Abstract
Long conserved mechanisms maintain homeostasis in living creatures in response to a variety of stresses. However, continuous exposure to stress can result in unabated production of stress hormones, especially catecholamines, which can have detrimental health effects. While the long-term effects of chronic stress have well-known physiological consequences, recent discoveries have revealed that stress may affect therapeutic efficacy in cancer. Growing epidemiological evidence reveals strong correlations between progression-free and long-term survival and β-blocker usage in cancer patients. In this review, we summarize the current understanding of how the catecholamines, epinephrine and norepinephrine, affect cancer cell survival and tumor progression. We also highlight new data exploring the potential contributions of stress to immunosuppression in the tumor microenvironment and the implications of these findings for the efficacy of immunotherapies.
Keywords: Stress, Catecholamines, Immunosuppression, Immunotherapies, Nervous system
Introduction
All organisms encounter obstacles or stressful situations which threaten their survival. These hazards can range from physical dangers such as predators or environmental conditions to psychological trauma such as isolation or emotional loss. To cope, every creature has evolved complex mechanisms to deal with the wide variety of stressors they may encounter. In multicellular organisms, these mechanisms often involve the coordinated response of several organ systems by the brain, resulting in a series of physiological responses.
Two branches of the nervous system govern an individual’s response to stress: the autonomic nervous system and the hypothalamic-pituitary adrenal axis. These two pathways produce several neurotransmitters and hormones that facilitate both behavioral and biochemical changes, i.e., “The Fight-or-Flight Response”, and bolster the odds of survival. Catecholamines, including norepinephrine (NE) and epinephrine (EPI), are the primary molecules involved in these responses and originate from the sympathetic nerves of the autonomic system and adrenal medulla. Cortisol (in humans) or corticosterone (in humans and rodents) are produced in the adrenal cortex [1, 2] and also participate in mediating stress responses. Depending on the target cells, these molecules can activate multiple intracellular signal transduction pathways that influence survival or apoptosis, protein production, and cellular replication. Additionally, NE and EPI regulate blood pressure, heart and respiratory rate, and body temperature (non-shivering thermogenesis) even during non-stressed states by binding to α- and β-adrenergic receptors on tissues [3]. The development of therapeutic agents to target these adrenergic receptors has provided valuable medications for use in both the clinic and the laboratory. In this review, we highlight the growing recognition of the relationships between stress and immunosuppression in the context of tumor biology and its potential impact on therapeutic efficacy.
Adrenergic receptors have emerged as an interest in cancer biology due to novel findings linking neurotransmitters and tumor progression. Further, intriguing epidemiological evidence has shown that patients taking drugs known as β-adrenergic antagonists (“β-blockers”), which are commonly prescribed to treat hypertension and anxiety, have significantly lower rates of several cancers [4–8]. As will be discussed, the anti-tumor effect of β-blockers involves the inhibition of multiple pro-survival pathways within tumor cells. In addition, these therapeutic agents may also improve outcomes for cancer patients by altering the host immune response.
In this review, we examine how stress activates adrenergic receptors and how the downstream pathways may impact both tumor and anti-immune cell activities. We discuss the effects of stress hormones on cancer cell survival, metastasis, and immunosuppression and detail current work exploring the effects of stress on the efficacy of immune-based therapies. Additionally, recent findings from our own laboratory have revealed that stress induced by the physical environment, i.e. temperature-induced cold stress, can significantly impair the efficacy of cytotoxic chemotherapies and the anti-tumor immune response.
The classical stress pathway—the sympathetic nervous system and the adrenergic receptors
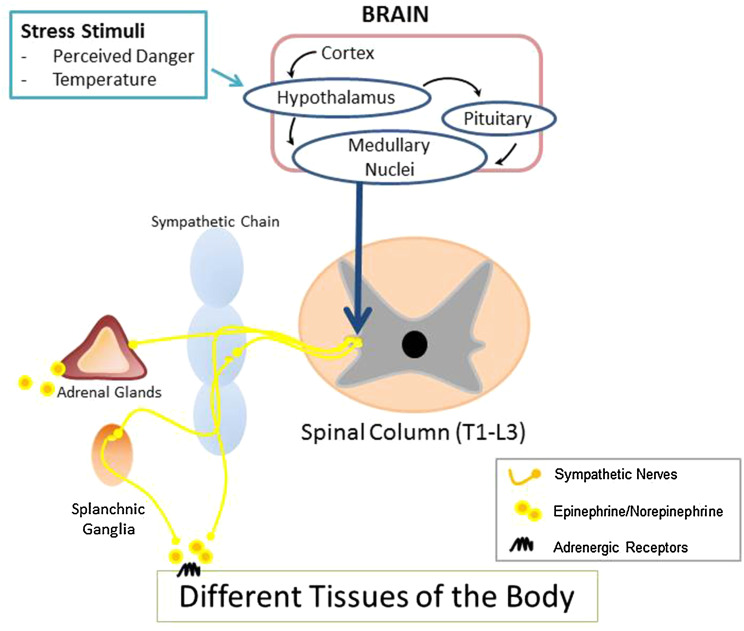
A wide variety of signals including fear, depression, anxiety, pain, and temperature can activate the stress pathways and elicit a physiological response. The resultant “fight-or-flight responses” require the systematic coordination of different tissues and organs to allow an organism to respond to danger. Following a perceived threat, signals relay from the limbic system, as well as the hypothalamus and pituitary gland, to nuclei in the medulla such as the locus coeruleus, a major hub of autonomic activity. Simultaneously, afferent signals from the periphery can also project back to and activate the nuclei in the medulla in order to re-establish homeostasis. From there, projections travel to the interomediolateral column of the spinal cord (T1–L3) where they synapse with preganglionic neurons. Axons from these cells relay signals to postganglionic neurons in the paravertebral ganglia of the sympathetic trunk, which then innervate distant tissue sites. In response to stress, NE is released locally from these sympathetic nerve endings in tissues and systemically from the adrenal medulla into the bloodstream, along with EPI, from the adrenal medulla (Fig. 1).
Fig. 1.
Physical dangers, such as predators or changes in the surrounding environment, induce signaling from the cortex or the hypothalamus to nuclei located in the medulla. These nuclei project axons onto cell bodies located in the intermediolateral column of the T1 and L3 spinal cord. From there, cells synapse with postsynaptic ganglia located in the sympathetic trunk or in splanchnic ganglia throughout the body. In addition, presynaptic neurons innervate cells of the adrenal medulla to induce hormone production. Therefore, signaling from the sympathetic nervous system can affect tissues and cells located throughout the body
Catecholamines are the major mediators of the acute stress response and function by binding to a class of seven-transmembrane, G-coupled protein receptors. These receptors are divided into two groups, α-adrenergic receptors and β-adrenergic receptors. These receptors share significant homology despite the higher affinity of α-adrenergic receptors for NE while the β-adrenergic receptors preferentially bind EPI [9]. The adrenergic receptors are expressed on many tissue types throughout the body, but the most detailed characterization of these receptors has been performed on cells of the cardiovascular system where they play a major role in regulating blood flow by modulating heart rate, myocardial contractility, and vascular smooth muscle cell constriction [10]. Additionally, the adrenergic receptors also modulate the function of hepatocytes, neurons, adipocytes, sweat glands, smooth muscle of the respiratory and gastrointestinal tracts, and even cells of the immune system [11]. Adrenergic receptors are 7-pass transmembrane G protein-coupled receptors. It is known that the α1-adrenergic receptors generally interact with the Gq subunit while α2-adrenergic receptors are inhibitory and prevent the release of NE at the presynaptic ganglion by downregulating the activity of adenylyl cyclase [12]. On the other hand, β1- and β2-adrenergic receptors signal through the Gs subunits inducing the activation of targets such as protein kinase A (PKA) to initiate gene expression by the transcription factor cAMP response element binding, CREB [12, 13]. In addition, other studies show that adrenergic receptors regulate numerous pathways, including mitogen-activated protein kinase (MAPK), Akt, NFκB [14], and Stat3 [15], which are important in the regulation of cellular functions related to apoptosis and survival, cellular mobilization, and inflammation.
