Abstract
The NIH has recently highlighted the importance of sexual dimorphisms and has mandated inclusion of both sexes in clinical trials and basic research. In this review we highlight new and novel ways sex hormones influence body adiposity and the metabolic syndrome. Understanding how and why metabolic processes differ by sex will enable clinicians to target and personalize therapies based on gender. Adipose tissue function and deposition differ by sex. Females differ with respect to distribution of adipose tissues, males tend to accrue more visceral fat, leading to the classic android body shape which has been highly correlated to increased cardiovascular risk; whereas females accrue more fat in the subcutaneous depot prior to menopause, a feature which affords protection from the negative consequences associated with obesity and the metabolic syndrome. After menopause, fat deposition and accrual shift to favor the visceral depot. This shift is accompanied by a parallel increase in metabolic risk reminiscent to that seen in men. A full understanding of the physiology behind why, and by what mechanisms, adipose tissues accumulate in specific depots and how these depots differ metabolically by sex is important in efforts of prevention of obesity and chronic disease. Estrogens, directly or through activation of their receptors on adipocytes and in adipose tissues, facilitate adipose tissue deposition and function. Evidence suggests that estrogens augment the sympathetic tone differentially to the adipose tissue depots favoring lipid accumulation in the subcutaneous depot in women and visceral fat deposition in men. At the level of adipocyte function, estrogens and their receptors influence the expandability of fat cells enhancing the expandability in the subcutaneous depot and inhibiting it in the visceral depot. Sex hormones clearly influence adipose tissue function and deposition, determining how to capture and utilize their function in a time of caloric surfeit, requires more information. The key will be harnessing the beneficial effects of sex hormones in such a way as to provide ‘healthy’ adiposity.
Keywords: Obesity; Sexual dimorphism; Estrogen, estrogen receptor alpha; Visceral fat depot; Subcutaneous fat depot; Browning of fat; Natriuretic peptides; PGC1 alpha; Prolyl hydroxylase domain; Hypoxia inducible factor
1. Introduction
Over the past 20 years, adult and childhood obesity rates have doubled, while adolescent obesity has tripled (Ford et al., 2014). Two-thirds of Americans are currently at-risk for obesity related mortality or morbidity however this differs by sex. While the connection between obesity and risk of heart disease, hypertension, cancer, stroke, and diabetes is well established in men, it is less so for women and the mechanisms underlying these sexually dimorphic influences remain poorly understood. Over the past decade adipose tissues have been determined to be more than a storage vessel for triglycerides, rather, these tissues actively contribute to metabolic homeostasis by secreting a wide variety of signaling molecules and hormones. An often underappreciated finding is that adipose tissue function and deposition differ by sex. Females have an overall higher total body fat content when compared to men. Importantly, females differ with respect to distribution of adipose tissues, males tend to accrue more visceral fat, leading to the classic android body shape which has been highly correlated to increased cardiovascular risk; whereas females accrue more fat in the subcutaneous depot prior to menopause, a feature associated with protection from the negative consequences associated with obesity and the metabolic syndrome (Fig. 1). After menopause, fat deposition and accrual shift to favor the visceral depot. This shift is accompanied by a parallel increase in metabolic risk reminiscent to that seen in men. A full understanding of the physiology behind why, and by what mechanisms, adipose tissues accumulate in specific depots and how these depots differ metabolically by sex is important in efforts of prevention of obesity and chronic disease. A review of sex differences in obesity/adipose tissue distribution is timely given that obesity has recently been classified as a disease, and that the National Institutes of Health has made it mandatory to explore gender differences in disease states.
