information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
Dr. Pollack was supported by a National Cancer Institute grant (K07CA151910-01A1) for work performed as part of the current study.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 3.Chia SK, Speers CH, D’yachkova Y, et al. The impact of new chemotherapeutic and hormonal agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 4.Loblaw DA, Walker-Dilks C, Winquist E, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: a systematic review. Clin Oncol (R Coll Radiol) 2013;25:406–430. doi: 10.1016/j.clon.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harboring ALK gene rearrangement: a retrospective analysis. Lancet Oncol. 2011;12:1004–1012. doi: 10.1016/S1470-2045(11)70232-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seal B, Sullivan S, Ramsey S, et al. Medical costs associated with use of systemic therapy in adults with colorectal cancer. J Manag Care Pharm. 2013;19:461–467. doi: 10.18553/jmcp.2013.19.6.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Temple LK, Hsieh WD, Saltz L, et al. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475–3484. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- 9.Mayo SC, Heckman JE, Shore AD, et al. Trends in liver-directed management of patients with colorectal liver metastasis: a population-based analysis. Surgery. 2011;150:204–216. doi: 10.1016/j.surg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Zouhairi M, Charabaty A, Pishvaian MJ. Molecularly targeted therapy for metastatic colon cancer: proven treatments and promising new agents. Gastrointest Cancer Res. 2011;4:15–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Meropol NJ, Schulman KA. The cost of cancer care: issues and implications. J Clin Oncol. 2007;25:180–186. doi: 10.1200/JCO.2006.09.6081. [DOI] [PubMed] [Google Scholar]
- 12.Portner ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 13.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 14.Paulson EC, Mitra N, Sonnad S, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. 2008;248:675–686. doi: 10.1097/SLA.0b013e318187a757. [DOI] [PubMed] [Google Scholar]
- 15.Birkmeyer NJ, Goodney PP, Stukel TA, et al. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005;103:435–441. doi: 10.1002/cncr.20785. [DOI] [PubMed] [Google Scholar]
- 16.Mechanic R, Coleman K, Dobson A. Teaching hospital costs: implications for academic missions in a competitive market. JAMA. 1998;280:1015–1019. doi: 10.1001/jama.280.11.1015. [DOI] [PubMed] [Google Scholar]
- 17.Fox PD, Wasserman J. Academic centers and managed care: uneasy partners. Health Aff (Millwood) 1993;12:85–93. doi: 10.1377/hlthaff.12.1.85. [DOI] [PubMed] [Google Scholar]
- 18.Wennberg JE. Unwarranted variations in healthcare delivery: implications for academic medical centres. BMJ. 2002;325:961–964. doi: 10.1136/bmj.325.7370.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wennberg JE, Thomson PY, Fisher ES, et al. Use of hospitals, physician visits, and hospice care during last 6 months of life among cohorts loyal to highly respected hospitals in the United States. BMJ. 2004;328:607–611. doi: 10.1136/bmj.328.7440.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138:288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 21.Ayanian JZ, Weissman JS. Teaching hospitals and quality of care: a review of the literature. Milbank Q. 2002;80:569–593. doi: 10.1111/1468-0009.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bynum JP, Bernal-Delgado E, Gottlieb D, Fisher E. Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population-based provider performance measurements. Health Serv Res. 2007;42:45–62. doi: 10.1111/j.1475-6773.2006.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz HL, Fehrenbacher W, Novotny T, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 25.Jirillo A, Vascon F, Giacobbo M. Bevacizumab in advanced cancer, too much or too little? Ann Oncol. 2008;19:1817–1818. doi: 10.1093/annonc/mdn564. [DOI] [PubMed] [Google Scholar]
- 26.Kemeny NE. Treatment of metastatic colon cancer: “the times they are A-changing”. J Clin Oncol. 2013;31:1913–1916. doi: 10.1200/JCO.2013.49.4500. [DOI] [PubMed] [Google Scholar]
- 27.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McArdle CS, Hole DJ. Influence of volume and specialization on survival following surgery for colorectal cancer. Br J Surg. 2004;91:610–617. doi: 10.1002/bjs.4476. [DOI] [PubMed] [Google Scholar]
- 29.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–1451. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 30.Ko C, Chaudhry S. The need for a multidisciplinary approach to cancer care. J Surg Res. 2002;105:53–57. doi: 10.1006/jsre.2002.6449. [DOI] [PubMed] [Google Scholar]
- 31.Chagpar R, Xing Y, Chiang YJ, et al. Adherence to stage-specific treatment guidelines for patients with colon cancer. J Clin Oncol. 2012;30:972–979. doi: 10.1200/JCO.2011.39.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Kenfield SA, Stampfer MJ, Chan JM, et al. Smoking and prostate cancer survival and recurrence. JAMA. 2011;305:2548–2555. doi: 10.1001/jama.2011.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–2769. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.