In addition to the “Fight-or-Flight Response,” the SNS controls the response to stressors, which interfere with normal homeostasis. These stressors include starvation, in which catecholamines regulate the mobilization of stored metabolites such as lipids and glycogen, and thermoregulation, in which NE induces heat production largely from brown adipose tissue. For example, upon exposure to cold ambient temperatures, afferent nerve fibers from the skin signal to pre-optic hypothalamic nuclei [16] and subsequent hypothalamus activation results in the signaling to the brown adipose tissue to induce adaptive thermogenesis [17]. This process, mediated primarily by NE, facilitates a rapid rise in metabolic rate and an elevation in body temperature [18].
While the acute activation of the stress [19] pathways has beneficial effects on the survival, continuous chronic activation of these pathways can have detrimental consequences as evidenced by the development of cardiovascular diseases and exacerbation of autoimmune diseases such as multiple sclerosis [10, 20, 21]. The formation of peptic ulcers in stressed humans and animals is an archetypical sign of sympathetic over activation. Thus, the activation of the SNS can have both advantageous and harmful effects on organisms depending on the duration of the response. Overall, the widespread, systemic nature of the sympathetic response supports the idea that tumor cells, and immune or stromal cells within the tumor microenvironment could be influenced by changes in secretion of stress hormones occurring at a distance, or perhaps locally. Some recently published evidence in support of this notion is presented below.
Stress and cancer
Many of the pathways involved in adrenergic receptor signaling regulate apoptosis, proliferation, and angiogenesis in normal tissues. Therefore, it should come as little surprise that many tumor types have been found to express functional, cell surface adrenergic receptors. Early studies in rodent tumor models identified adrenergic receptor expression on various cancer cells including those in carcinogen-induced mammary tumors [22, 23], melanoma [24], and pituitary tumors [25]. The relevance of these findings was confirmed in samples from human patients which showed that cancers including Ewing sarcoma, neuroblastoma, rhabdomyosarcoma, lymphoma and other pediatric tumors also expressed adrenergic receptors [19, 26]. Furthermore, investigations by later groups revealed that human pancreatic [27], lung [28], breast [29], melanoma [30], and prostate cancer cells [31] all displayed detectable levels of the receptors.
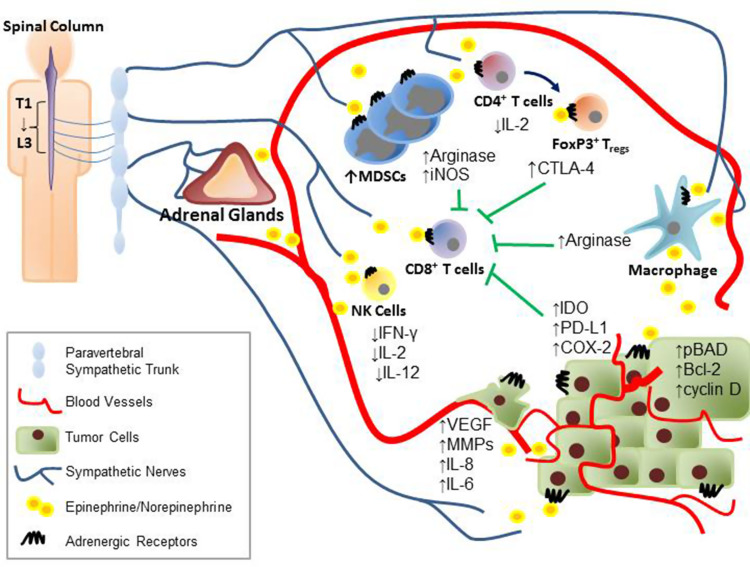
To determine the effect of adrenergic receptor signaling in tumor cells, many researchers have utilized various physiological stress models, e.g., exposing rodents to adverse stimuli that promote fear or anxiety. Many of the common stimuli include (1) social isolation where laboratory rodents, which are highly social creatures, are housed individually in cages for extended periods to elicit loneliness, (2) restraint stress in which the animals are immobilized or confined to small spaces, or (3) intimidation induced stress which involves placing rodents into another animal’s cage [32]. All of these methods have been confirmed to induce stress by measurements of circulating hormones including EPI and NE. In addition, recent studies in murine tumor models have correlated elevated levels of stress hormones with increased tumor progression [33–35]. Currently, new findings indicate that both catecholamines and adrenergic receptors can promote cancer survival and induce metastasis (Fig. 2).
Fig. 2.
Stress induces production of several neurotransmitters and hormones which can enter the tumor microenvironment through vasculature, innervation, or even locally by infiltrating immune cells. Activation of the adrenergic receptors on immune cells results in the production of immunosuppressive molecules such as CTLA-4, arginase, and iNOS. Simultaneously, the catecholamines can also inhibit the production of important activation cytokines such as IFN-γ, IL-2, and IL-12. In addition, increased levels of stress hormones in the tumor microenvironment can also increase the levels of IDO, PD-L1, and COX-2 expressed by the tumor, which facilitates immune dysfunction and increases expression of pro-survival and pro-metastatic molecules
Stress and tumorigenesis
Several findings have suggested that catecholamines and adrenergic receptors play a role in facilitating tumorigenesis. Work by multiple groups has shown increased expression of β-adrenergic receptors on high-grade patient tumors compared with lower-stage disease [27, 36, 37]. In addition, data have shown that the levels of the β-adrenergic receptors are significantly higher in invasive melanoma compared with premalignant nevi, suggesting that the receptors have a role in driving tumor progression [30].
Current studies demonstrate that activation of adrenergic receptors can promote tumor progression. Early studies have indicated that chronic activation of G protein-coupled receptors, such as the α1B-adrenergic receptor, can induce malignant transformation in normal cell lines including Rat-1 and NIH3T3 cell lines [38]. Similar findings were reported by other groups who also showed that prolonged exposure of NIH3T3 cells to NE and EPI promoted DNA damage and enhanced tumor formation in vivo [39]. Hara et al. later elucidated the mechanism by which stress hormones induce DNA damage. Using β-arrestin-1 knockout mice, they determined that β-arrestin-1 induced MDM2-mediated p53 degradation in both cell lines as well as in the thymus of mice receiving infusions of the β-adrenergic receptor agonist, isoproterenol [40]. Furthermore, they also determined that activation of PKA by β2-adrenergic receptor promoted the development of reactive oxygen species, resulting in increased DNA damage [40]. This study strongly demonstrated that catecholamines could induce DNA damage in normal cells and lead to the development of cancer.
Furthermore, work from Al-Wadei et al. [41] demonstrated that stimulation of normal pancreatic duct epithelial cells by nicotine could induce production of catecholamines. Activation of adrenergic receptors by autocrine signaling on non-transformed cells resulted in increased cell proliferation and activation of oncogenic proteins including epithelial growth factor receptor (EGFR). These findings suggest that continuous activation of β-adrenergic receptors by external factors can promote healthy cells to undergo transformation.
Stress and tumor survival mechanisms
The majority of work linking stress and cancer has centered on the ability of the stress molecules to support tumor survival and growth. In many studies, increased expression of the receptors correlated with increased malignancy, implying that these receptors have a role in tumor progression. Recent data have demonstrated that stimulation of these receptors can have dramatic effects on multiple parameters of cancer cell biology, particularly metastasis. A recent study by Shan et al. in pancreatic cancer cell lines (in vitro) has shown that inhibition of adrenergic receptors leads to better responses to therapy and simultaneously to decreased activation of pathways regulating survival [42]. Specifically, these authors observed decreased expression of molecules such as Bcl-2 upon blockade of β2-adrenergic receptors in vitro on human pancreatic cancer cell lines MIA PaCa-2 and BxPC-3, which correlated with increased killing by gemcitabine [42]. In addition to apoptotic pathways, data from Zhang et al. [27, 43] showed that β-adrenergic receptors regulate cyclin expression as well as NFκB, Akt, and Erk1/2 pathways which all play important roles in tumor survival and proliferation. Interestingly, the authors further demonstrated that compared to β1-adrenergic receptors, β2-adrenergic receptors contribute disproportionately to the regulation of these pathways. While use of the β1-specific antagonist, metoprolol, was able to effectively reduce proliferation and induce cell death by inhibiting cyclin D, Erk1/2 activation, and increasing Bax expression, it did not affect Bcl-2 or caspase-3/9 and had only modest effects on the phosphorylation of Akt and NFκB in different cells [27]. However, use of a β2-adrenergic receptor antagonist decreased the expression of the pro-survival molecules and reduced the spread of pancreatic tumor cells [27]. These findings suggest that β2-adrenergic receptor signaling plays a more prominent role in the survival of these cells.