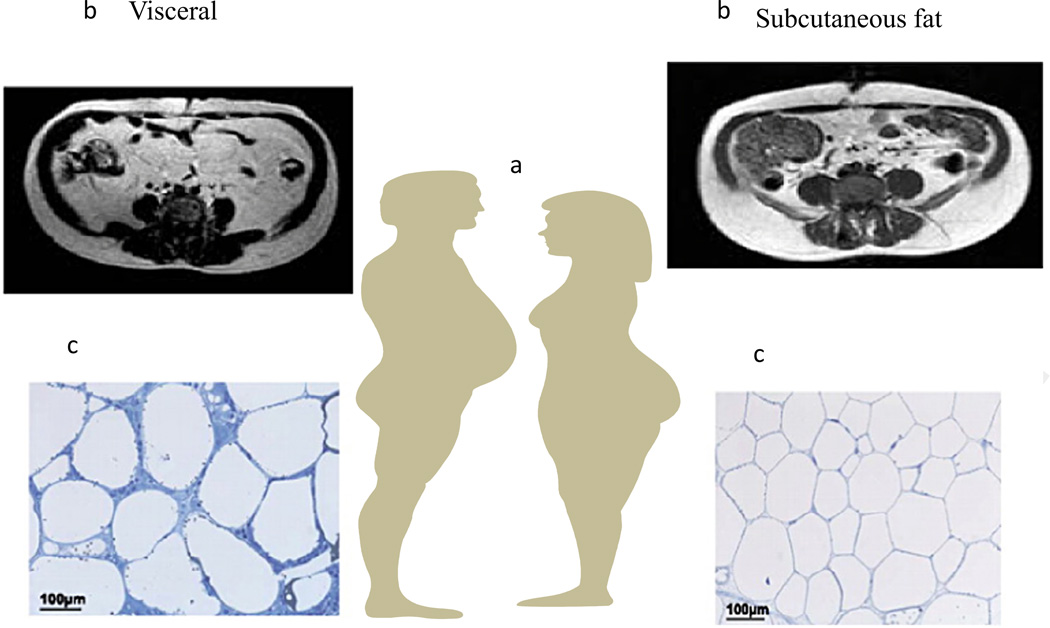
Fig. 1.
Approximately 80% of all body fat is in the subcutaneous depot and lies just under the skin primarily around the waist, in the subscapular area, and in the gluteal and femoral (thigh) areas. Visceral fat, accounting for 10–20% of total fat, is in the abdomen primarily in the omentum and mesentery but also in perirenal, gonadal, epicardial, and retroperitoneal depots. Visceral fat accounts for a higher percentage of total fat in men than in women. In men adipose tissue preferentially accumulates in the visceral depot while fat accumulation is primarily in the subcutaneous depot in women. The magnitude of this difference is amplified from late puberty to early adulthood as men develop the typical android body shape while women a more gynoid shape. Menopause is followed by redistribution of adipose tissue to the visceral depots leading to a more central or android shape in post-menopausal women who are not hormone replaced. The timing of these changes implicates involvement of sex hormones. Up to the transition through menopause, women tend to accrue adipose tissue preferentially in the subcutaneous depot due to its greater storage capacity, and the expandability of subcutaneous fat can be traced to a greater degree of hyperplasia of fat cells. Men accrue adipose tissue preferentially in the visceral depot, and the accumulation of excess fat in the visceral depot is primarily achieved by hypertrophy of fat cells. Once storage capacity is exceeded, visceral adipose tissue is characterized by fibrotic and inflamed adipose tissue which is highly correlated with the metabolic syndrome. (a) A cartoon depicting android and gynoid deposition of adipose tissue in males and females. (b) Representative coronal midsection MRI images of a BMI-matched male and female demonstrating fat distribution with the white matter depicting adipose tissues. (c) Representative histologic adipose tissue sections from subcutaneous or visceral adipose tissues. The subcutaneous adipose tissue has smaller more ‘plastic’ adipocytes whereas the visceral adipose tissue is characterized by larger adipocytes encased in fibrotic tissues.
2. Estrogens and adiposity
Obesity is influenced by a number of variables such as ethnicity, socioeconomic status and education which makes it difficult in humans to determine whether a biological difference per se exists regarding the propensity to gain weight between men and women. By contrast, in animal models where non-biological factors are excluded, studies suggest the propensity toward development of obesity differs between the sexes and this is directly due to sex hormones. For example, female rats gain less weight compared to males when presented with a metabolic challenge such as a high fat diet, a difference no longer seen following ovariectomy (Stubbins et al., 2012). Estrogens protect against increased body adiposity/obesity through their effects to suppress appetite and increase energy expenditure. Estradiol suppresses feeding by enhancing the potency of other anorectic signals, such as cholecystokinin, apolipoprotein A-IV, leptin, brain derived neurotrophic factor (BDNF), and by decreasing the potency of orexigenic signals such as melanin-concentrating hormone and ghrelin (Clegg et al., 2006, 2007; Geary, 2001; Messina et al., 2006; Shen et al., 2010; Zhu et al., 2013).