Studies in both transgenic and xenograft models revealed that prostate carcinomas are highly enriched in adrenergic receptors. Findings demonstrated that β2-adrenergic receptor activation of the classical PKA pathway leads to phosphorylation of the anti-apoptotic molecule, Bcl2-associated death promoter (BAD) [44]. BAD functions by sequestering Bcl-2 and Bcl-xL in order to facilitate the translocation of Bak and Bax to the mitochondria. However, the pro-apoptotic function of BAD can be abrogated by the phosphorylation of several amino acid residues including S112, S136 [45], S155 [46], and S170 [47]. PKA, in particular, can modify the S112 and S136 sites leading to inhibition of BAD function [34]. Remarkably, the authors also discovered that phosphorylation of BAD alone at S112 determined the survival of prostate tumor cells in response to β2-adrenergic receptor activation. Upon mutation of this phosphorylation site, apoptosis was restored in tumor cells in spite of other possible downstream targets of PKA signaling that could also regulate survival. Most notably, the classical transcription factor associated with β2-adrenergic receptor activation, CREB, which drives transcription of other anti-apoptotic Bcl-2 family members, could not compensate for the loss of BAD inhibition [34]. In addition to prostate and pancreatic cancers, similar findings have been reported in melanoma, breast, ovarian, and leukemia, demonstrating the broad effect that catecholamines have on multiple forms of cancer.
Work from our own laboratory reveals that NE-driven stress responses induced in mice through use of mildly cool housing temperatures can also influence therapeutic sensitivity (Eng et al. submitted). In response to chronic cold stress induced simply by the standard housing temperatures (approximately 21–23 °C) used in all animal research facilities, mice produce increased levels of NE [48, 49], compared with mice housed at 30 °C [50, 51]. At this warmer temperature, NE levels decline because baseline metabolism generates enough heat to maintain core body temperatures at 37 °C. We observe that tumors grown in mice housed at 30 °C have significantly lower levels of pro-survival molecules, including phosphorylated BAD, and are significantly more sensitive to apoptosis-inducing therapies (Eng et al. submitted). Overall, these findings show that signaling through β-adrenergic receptor can enhance tumor survival through multiple intracellular pathways.
Stress and changes in metastatic potential
In addition to a role in cell survival, adrenergic receptor signaling has also been shown to mediate metastasis. The regulation of metastasis by adrenergic receptors occurs at multiple levels and involves not only cancer cells, but also cells in the tumor microenvironment and in the metastatic niche. Within tumor cells, activation of adrenergic receptors drives the production of cytokines that promote angiogenesis and mobilization. Work from our laboratory has shown that mice housed under conditions of chronic cold stress (which increases systemic NE levels) have a significantly higher metastatic burden [52]. In particular, mice housed at standard 22 °C implanted orthotopically with 4T1 murine mammary carcinoma cells had greater amounts of metastatic lung nodules compared with mice housed at 30 °C [52]. Recent work in ovarian cancer shows that catecholamines can protect cells from anoikis, apoptosis resulting loss of contact from the extracellular matrix, a major checkpoint that metastatic cells must overcome [53]. These molecular changes resulted from β-adrenergic receptor signaling and the activation of the downstream kinase, Src [53]. Later studies revealed that Src kinase activates a complex network of molecular signals, which ultimately foster a metastatic phenotype in ovarian cancer [54]. In particular, the expression of vascular endothelial growth factor and matrix metalloproteinases in tumors significantly increases following treatment with catecholamines or adrenergic agonists. In conjunction with elevated levels of pro-inflammatory factors such as IL-8 and IL-6 [55, 56], these molecules are believed to be the major initiators of stress-induced metastasis or the “metastatic switch” controlled by adrenergic signaling.
However, the actions of tumor cells alone are not believed to be sufficient to drive metastasis. Several groups have discovered that host cells in both the metastatic niche and the primary tumor microenvironment are also affected by SNS signaling. For instance, it was recently observed that catecholamines induced osteoprotegerin production in the bone marrow allowing for the dissemination of breast tumor cells [57]. In addition, findings from Magnon et al. [58] showed that signals from both the sympathetic and the parasympathetic nervous system increased prostate tumor development and metastasis. Specifically, these studies demonstrated that sympathetic nerves are essential for the engraftment of prostate tumor cells and for the development of spontaneous tumors [58]. In these experiments, inhibition of catecholamine production by reserpine, as well as use of β2- and β3-knockout animals, prevented tumor formation, demonstrating that β-adrenergic receptors were important for establishing the proper “soil” for tumor development [58]. However, the authors discovered that metastasis of the tumors was governed by activation of stromal type 1 cholinergic receptors by acetylcholine from parasympathetic nerve fibers [58].
Magnon et al. [58] also brought to light a surprising aspect of the autonomic nervous system’s role in early tumor formation and metastatic progression. First, the data identified a positive clinical correlation between the infiltration of nerve fibers into the tumor microenvironment and the poorer prognosis of prostate cancer patients [58]. Additionally, they demonstrated a previously unknown role for sympathetic nerves in tumor initiation and development [58]. As both injected cell lines and spontaneous tumors failed to grow, this suggests that the SNS plays a role in establishing the initial niche for cancer cells to survive. Lastly, they showed a remarkable role for parasympathetic nerves in the later stages of tumor growth [58]. While much of the work on neurotransmitters and cancer has focused on the interaction of hormones directly on tumor cells, this work demonstrated that the parasympathetic nervous system’s influence on the surrounding stroma has an equally important effect on the tumor progression [58]. Taken together, these findings highlight the complex mechanisms by which neurotransmitters and stress molecules exert their effect on tumors to promote the growth and spread of tumors.
Stress and immunosuppression
Stress and immunity
Stress has long been identified as a determinant of immune function [59, 60], and many studies have revealed that both lymphoid and myeloid cells possess the receptors necessary to respond to stress. During exposure to acute stress, stress hormones stimulate myelopoiesis and promote the egress of immune cells from the bone marrow into the blood. Additionally, previous studies have demonstrated that activation of adrenergic receptors on CD4+ T cells and B cells can facilitate interferon and IgG1 production, respectively [61–64]. The resulting increase in circulating immune cells and the enhanced lymphocyte function are believed to be a preemptive response against potential infections, which may result during an encounter with a threat.
On the other hand, chronic, continuous exposure of immune cells to stress hormones actually diminishes their activity and induces their apoptosis. Glucocorticoids, such as corticosterone and cortisone, have well-characterized effects on dampening the immune response and have been used as immunosuppressive agents for the treatment of autoimmune and inflammatory diseases since the late 1940s [65, 66]. However, the effects of the SNS neurotransmitters on immune cells are much less well understood. While some evidence suggests that activation of adrenergic receptors may improve immune cell function, other observations indicate that long-term stimulation by catecholamines may actually have detrimental effects on key immune cells including lymphocytes, macrophages, and natural killer cells [67–69]. Reports have suggested that NE can inhibit the expression of MHC class II on astrocytes [70]. Activation of the β2-adrenergic receptor impaired interferon-γ-induced expression of MHC class II molecules and could be reversed with propranolol, but not by β1-adrenergic receptor antagonists [70]. Furthermore, spinal cord injury leading to autonomic dysreflexia can result in severe immunosuppression as a result of unfettered accumulation of NE from the damaged nerves [71].
Moreover, recent studies find that stress can also alter the inflammatory process and enhance the development of chronic illness. For instance, the stress of cohabitating with sick mice can incite an inflammatory lung disease in previously healthy animals [72]. Mice challenged with intranasal ovalbumin developed allergic lung inflammation when housed with mice inoculated with tumors but not when housed with healthy animals [72]. In the mice housed with sick animals, the development of lung inflammation stemmed from elevated serum NE leading to increased production of IL-4, IL-5, and ultimately anti-ovalbumin antibodies in the challenged animals [72].
These findings are further supported by work that demonstrated that chronic, intermittent stress resulted in elevated levels of circulating leukocytes in individuals exposed to high-stress situations compared with unstressed individuals, such as medical residents tested pre- and post-call from the intensive care unit [73]. Companion murine studies revealed that mice exposed to stress, which resulted in elevated NE levels in the bone marrow, had increased proliferation of cells, including hematopoietic stem cells, granulocyte progenitors, myeloid dendritic cell (DC), and lymphoid progenitors resulting in stress-induced leukocytosis [73]. Using ApoE−/− mice, which have an increased susceptibility to atherosclerosis, they found that increased leukocytosis enhanced the rate of arterial plaque formation and that blockade of the β3-adrenergic receptor on bone marrow abrogated these effects in a CXCL12-dependent manner [73]. This indicated that adrenergic receptor signaling in the bone marrow could facilitate a premature release of immune cells into the circulation. Overall, these findings showed that stress has a strong role in promoting the development of various illnesses. By enhancing systemic inflammation and suppressing adaptive immunity, catecholamines can significantly enhance chronic diseases, as well as infectious and neoplastic conditions.
Stress and immunosuppressive cells
Studying the effects of stress hormones on immunosuppressive cells has produced conflicting results. For instance, CD4+CD25+ regulatory T (Treg) cells were discovered to express tyrosine hydroxylase and produce catecholamines. The release of catecholamines by these cells actually decreased the production of immunosuppressive cytokines such as TGF-β through the activation of dopamine receptors [74]. These findings that Treg were inhibited by signals from the SNS were later confirmed in studies in which systemic blockade of catecholamine released from nerve endings improved Treg function and decreased autoimmune encephalomyelitis severity in mouse studies [75].
In contrast, Guereschi et al. reported that activation of the β2-adrenergic receptor on cells expressing FoxP3+, the major transcription factor driving Treg differentiation, enhanced their suppressive properties by decreasing IL-2 transcription and increasing cell surface expression of CTLA-4, a molecule that promotes T cell anergy. Even more surprising, activation of the β2-adrenergic receptor on CD4+ FoxP3− cells induced expression of FoxP3, suggesting that β2 activation could drive immune cell differentiation [76]. However, these studies were done either in vitro or in non-tumor systems, making these findings difficult to extrapolate to situations involving cancers. Overall, these data highlight the complex responses that stress has on regulatory lymphocyte populations.
Another population of suppressive cells in the tumor microenvironment which can also affect tumor progression is the myeloid cells. These cells broadly include tumor-associated DCs, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). Our recent review details literature discussing the effects of stress on DCs [77]; therefore, these cells will not be covered in depth in this review. Briefly, DCs are also extremely responsive to stress hormones. Their polarization and function can be skewed depending on the duration of the stress and the environmental context, such as in response to mild cold stress as shown in [77].
The most abundant immune cells in the tumor microenvironment are TAMs. These cells have been implicated in facilitating tumor progression through multiple mechanisms including suppressing tumoricidal immune cells, inducing vascularization, and promoting metastasis. While current understanding of the effects of stress on macrophages is poor, work has shown that stress can lead to the recruitment of these cells to tumors [58, 78]. Although little is known about the direct effects of catecholamines on macrophages in cancer, the impact of stress hormones on these cells has been demonstrated in non-tumor models. One study demonstrated that catecholamines facilitate the production of arginase from macrophages in a murine trauma model [79]. Furthermore, other work reveals that macrophages may also be a major source of catecholamines, which may also drive tumor progression in response to stress [48, 80]. While further investigation is needed, growing evidence implicates macrophages as a major cell type linking the stress and immune responses.
In addition to TAMs, another major immunosuppressive myeloid cell population that has only recently been identified in tumors is the MDSCs. Work by several groups has demonstrated that a plethora of tumor-derived factors can induce the development of these cells, including G-CSF, GM-CSF, IL-6, IL-1β, and cyclo-oxygenase (COX)-2 [81–83]. However, new data suggest that stress alone can lead to the accumulation of MDSCs, which are broadly defined as CD11b+GR1+ cells. Jin et al. [84] demonstrated that these cells have the potential to suppress immunity by producing factors such as nitric oxide and arginase. These studies are in agreement with others showing a strong correlation between stress and immune suppression in breast cancer patients [85]. In this work, MDSCs were increased in patients who reported high levels of stress, suggesting that they may be a contributing factor to the immune suppression observed in breast cancer cases [85]. Taken together, these new studies indicate that stress can lead to significant dysregulation of immune cell function and potentially contribute to a pro-tumorigenic environment in patients by suppressing host immunity.
Stress and the immunosuppressive tumor microenvironment
The full impact of catecholamines on immune cells in the tumor microenvironment is currently unknown. Nevertheless, the idea that chronic, elevated levels of NE and EPI can result in immune suppression is supported by information from fields outside of cancer biology. Studies, which examined physical stress induced by cool ambient temperatures, showed that the ensuing increase in body heat production (thermogenesis) was due to both an increased production of NE and an increased skewing of macrophages toward an M2 phenotype [48, 86]. Surprisingly, the authors also found that these cold-induced M2 macrophages themselves were the major source of catecholamines in the brown fat, and subsequent depletion of these cells resulted in the loss of body temperature maintenance [48]. Although performed in the absence of a disease, this study also demonstrated that the cold stress experienced by laboratory mice alters immune cell phenotypes. If skewing of macrophage in response to cold stress also occurs in M2 TAMs, it could potentially promote an immunosuppressive environment within the tumor. These findings correlate with recent work by our group, demonstrating that mice housed at their thermoneutral temperature (30 °C) are able to control tumor growth better than mice maintained at standard temperatures (22 °C) [52]. It is clear that although mice at 22 °C are able to maintain a normal body temperature, this comparatively cool temperature causes adrenergic stress, which affects basal metabolic rate and various other physiological processes. In our study, the improved anti-tumor control was dependent on the immune response; CD8+ T cells displayed improved functionality and were present in larger numbers within the tumors of mice at 30 °C [52]. In addition, immunosuppressive cells were diminished in mice maintained at thermoneutrality, indicating decreased immunosuppression [52].
Substantial investigation of stress and cancer in patients has been performed in the context of postsurgical immunosuppression. Studies have demonstrated that the release of catecholamines following surgery significantly impairs the anti-tumor immune response and can greatly increase the chances of recurrence [87]. This major clinical problem was caused by increased levels of circulating catecholamines in postoperative patients [87]. Experiments performed in rodent models treated with β-blockers and anti-inflammatory, cyclo-oxygenase (COX) inhibitors revealed a vast improvement in long-term, tumor-free survival in these animals, indicating that a major driver of this relapse was the stress hormones [87]. Further investigation revealed that the elevated levels of NE and EPI impaired natural killer (NK) cell function during the postsurgical period allowing for residual tumor cells to re-establish [88, 89].
Stress and therapeutic responses
In recent years, immune-based therapies have shown exciting promise in the clinic. The growing interest in these novel agents is due not only to their ability to facilitate an effective immune response against both the primary tumor and metastases, but also for their potential to induce long-term, durable remission in patients. Unfortunately, the number of patients who ultimately respond to these treatments is still small, and the reasons behind these differences are not completely known. As discussed, stress hormones can significantly impair the immune response by increasing the levels of immunosuppressive cytokines, promoting immune effector cell dysfunction, and enhancing tumor evasion of the immune response. These processes can have profound implications for therapeutic efficacy in patients and contribute to the eventual loss of response in many tumor types. Despite the potential significance, only a few studies have investigated the effects of catecholamines on immunogenicity or on the efficacy of immunotherapies, and these are summarized in the following sections.
Stress and immuno-editing of tumors
As recently revised by Hanahan and Weinberg [90], the “Hallmarks of Cancer” now include immune evasion as a major characteristic of malignancies. In order to survive, cancer cells employ multiple strategies to escape recognition by the endogenous host immune system including downregulating antigen expression, increasing the production of inhibitory cytokines, and recruiting immune suppressing cells [90]. The extent to which the nervous system and stress may contribute to this immunosuppressive environment is poorly understood; however, a recent study demonstrated that NE acting on tumor cells promoted immunoediting and altered the phenotype of the cancer cells to promote immune evasion. One study observed that pancreatic tumor cells exposed to NE had decreased expression of MHC class I molecules as well as the co-stimulatory ligand B7-1, and increased levels of immunosuppressive indolamine-2,3-oxygenase (IDO) and B7H-1 (PD-L1), the ligand for the programmed cell death receptor (PD-1). While these changes only persisted for a short period of time, these phenotypic changes on cancer cells would not only prevent T cell recognition, but also impair their function by depleting essential nutrients such as tryptophan (by IDO) and inducing exhaustion even in activated lymphocytes [91].