In women, caloric intake varies across the menstrual cycle. Women tend to eat less during the 4-day periovulatory phase of the menstrual cycle when estradiol reaches its peak and these cyclic changes in feeding are absent in women with anovulatory cycles (Barr et al., 1995; Buffenstein et al., 1995; Davidsen et al., 2007; Lissner et al., 1988). Consistently, cycling female rodents consume different amounts of food across their 4-day ovarian cycles, consuming the least during diestrus, which occurs right after preovulatory rise in estradiol secretion, and consuming the most during estrus when estradiol levels are lower indicating physiologic estradiol levels are negatively correlated with food intake (Asarian and Geary, 2013; Tarttelin and Gorski, 1971).
Estrogens also protect against weight gain by increasing energy expenditure. Many postmenopausal women gain body weight due the natural decrease in endogenous estradiol levels during menopause and reductions in energy expenditure can be prevented by estrogen replacement therapy (Gambacciani et al., 1997). Additionally, postmenopausal women have a lower fat oxidation and energy expenditure during exercise and sleep when compared to premenopausal women (Abildgarrd et al., 2013; Lovejoy et al., 2008). Rodent studies have confirmed these findings and identified that activation of the estrogen receptors in the ventral medial nucleus of the hypothalamus results in increased energy expenditure (Musatov et al., 2007; Xu et al., 2011). Combined, these observations demonstrate that estrogens suppress food intake and increase energy expenditure in women.
3. Sexual dimorphism and fat distribution
Premenopausal women tend to store fat on the hips, thighs and buttocks, giving them a pear shape also called gynoid, or gluteal–femoral pattern of adipose tissue distribution. Men accumulate fat predominately in the abdominal region giving them an apple shape also referred to as android, or abdominal pattern of fat accrual (Fig. 1). Even lean men carry a greater proportion of their body fat in the visceral depot as compared to lean women.
Differences in adipose tissue distribution are tied to adipose depot specific differences in the determinants of fat uptake and storage. Lipoprotein lipase activity is the rate limiting step in the accumulation of fat derived from circulating fatty acids and triglycerides. Activity of the enzyme is higher in gluteal (subcutaneous) as compared to abdominal (visceral) fat in women facilitating their gynoid distribution. By contrast enzyme activity is higher in abdominal/visceral adipose tissues in men (Arner et al., 1991). These sex differences in adipose tissue distribution are accentuated through a suppressive effect of testosterone on lipoprotein lipase activity in femoral subcutaneous fat in men (Ramirez et al., 1997).
Human fat cells express lipolytic β1–2 and antilipolytic α2-adrenergic receptors providing an additional mechanism to regulate lipolysis/lipogenesis and the filling of adipose tissues. A sexual dimorphism exists in the distribution of these receptors to account for sex- and depot-specific differences in the modulation of lipolysis (Richelsen, 1986; Richelsen et al., 1991). Estradiol increases α2-adrenergic receptors in subcutaneous adipose tissue but has no effect on adrenergic receptors in intraabdominal adipocytes (Pedersen et al., 2004). The ratio of α2 to β1–2-adrenergic receptors in the subcutaneous depot in premenopausal women is increased and accounts for the lower lipolytic response to epinephrine and norepinephrine as compared to adipocytes from men. This balance of adrenergic receptors in reversed in the visceral depot of women favoring lipolysis. In men as well as postmenopausal women the adrenergic receptor ratios are reversed potentially explaining the preferential accumulation of fat in the visceral depot (Gavin et al., 2013; Richelsen, 1986; Richelsen et al., 1991).
Estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) are estrogen receptors on adipocytes which influence adiposity. Studies in transgenic animals suggest the distribution of these receptors contribute to the ability of estrogens to modulate distribution of fat between the depots. The total body ERα knockout mouse has increased adiposity, increased visceral fat accumulation, and the metabolic syndrome (Davis et al., 2013). Following ovariectomy, estrogens reduce visceral fat mass in wild type and ERβ knockout but not ERα knockout female mice indicating the lipolytic effect of estrogens are primarily mediated through ERα (Gavin et al., 2013; Lindberg et al., 2002). Loss in fat mass following administration of estrogens is greater in ERβ knockout mice suggesting ERβ may act as a repressor of, or in opposition to, the ERα mediated effects on fat mass (Gavin et al., 2013; Lindberg et al., 2002; Naaz et al., 2002). To the extent ERβ opposes the actions of ERα, a higher ERα/β ratio in the abdominal visceral fat serves to limit adipose accumulation in this depot whereas a lower ERα/ERβ ratio in gluteal fat provides a more favorable environment for adipose accumulation and storage in premenopausal women. Males have a relative lack of ERα in the visceral depot and are therefore primed to store more fat viscerally. Deletion of ERα from adipocytes in males and females causes increased adiposity specifically in the visceral depot (Davis et al., 2013).
ERs, specifically ERα, located in the central nervous system plays a role in determining adipose tissue distribution. Targeted disruption of ERα in the ventromedial nucleus of the hypothalamus leads to visceral obesity in females (Xu et al., 2011). Expression of ERα is preferentially associated with neurons directed to the visceral depot providing a pathway by which administration of exogenous estrogens into the CNS dramatically reduces visceral adiposity (Adler et al., 2012; Clegg et al., 2006). This effect may be related to changes in sympathetic input to fat depots as there is a sexual dimorphism in the sympathetic innervation of adipose tissue. Estrogens have been shown to promote lipolysis through increased adipose tissue sympathetic nerve activity. Male rats have more neuronal projections into visceral fat, whereas females have more projections to the subcutaneous depot (Adler et al., 2012). In addition, the number of ERα receptor expressing neurons projecting to subcutaneous fat is lower in males compared to females. Whether central input via these differences in innervation or adrenergic receptor makeup at the level of the fat depots or both ultimately dictate where adipose tissue preferentially accumulates is not known. In a ovariectomized rat model where the retroperitoneal fat pad is unilaterally denervated, administration of estrogens resulted in significantly more adipose tissue loss in the intact fat pad as compared to the denervated pad (Lazzarini and Wade, 1991). Central administration of estrogen, acting through ERα in the ventromedial nucleus, activates the sympathetic nervous system and increases thermogenesis (Martinez de Morentin et al., 2014).
4. Visceral vs subcutaneous adipose tissue and metabolic function
Visceral fat is a source of proinflammatory cytokines that contribute to insulin resistance. In addition, the high lipolytic rate of visceral fat generates large amounts of free fatty acids that are delivered to the liver causing increased hepatic glucose production, hyperinsulinemia, and other features of the metabolic syndrome (Shulman, 2014). By contrast, accumulation of fat in the subcutaneous depot is an independent predictor of lower cardiovascular and diabetes-related mortality, and protects against impaired glucose metabolism (Tanko et al., 2003; Van Pelt et al., 2002). These findings are consistent with the fact that women, with higher levels of subcutaneous fat, are protected from diseases associated with obesity whereas men, with higher amounts of visceral fat deposition, are at a heighten risk for diseases associated with obesity.
Surgical removal of visceral adipose tissues in animals and humans improves insulin resistance and diabetes (Gabriely et al., 2002). In a study of obese subjects undergoing adjustable gastric banding, a comparison was made between the procedure alone or banding plus surgical removal of the omentum (a component of visceral fat) (Thorne et al., 2002). Despite comparable weight loss, change in waist hip ratio, and sagittal diameter at 2 years of follow up, the improvements in oral glucose tolerance, insulin sensitivity, and fasting plasma glucose and insulin were 2–3 times greater in the omentectomized group. These findings strongly connect reductions in insulin sensitivity with visceral fat accrual.