Stress and CpG adjuvants
Recent breakthroughs have demonstrated that cancer vaccines can have safe and durable outcomes in the clinic. These strategies involve the ex vivo activation of a patient’s own immune cells, most often DCs, by priming them with tumor antigens such as prostatic alkaline phosphatase in prostate cancer or NY-ESO-1, which is found in many tumor types [92–94]. These cells are then re-infused back into patients and allowed to stimulate the adaptive immune system to mount a response against the tumor.
To further enhance the therapeutic efficacy, adjuvants have been widely explored as a means of bolstering the ability of DCs to activate tumor-specific T cells. Nucleic acid-based adjuvants, such as CpG oligonucleotides, have been especially effective at enhancing the production of interferon-γ, interferon-α, IL-12, and IL-6 from innate immune cells in vitro and in vivo. Recent work has shown that the stimulatory effect of CpG adjuvants was diminished in mice subjected to stress as demonstrated by decreased IL-12. Further studies by this group suggest that the major mediator of this stress-induced suppression of IL-12 was due to the effects of prostaglandins and glucocorticoids, and not catecholamines. However, pharmacological administration of EPI was able to reduce IL-12 levels through a glucocorticoid-driven mechanism suggesting that that catecholamines may still play a role in the decreased efficacy of CpG therapies [95, 96].
Stress and rhIL-2 therapy
Recombinant human IL-2 (rhIL-2) is one of the earliest developed immunotherapies for cancer and continues to be used in the treatment of melanoma and renal cell carcinoma. Unfortunately, treatment-related toxicities as well as its potential role in enhancing Treg activity have limited the use of rhIL-2 in the clinic [97, 98]. However, the development of new combination therapies to enhance the anti-tumor effect of rhIL-2 while limiting the adverse effects has renewed interest in the therapy. One possible avenue to improve the therapeutic efficacy of rhIL-2 may be to combine it with β-blockers, since early studies have demonstrated that the cellular effects of IL-2 can be altered by stress. It has been shown that increased stress hormones can significantly reduce the production of IL-2 from immune cells as well as the expression of IL-2 receptor (IL-2R) on T lymphocytes [99]. In addition to impairing the function of immune cells, loss of the IL-2R also indicates that the efficacy of rhIL-2 therapy in patients under immense stress will be greatly diminished as these cells cannot respond to the treatment either. Clinically, psychological stress of patients with metastatic renal cell carcinoma, as assessed by the status of their sexual identity, correlated with degree of response to rhIL-2 [100]. Patients were deemed to have either a maintained sexual identity or lack a sexual identity based on their response to Rorschach test. Based on their findings, it was discovered that patients who lacked sexual identity, and were thus under a higher degree of stress, had poorer response to rhIL-2 therapy compared with unstressed patients [100]. In addition, patients with no sexual identity had fewer circulating lymphocytes following rhIL-2 treatment, suggesting that stress impaired cellular expansion. Overall, these studies indicate that the immunotherapy efficacy is altered by stress hormones and that these hormones can significantly impair normal immune cell function in response to the treatment.
Stress and targeted immunotherapies
Targeted therapies have been extremely successful in treating tumors, which previously had left patients with few medical options. This class of drugs encompasses a diverse array of agents including therapeutic monoclonal antibodies and small molecules. Similarly, they also act through diverse mechanisms including inhibiting tumor-specific proteins, such as overactive cell surface receptors, or by inducing antibody-dependent cell-mediated cytotoxicity (ADCC), as in the case of therapeutic antibodies. While these therapies are extremely effective, their efficacy depends on the continued expression of their target proteins by tumor cells as well as the ability for immune cells to bind many of these antibodies to trigger killing. Recently, studies showed that adrenergic receptor signaling is capable of altering the expression and activity of common tumor targets, particularly EGFR, and potentially impairing the efficacy of these therapies. In both breast cancer and oral squamous cell carcinoma, stimulation of EGFR led to the activation of key survival pathways as well as the upregulation of β-adrenergic receptors. These studies also revealed that stimulation of β-adrenergic receptors could induce further expression of EGFR on the tumor cell surface. Thus, these two receptors appeared to work in concert via a feedback loop to further activate each other and their downstream targets. One major consequence of this interconnected regulation was revealed by demonstrating that that activation of the β2-adrenergic receptor on gastric cancer cells resulted in decreased responses to trastuzumab, a targeted therapy against human epidermal growth factor receptor 2 (Her2) [101]. Mechanistically, activation of the β2-adrenergic receptor increased MUC4 by Stat3 and ERK, leading to impaired binding of trastuzumab to Her2 [101]. As trastuzumab inhibits tumor progression through cell cycle arrest and through NK cell-mediated ADCC, masking of the target epitope enhanced the tumor survival [102]. Ultimately, these findings highlight the surprising effect that catecholamines have on impairing the efficacy of certain targeted therapies.
Conclusions
The stress response is one of the most highly conserved and fundamental biological processes in living creatures. Evolutionarily, this response developed as a means for coping with many forms of imminent danger; however, the remarkable features of the stress response include the fact that not only are all forms of stress regulated by a small number of molecules and pathways, but that the stress response can also coordinate the activities of multiple organ systems. In fact, the signals produced in response to stress effectively prioritize resource use by certain tissues such as the cardiovascular and musculoskeletal systems, which can have a direct impact on the outcome for escape and survival. As such, the stress response is a prime example of the physiological trade-off in regard to resource allocation that organisms must make in order to survive.
Unfortunately, any large, prolonged deviation from normal homeostasis also has consequences. The chronic activation of this pathway and the continued production of molecular stress mediators, including secretion of the catecholamines, have profound ramifications on health and disease. New insights and discoveries have finally brought the potential effects of chronic stress to the forefront of cancer research. Recent work has highlighted the fundamental role that stress hormones and their receptors play in tumorigenesis, tumor growth, and metastasis [103]. In addition, studies have long established a negative effect of stress on basic immune cell biology and their function in autoimmunity or infection, but very little research has currently been done to explore the role of stress on immune cells in cancer. This area of research will become especially important as a result of the growing interest in immune-based therapies and vaccines. These new immunomodulatory agents aimed not at the tumor cells, but toward the host immune cells have shown tremendous clinical promise. In particular, the successes of ipillimumab (anti-CTLA4) and nivolimumab (anti-PD-1), which prevent the engagement of molecules on T cells that induce anergy and exhaustion, have spurred excitement in the field and highlighted the enormous potential that the host immune response alone has in controlling tumor growth [104]. Furthermore, progress continues to be made in developing strategies to optimize well-established immunotherapies such as rhIL-2, interferon, and adoptive T cell therapy [105, 106]. Yet all of these treatment strategies are still greatly dependent on factors in the tumor. Therefore, any means of optimizing the host environment to prevent T cell dysfunction or death and to reduce the degree of immunosuppression could have profound effects on the therapeutic response in patients.
The growing epidemiological evidence hints toward the possibility that α- and β-blockers may be one method of improving therapeutic response by targeting multiple parameters in the tumor and the host. Already, studies have begun to emerge, indicating that concomitant use of β-blockers can potentiate the effects of radiation or chemotherapy in select groups of individuals [107]. In several clinical studies examining breast cancer, melanoma, and ovarian cancer patients, researchers have found that use of β-blockers improved long-term patient survival independent of other medications taken [6, 7, 108, 109]. However, further studies are needed to fully confirm the effects of β-blockers on cancer progression and patient survival as discordant findings from other studies have made the interpretation of the effects of adrenergic blockade in patients difficult [110–112]. The discrepancies may ultimately be due to multiple factors including the class of β-blockers used by patients, the initial treatment regimens used on the patients and potential biases in the studies, such as selection bias. As a result, carefully designed clinical trials to study the effects of β-blockers in patients, especially in combination with other therapies, should be performed in order to determine their clinical benefits.
In conclusion, studying the interaction between stress and the immune response in cancer is a promising new area of research. While catecholamines are known to impair the anti-tumor immune response, the exact mechanism(s) of how stress hormones affect immune cells in the setting of cancer is not completely understood due to the complex signal transduction pathways and responses that can be elicited through adrenergic receptor activation. Fortunately, the wide assortment of inhibitors, β-blockers and α-blockers, developed for the routine treatment of hypertension and other disorders provides an opportunity for immediate translational research. Several outstanding questions remain including pinpointing the source of catecholamines in the tumor microenvironment (i.e., vasculature, direct innervation, and autocrine production by tumor cells). As patients undergoing cancer treatment are under considerable emotional stress, the combination of these stress alleviating therapies with immunotherapies could have significant benefit. Further research is required, but the opportunity for enormous clinical advancement is evident even now at these early stages.
Acknowledgments
We thank Jeanne Prendergast for editorial assistance. This work was supported by National Institute of Health Grants R01 CA135368 and T32 CA 085183 and Breast Cancer Research and Education Fund through NY State Department of Health contract # C028252.
Conflict of interest
The authors declare that they have no conflicts of interest.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- COX
Cyclo-oxygenase
- DC
Dendritic cell
- EGFR
Epithelial growth factor receptor
- EPI
Epinephrine
- IDO
Indolamine-2,3-oxygenase
- MAPK
Mitogen-activated protein kinase
- MDSC
Myeloid-derived suppressor cell
- NE
Norepinephrine
- NK
Natural killer
- PKA
Protein kinase A
- rhIL-2
Recombinant human IL-2
- SNS
Sympathetic nervous system
- TAM
Tumor-associated macrophage
- Treg
Regulatory T cell
References
- 1.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein DS. Adrenal responses to stress. Cell Mol Neurobiol. 2010;30:1433–1440. doi: 10.1007/s10571-010-9606-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chruscinski A, Brede ME, Meinel L, Lohse MJ, Kobilka BK, Hein L. Differential distribution of beta-adrenergic receptor subtypes in blood vessels of knockout mice lacking beta(1)- or beta(2)-adrenergic receptors. Mol Pharmacol. 2001;60:955–962. doi: 10.1124/mol.60.5.955. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald PJ. Beta blockers, norepinephrine, and cancer: an epidemiological viewpoint. Clin Epidemiol. 2012;4:151–156. doi: 10.2147/CLEP.S33695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 6.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemeshow S, Sorensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, Lesinski GB, Jackson R, Glaser R. Beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev. 2011;20:2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, Brenner H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer. 2014;120:1178–1186. doi: 10.1002/cncr.28546. [DOI] [PubMed] [Google Scholar]
- 9.Strosberg A. Structure, function, and regulation of adrenergic receptors. Protein Sci. 1993;2:1198–1209. doi: 10.1002/pro.5560020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein DS. Catecholamines and stress. Endocr Regul. 2003;37:69–80. [PubMed] [Google Scholar]
- 11.Summers RJ, McMartin LR. Adrenoceptors and their second messenger systems. J Neurochem. 1993;60:10–23. doi: 10.1111/j.1471-4159.1993.tb05817.x. [DOI] [PubMed] [Google Scholar]
- 12.Hein L, Kobilka B. Adrenergic receptor signal transduction and regulation. Neuropharmacology. 1995;34:357–366. doi: 10.1016/0028-3908(95)00018-2. [DOI] [PubMed] [Google Scholar]
- 13.Barnes PJ. Beta-adrenergic receptors and their regulation. Am J Respir Crit Care Med. 1995;152:838–860. doi: 10.1164/ajrccm.152.3.7663795. [DOI] [PubMed] [Google Scholar]
- 14.Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B, Pei G. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-κB pathways. Mol Cell. 2004;14:303–317. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Ho Y, Guo H, Wang Y. Rapid activation of Stat3 and ERK1/2 by nicotine modulates cell proliferation in human bladder cancer cells. Toxicol Sci. 2008;104:283–293. doi: 10.1093/toxsci/kfn086. [DOI] [PubMed] [Google Scholar]
- 16.Myers R. Catecholamines and the regulation of body temperature. In: Szekeres L, editor. Adrenergic activators and inhibitors. Berlin: Springer; 1980. pp. 549–567. [Google Scholar]
- 17.Morrison SF. Central pathways controlling brown adipose tissue thermogenesis. News Physiol Sci. 2004;19:67–74. doi: 10.1152/nips.01502.2003. [DOI] [PubMed] [Google Scholar]
- 18.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 19.Whitsett JA, Burdsall J, Workman L, Hollinger B, Neely J. Beta-adrenergic receptors in pediatric tumors: uncoupled beta 1-adrenergic receptor in Ewing’s sarcoma. J Natl Cancer Inst. 1983;71:779–786. [PubMed] [Google Scholar]
- 20.Heesen C, Schulz H, Schmidt M, Gold S, Tessmer W, Schulz K. Endocrine and cytokine responses to acute psychological stress in multiple sclerosis. Brain Behav Immun. 2002;16:282–287. doi: 10.1006/brbi.2001.0628. [DOI] [PubMed] [Google Scholar]
- 21.Flachenecker P, Reiners K, Krauser M, Wolf A, Toyka KV. Autonomic dysfunction in multiple sclerosis is related to disease activity and progression of disability. Mult Scler. 2001;7:327–334. doi: 10.1177/135245850100700509. [DOI] [PubMed] [Google Scholar]
- 22.Vandewalle B, Revillion F, Lefebvre J. Functional β-adrenergic receptors in breast cancer cells. J Cancer Res Clin Oncol. 1990;116:303–306. doi: 10.1007/BF01612908. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti B, Spinola PG, Plante M, Poyet P, Folléa N, Pelletier G, Labrie F. Beta-adrenergic receptors in DMBA-induced rat mammary tumors: correlation with progesterone receptor and tumor growth. Breast Cancer Res Treat. 1989;13:251–263. doi: 10.1007/BF02106575. [DOI] [PubMed] [Google Scholar]
- 24.Valles SL, Benlloch M, Rodriguez ML, Mena S, Pellicer JA, Asensi M, Obrador E, Estrela JM. Stress hormones promote growth of B16-F10 melanoma metastases: an interleukin 6-and glutathione-dependent mechanism. J Transl Med. 2013;11:72. doi: 10.1186/1479-5876-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reisine TD, Heisler S, Hook VY, Axelrod J. Activation of beta 2-adrenergic receptors on mouse anterior pituitary tumor cells increases cyclic adenosine 3′:5′-monophosphate synthesis and adrenocorticotropin release. J Neurosci. 1983;3:725–732. doi: 10.1523/JNEUROSCI.03-04-00725.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sardi I, Giunti L, Bresci C, Buccoliero AM, Degl’innocenti D, Cardellicchio S, Baroni G, Castiglione F, Da Ros M, Fiorini P. Expression of β-adrenergic receptors in pediatric malignant brain tumors. Oncol Lett. 2013;5:221–225. doi: 10.3892/ol.2012.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang D, Ma Q, Wang Z, Zhang M, Guo K, Wang F, Wu E. b2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NF B pathway. Mol Cancer. 2011;10:146. doi: 10.1186/1476-4598-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondratenko TY, Zacharova IV, Kuzina NV, Katukov VY, Severin ES, Kornilova ZC, Perelman MI. Alterations in human lung adrenergic receptors in cancer. Biochem Mol Biol Int. 1993;29:123–130. [PubMed] [Google Scholar]
- 29.Draoui A, Vandewalle B, Hornez L, Revillion F, Lefebvre J. Beta-adrenergic receptors in human breast cancer: identification, characterization and correlation with progesterone and estradiol receptors. Anticancer Res. 1991;11:677–680. [PubMed] [Google Scholar]
- 30.Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, Cecchi R, Chiarugi P. β-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013;93:279–290. doi: 10.1038/labinvest.2012.175. [DOI] [PubMed] [Google Scholar]
- 31.Nagmani R, Pasco DS, Salas RD, Feller DR. Evaluation of beta-adrenergic receptor subtypes in the human prostate cancer cell line-LNCaP. Biochem Pharmacol. 2003;65:1489–1494. doi: 10.1016/s0006-2952(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 32.Heinrichs SC, Koob GF. Application of experimental stressors in laboratory rodents. Curr Protoc Neurosci. 2006 doi: 10.1002/0471142301.ns0804s34. [DOI] [PubMed] [Google Scholar]