By contrast, selective removal of subcutaneous fat as with liposuction confers no significant improvement in obesity-associated metabolic abnormalities and in animal models is associated with a worse metabolic profile (Klein et al., 2004; Weber et al., 2000). Lipectomized hamsters where >50% of subcutaneous adipose tissue is removed lead to more intra-abdominal visceral adipose tissues as a percentage of total body fat, higher insulinemic index, and a strong trend toward increased liver fat content when compared to sham operated controls (Weber et al., 2000). Lipectomy reduces the number of subcutaneous adipocytes causing excess fat to accumulate in the visceral depot and ectopically in the liver resulting in deleterious metabolic consequences. The reaccumulation of body fat following liposuction in humans is also associated with redistribution from subcutaneous to visceral fat deposition (Hernandez et al., 2011). These findings suggest removal of subcutaneous adipose tissue increases metabolic risk by removing a buffer or sink for peripheral TGs, analogous to changing ‘good adipose tissue’ deposition as seen in females, to unhealthy adipose tissue deposition as seen in males.
5. Visceral and subcutaneous adipocytes differ
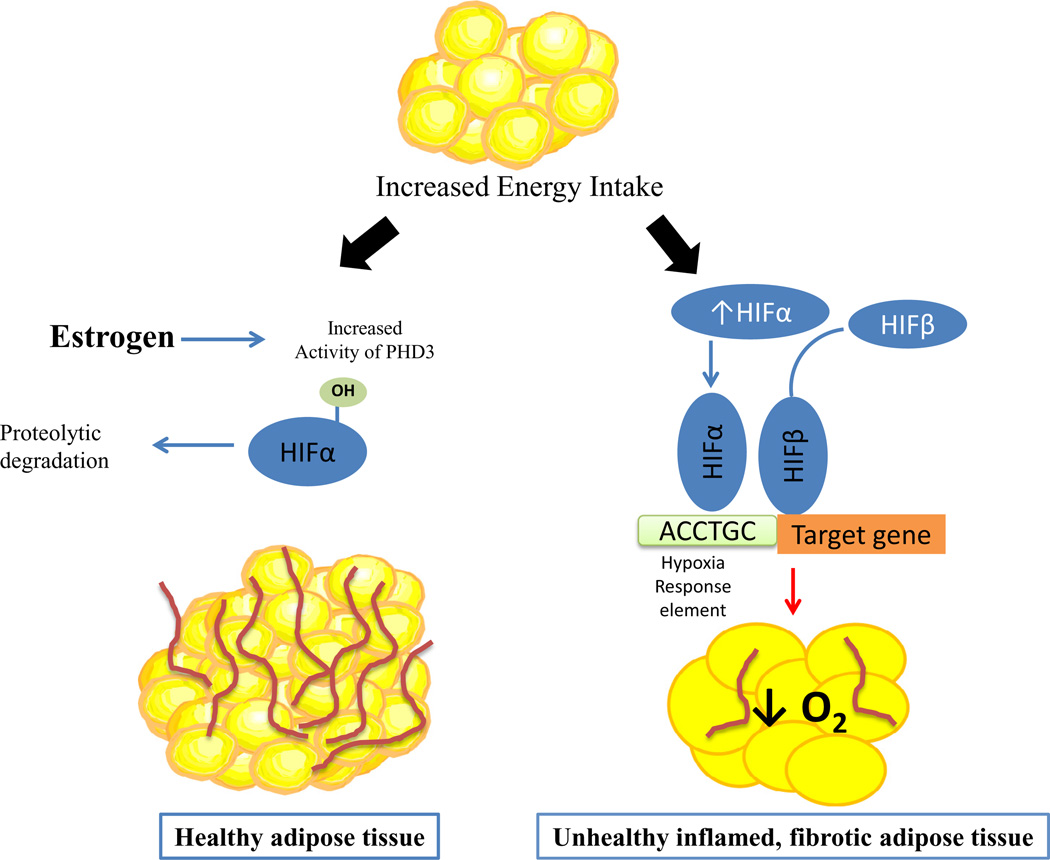
Accumulating evidence suggests obesity complications result from the inability of fat cells to expand and safely store lipids (Fig. 2), which leads to ectopic deposition of lipids in other tissues termed lipotoxicity which results in insulin resistance (Shulman, 2014). Expansion of fat mass can occur either by an increase in volume of preexisting adipocytes (hypertrophy) or by hyperplasia in which an increase in fat mass occurs through recruitment of new preadipocytes. When fat cells surpass their storage capacity and lack the ability to expand further, adipocyte homeostasis is altered leading to metabolic dysregulation (Fig. 2). Studies in rodents suggest the visceral fat expands predominantly by adipocyte hypertrophy, while the subcutaneous depot expands by adipocyte hyperplasia following exposure to a high-fat diet (Wang et al., 2013). Adipocyte hyperplasia leads to small adipocytes which are not only more insulin sensitive, but also have enhanced storage capacity. The number of small, early-differentiated adipocytes, isolated from the stromal–vascular fraction (SVF) in subcutaneous depots of normal weight men and women, correlates positively with subcutaneous adiposity (particularly in the femoral subcutaneous depot), and negatively with visceral fat accumulation suggesting the abundance of adipocytes in subcutaneous fat is an important predictor for adipose tissue expandability (Tchoukalova et al., 2010). The precise mechanism by which sex hormones determine adipocyte hyperplasia or hypertrophy is unknown; however preliminary data suggest estrogens influence hyperplasia by increasing adipocyte progenitor cells.