- 33.Szpunar MJ, Burke KA, Dawes RP, Brown EB, Madden KS. The antidepressant desipramine and alpha2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev Res (Phila) 2013;6:1262–1272. doi: 10.1158/1940-6207.CAPR-13-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R, Jr, Danial N. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123:874. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutgendorf SK, DeGeest K, Dahmoush L, Farley D, Penedo F, Bender D, Goodheart M, Buekers TE, Mendez L, Krueger G, Clevenger L, Lubaroff DM, Sood AK, Cole SW. Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain Behav Immun. 2011;25:250–255. doi: 10.1016/j.bbi.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sardi I, Giunti L, Bresci C, Buccoliero AM, Degl’innocenti D, Cardellicchio S, Baroni G, Castiglione F, Ros MD, Fiorini P, Giglio S, Genitori L, Arico M, Filippi L. Expression of beta-adrenergic receptors in pediatric malignant brain tumors. Oncol Lett. 2013;5:221–225. doi: 10.3892/ol.2012.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Powe DG, Voss MJ, Habashy HO, Zanker KS, Green AR, Ellis IO, Entschladen F. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: an immunohistochemical study. Breast Cancer Res Treat. 2011;130:457–463. doi: 10.1007/s10549-011-1371-z. [DOI] [PubMed] [Google Scholar]
- 38.Allen LF, Lefkowitz RJ, Caron MG, Cotecchia S. G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the alpha 1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc Natl Acad Sci USA. 1991;88:11354–11358. doi: 10.1073/pnas.88.24.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flint MS, Baum A, Episcopo B, Knickelbein KZ, Liegey Dougall AJ, Chambers WH, Jenkins FJ. Chronic exposure to stress hormones promotes transformation and tumorigenicity of 3T3 mouse fibroblasts. Stress. 2013;16:114–121. doi: 10.3109/10253890.2012.686075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara MR, Kovacs JJ, Whalen EJ, Rajagopal S, Strachan RT, Grant W, Towers AJ, Williams B, Lam CM, Xiao K, Shenoy SK, Gregory SG, Ahn S, Duckett DR, Lefkowitz RJ. A stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1. Nature. 2011;477:349–353. doi: 10.1038/nature10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Wadei MH, Al-Wadei HA, Schuller HM. Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors alpha3, alpha5, and alpha7. Mol Cancer Res. 2012;10:239–249. doi: 10.1158/1541-7786.MCR-11-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shan T, Ma Q, Zhang D, Guo K, Liu H, Wang F, Wu E. β2-adrenoceptor blocker synergizes with gemcitabine to inhibit the proliferation of pancreatic cancer cells via apoptosis induction. Eur J Pharmacol. 2011;665:1–7. doi: 10.1016/j.ejphar.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D, Ma QY, Hu HT, Zhang M. beta2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFkappaB and AP-1. Cancer Biol Ther. 2010;10:19–29. doi: 10.4161/cbt.10.1.11944. [DOI] [PubMed] [Google Scholar]
- 44.Sastry KSR, Karpova Y, Prokopovich S, Smith AJ, Essau B, Gersappe A, Carson JP, Weber MJ, Register TC, Chen YQ, Penn RB, Kulik G. Epinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylation. J Biol Chem. 2007;282:14094–14100. doi: 10.1074/jbc.M611370200. [DOI] [PubMed] [Google Scholar]
- 45.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 Not BCL-XL . Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 46.Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000;275:25865–25869. doi: 10.1074/jbc.M004199200. [DOI] [PubMed] [Google Scholar]
- 47.Dramsi S, Scheid MP, Maiti A, Hojabrpour P, Chen X, Schubert K, Goodlett DR, Aebersold R, Duronio V. Identification of a novel phosphorylation site, Ser-170, as a regulator of bad pro-apoptotic activity. J Biol Chem. 2002;277:6399–6405. doi: 10.1074/jbc.M109990200. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uchida K, Shiuchi T, Inada H, Minokoshi Y, Tominaga M. Metabolic adaptation of mice in a cool environment. Pflügers Arch Eur J Physiol. 2010;459:765–774. doi: 10.1007/s00424-010-0795-3. [DOI] [PubMed] [Google Scholar]
- 50.Spector WS. Handbook of biological data. Philadelphia: W. B. Saunders Co; 1956. [Google Scholar]
- 51.Gordon CJ. Thermal physiology of laboratory mice: defining thermoneutrality. J Therm Biol. 2012;37:654–685. [Google Scholar]
- 52.Kokolus KM, Capitano ML, Lee CT, Eng JW, Waight JD, Hylander BL, Sexton S, Hong CC, Gordon CJ, Abrams SI, Repasky EA. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci USA. 2013;110:20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sood AK, Armaiz-Pena GN, Halder J, Nick AM, Stone RL, Hu W, Carroll AR, Spannuth WA, Deavers MT, Allen JK, Han LY, Kamat AA, Shahzad MM, McIntyre BW, Diaz-Montero CM, Jennings NB, Lin YG, Merritt WM, DeGeest K, Vivas-Mejia PE, Lopez-Berestein G, Schaller MD, Cole SW, Lutgendorf SK. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120:1515–1523. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armaiz-Pena GN, Allen JK, Cruz A, Stone RL, Nick AM, Lin YG, Han LY, Mangala LS, Villares GJ, Vivas-Mejia P, Rodriguez-Aguayo C, Nagaraja AS, Gharpure KM, Wu Z, English RD, Soman KV, Shahzad MM, Zigler M, Deavers MT, Zien A, Soldatos TG, Jackson DB, Wiktorowicz JE, Torres-Lugo M, Young T, De Geest K, Gallick GE, Bar-Eli M, Lopez-Berestein G, Cole SW, Lopez GE, Lutgendorf SK, Sood AK. Src activation by beta-adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. doi: 10.1038/ncomms2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shahzad MMK, Arevalo JM, Armaiz-Pena GN, Lu C, Stone RL, Moreno-Smith M, Nishimura M, Lee J, Jennings NB, Bottsford-Miller J, Vivas-Mejia P, Lutgendorf SK, Lopez-Berestein G, Bar-Eli M, Cole SW, Sood AK. Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasis. J Biol Chem. 2010;285:35462–35470. doi: 10.1074/jbc.M110.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, Jennings N, Arevalo J, Lutgendorf SK, Gallick GE, Sanguino AM, Lopez-Berestein G, Cole SW, Sood AK. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 57.Campbell JP, Karolak MR, Ma Y, Perrien DS, Masood-Campbell S, Penner NL, Munoz SA, Zijlstra A, Yang X, Sterling JA, Elefteriou F. stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 59.Glaser R, Kiecolt-Glaser J. How stress damages immune system and health. Discov Med. 2005;5:165–169. [PubMed] [Google Scholar]
- 60.Glaser R, Kiecolt-Glaser JK (2005) Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 5:243–251. doi:10.1038/nri1571 [DOI] [PubMed]
- 61.Podojil JR, Sanders VM. Selective regulation of mature IgG1 transcription by CD86 and beta 2-adrenergic receptor stimulation. J Immunol. 2003;170:5143–5151. doi: 10.4049/jimmunol.170.10.5143. [DOI] [PubMed] [Google Scholar]
- 62.Swanson MA, Lee WT, Sanders VM. IFN-gamma production by Th1 cells generated from naive CD4 + T cells exposed to norepinephrine. J Immunol. 2001;166:232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- 63.Kohm AP, Tang Y, Sanders VM, Jones SB. Activation of antigen-specific CD4 + Th2 cells and B cells in vivo increases norepinephrine release in the spleen and bone marrow. J Immunol. 2000;165:725–733. doi: 10.4049/jimmunol.165.2.725. [DOI] [PubMed] [Google Scholar]
- 64.Kohm AP, Sanders VM. Norepinephrine and beta 2-adrenergic receptor stimulation regulate CD4 + T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53:487–525. [PubMed] [Google Scholar]
- 65.Stahn C, Löwenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275:71–78. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 66.Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335:2–13. doi: 10.1016/j.mce.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhabhar FS, Mcewen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 68.Dhabhar FS. Stress-induced augmentation of immune function—the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 69.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frohman EM, Vayuvegula B, Gupta S, van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc Natl Acad Sci USA. 1988;85:1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Guan Z, Reader B, Shawler T, Mandrekar-Colucci S, Huang K, Weil Z, Bratasz A, Wells J, Powell ND, Sheridan JF, Whitacre CC, Rabchevsky AG, Nash MS, Popovich PG. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci. 2013;33:12970–12981. doi: 10.1523/JNEUROSCI.1974-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamasato EK, Ligeiro de Oliveira AP, Alves GJ, Palermo-Neto J. The influence of cohabitation with a sick cage mate on pulmonary allergic inflammatory response in mice. Brain Behav Immun. 2013;32:e17–e18. [Google Scholar]