Fig. 2.
When faced with chronic, excessive energy intake resulting in obesity, visceral adipose tissue (AT) becomes hypoxic due to increased adipocyte size and tissue mass which outpaces the supporting vascular supply resulting in inflamed and fibrotic adipose tissues which are co-localized with macrophages suggesting an immediate link between hypoxia and the inflammatory response. Both ERα and HIF-1 are related to fibrosis and inflammation in adipose tissue, albeit in opposite ways. ERα signaling improves adipose tissue function by decreasing adipose tissue inflammation and improving adipose tissue insulin sensitivity while HIF-1 worsens it through up-regulation of inflammatory mediators to include IL-6, NF-kB and TNFα and markers of fibrosis such as Col-6. It has recently been demonstrated E2/ERα regulates HIF-1 activity in adipose tissues by promoting transcription of a specific prolyl hydroxylase domain enzyme (PHD3). Increased activity of PHD3 through hydroxylation targets HIF for ubiquitination and degradation, thus providing a mechanistic explanation for the protective effect of E2/ERα against the metabolic impact of HIF-1 activation in adipose tissue and contributing to the reduction in adipose tissue inflammation and fibrosis seen in female adipose tissues.
An additional way estrogens may influence adipose tissues is through regulation of the vascular supply into adipose tissues (Elias et al., 2012; Gealekman et al., 2011). Fat cell hypertrophy or hyperplasia is influenced by vascular supply. Studies in samples taken from humans show subcutaneous fat has a higher capillary density and angiogenic growth capacity when compared to samples taken from the visceral depot (Gealekman et al., 2011). When vascular supply is limited adipose tissue expansion leads to hypoxia and activation of hypoxia inducible factor (HIF) (Fig. 2). Stabilization of HIF in turn has been linked to altered adipokine expression, proinflammatory macrophage recruitment, and insulin resistance, features typical of visceral fat. Estrogens attenuate activation of HIF by transcriptionally upregulating one of the prolyl hydroxylase enzymes which targets HIF for ubiquitination and degradation providing a mechanism by which estrogens reduce adipose tissue inflammation and fibrosis (Fig. 2) (Kim et al., 2014; Palmer and Clegg, 2014).
6. Estrogens and ‘browning’ of adipose tissues
Estrogens not only influence adipose tissue hyperplasia/hypertrophy and distribution, but they also influence the metabolic activity of adipose tissues by regulating ‘browning’, or enhancing the metabolic activity of adipose tissues (Fig. 3). Brown adipose tissue is metabolically more active due to the increased number of mitochondria. Recent data suggest the metabolic rate per kilogram adipose tissue is higher in women than men due to higher levels of brown adipose tissue in women and increased expression of genes involved in mitochondrial function to include uncoupling protein one (UCP-1) (Cypess et al., 2009; Nookaew et al., 2013).