- 73.Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von zur Muhlen C, Bode C, Fricchione GL, Denninger J. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20:754–758. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S. Human CD4 + CD25 + regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109:632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- 75.Bhowmick S, Singh A, Flavell RA, Clark RB, O’Rourke J, Cone RE. The sympathetic nervous system modulates CD4(+)FoxP3(+) regulatory T cells via a TGF-beta-dependent mechanism. J Leukoc Biol. 2009;86:1275–1283. doi: 10.1189/jlb.0209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guereschi MG, Araujo LP, Maricato JT, Takenaka MC, Nascimento VM, Vivanco BC, Reis VO, Keller AC, Brum PC, Basso AS. Beta2-adrenergic receptor signaling in CD4 Foxp3 regulatory T cells enhances their suppressive function in a PKA-dependent manner. Eur J Immunol. 2013;43:1001–1012. doi: 10.1002/eji.201243005. [DOI] [PubMed] [Google Scholar]
- 77.Kokolus KM, Spangler HM, Povinelli BJ, Farren MR, Lee KP, Repasky EA. Stressful presentations: mild chronic cold stress in mice Influences baseline properties of dendritic cells. Front Immunol. 2014;5:23. doi: 10.3389/fimmu.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JM, Morizono K, Karanikolas BD, Wu L. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–7052. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bernard AC, Fitzpatrick EA, Maley ME, Gellin GL, Tsuei BJ, Arden WA, Boulanger BR, Kearney PA, Ochoa JB. Beta adrenoceptor regulation of macrophage arginase activity. Surgery. 2000;127:412–418. doi: 10.1067/msy.2000.104115. [DOI] [PubMed] [Google Scholar]
- 80.Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, Day DE, Lentsch AB, Huber-Lang MS, Ward PA. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One. 2009;4:e4414. doi: 10.1371/journal.pone.0004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waight JD, Hu Q, Miller A, Liu S, Abrams SI. Tumor-derived G-CSF facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS One. 2011;6:e27690. doi: 10.1371/journal.pone.0027690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–281. doi: 10.1016/j.semcancer.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X, Zhu T, Zhang D, Wang W, Wang J, Shen B, Gao X, Shi Y, Zhang J. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One. 2013;8:e74497. doi: 10.1371/journal.pone.0074497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mundy-Bosse BL, Thornton LM, Yang HC, Andersen BL, Carson WE. Psychological stress is associated with altered levels of myeloid-derived suppressor cells in breast cancer patients. Cell Immunol. 2011;270:80–87. doi: 10.1016/j.cellimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Karp CL. Unstressing intemperate models: how cold stress undermines mouse modeling. J Exp Med. 2012;209:1069–1074. doi: 10.1084/jem.20120988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Benish M, Bartal I, Goldfarb Y, Levi B, Avraham R, Raz A, Ben-Eliyahu S. Perioperative use of β-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15:2042–2052. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenne E, Sorski L, Shaashua L, Neeman E, Matzner P, Levi B, Ben-Eliyahu S. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2014;37:207–219. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253:798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 90.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Wang L, Liu H, Chen X, Zhang M, Xie K, Ma Q. Immune Sculpting of Norepinephrine on MHC-I, B7-1, IDO and B7-H1 Expression and Regulation of Proliferation and Invasion in Pancreatic Carcinoma Cells. PLoS One. 2012;7:e45491. doi: 10.1371/journal.pone.0045491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA. 2007;104:8947–8952. doi: 10.1073/pnas.0703395104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, Richardson RL, Valone FH, Vuk-Pavlović S. Immunotherapy (APC8015, Provenge®) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 94.Jungbluth AA, Chen Y, Stockert E, Busam KJ, Kolb D, Iversen K, Coplan K, Williamson B, Altorki N, Old LJ. Immunohistochemical analysis of NY-ESO-1 antigen expression in normal and malignant human tissues. Int J Cancer. 2001;92:856–860. doi: 10.1002/ijc.1282. [DOI] [PubMed] [Google Scholar]
- 95.Shaashua L, Rosenne E, Neeman E, Sorski L, Sominsky L, Matzner P, Page GG, Ben-Eliyahu S. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology. 2014;42:11–23. doi: 10.1016/j.psyneuen.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shaashua L, Sominsky L, Levi B, Sorski L, Reznick M, Page G, Ben-Eliyahu S. In vivo suppression of plasma IL-12 levels by acute and chronic stress paradigms: potential mediating mechanisms and sex differences. Brain Behav Immun. 2012;26:996–1005. doi: 10.1016/j.bbi.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- 98.West WH, Tauer KW, Yannelli JR, Marshall GD, Orr DW, Thurman GB, Oldham RK. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987;316:898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- 99.Glaser R, Kennedy S, Lafuse WP, Bonneau RH, Speicher C, Hillhouse J, Kiecolt-Glaser JK. Psychological stress-induced modulation of interleukin 2 receptor gene expression and interleukin 2 production in peripheral blood leukocytes. Arch Gen Psychiatry. 1990;47:707–712. doi: 10.1001/archpsyc.1990.01810200015002. [DOI] [PubMed] [Google Scholar]
- 100.Messina G, Lissoni P, Bartolacelli E, Fumagalli L, Brivio F, Colombo E, Gardani GS. Efficacy of IL-2 immunotherapy in metastatic renal cell carcinoma in relation to the psychic profile as evaluated using the Rorschach test. Anticancer Res. 2007;27:2985–2988. [PubMed] [Google Scholar]
- 101.Shi M, Yang Z, Hu M, Liu D, Hu Y, Qian L, Zhang W, Chen H, Guo L, Yu M, Song L, Ma Y, Guo N. Catecholamine-Induced beta2-adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J Immunol. 2013;190:5600–5608. doi: 10.4049/jimmunol.1202364. [DOI] [PubMed] [Google Scholar]
- 102.Collins DM, O’Donovan N, McGowan PM, O’Sullivan F, Duffy MJ, Crown J. Trastuzumab induces antibody-dependent cell-mediated cytotoxicity (ADCC) in HER-2-non-amplified breast cancer cell lines. Ann Oncol. 2012;23:1788–1795. doi: 10.1093/annonc/mdr484. [DOI] [PubMed] [Google Scholar]
- 103.Cole SW, Sood AK. Molecular pathways: beta-adrenergic signaling in cancer. Clin Cancer Res. 2012;18:1201–1206. doi: 10.1158/1078-0432.CCR-11-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.List T, Neri D. Immunocytokines: a review of molecules in clinical development for cancer therapy. Clin Pharmacol. 2013;5(Suppl 1):29–45. doi: 10.2147/CPAA.S49231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang HM, Liao ZX, Komaki R, Welsh JW, O’Reilly MS, Chang JY, Zhuang Y, Levy LB, Lu C, Gomez DR. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol. 2013;24:1312–1319. doi: 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, Zanelotti A, Adamoli L, Colleoni M, Viale G, Goldhirsch A, Gandini S. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013;140:567–575. doi: 10.1007/s10549-013-2654-3. [DOI] [PubMed] [Google Scholar]
- 109.Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2012;127:375–378. doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 110.Johannesdottir SA, Schmidt M, Phillips G, Glaser R, Yang EV, Blumenfeld M, Lemeshow S. Use of beta-blockers and mortality following ovarian cancer diagnosis: a population-based cohort study. BMC Cancer. 2013;13:85. doi: 10.1186/1471-2407-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Livingstone E, Hollestein LM, van Herk-Sukel MP, van de Poll-Franse L, Nijsten T, Schadendorf D, de Vries E. Beta-blocker use and all-cause mortality of melanoma patients: results from a population-based Dutch cohort study. Eur J Cancer. 2013;49:3863–3871. doi: 10.1016/j.ejca.2013.07.141. [DOI] [PubMed] [Google Scholar]
- 112.Shah SM, Carey IM, Owen CG, Harris T, DeWilde S, Cook DG. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]