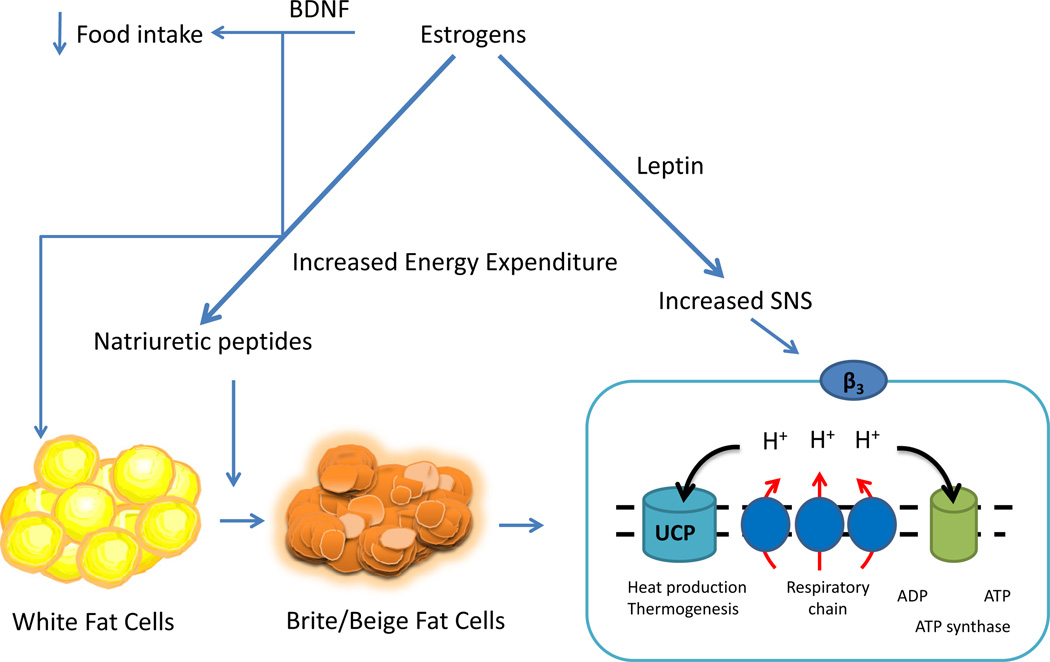
Fig. 3.
Estrogens protect against increased body adiposity/obesity through their effects to suppress appetite and increase energy expenditure. In addition to enhancing potency of other anorectic signals and decreasing the potency of orexigenic signals, ERα signaling enhances leptin-induced satiety and increased energy expenditure. Leptin also induces activation of peripheral sympathetic nerve activity leading to increased energy expenditure. Estrogens/ERα enhance the release of natriuretic peptides, ANP and BNP, which have been demonstrated to act on adipose tissues to facilitate ‘browning’ through upregulation of UCP-1 which uncouples oxidative phosphorylation causing an increase in thermogenesis and increased energy expenditure. Estrogens also upregulate BDNF which has been demonstrated to play a role in suppressing appetite and increasing energy expenditure through effects associated with browning of adipose tissue. Therefore, estrogens through their activation of ERα have a pleiotropic effect on energy expenditure by increasing leptin induced activation of SNS, by upregulating BDNF, and by increasing ANP/BNP which facilitate the transition from white adipocytes to brown which are metabolically more energetic.
Estrogens through ERα regulate both the gene and protein expression of BDNF in the hypothalamus which in turn has been linked to selective neural modulation of white fat to induce browning (Cao et al., 2011; Solum and Handa, 2002). Estrogens may also increase browning through regulation of natriuretic proteins ANP (atrial natriuretic peptide) and BNP (brain natriuretic peptide) (Fig. 3), which have recently been demonstrated to facilitate browning of white adipose tissues by up regulation of UCP-1 and by activating mitochondrial biogenesis and uncoupling in white adipose tissues (Bordicchia et al., 2012; Collins, 2014). Circulating levels of ANP and BNP are twofold higher in premenopausal women as compared to men due to a stimulatory effect of estrogens on cardiomyocytes to release ANP/BNP (Clark et al., 1990; Hong et al., 1992; Jankowski et al., 2001; Wang et al., 2002). These findings suggest gender differences in levels of natriuretic peptides may contribute to differences in metabolic rate due to differences in accumulation of brown adipose tissues. After menopause, the difference in natriuretic peptide values between the sexes decreases consistent with reductions in brown adipose tissues following menopause.
7. Teleological explanation for sex differences in adipose tissue deposition
The evolutionary basis as to why women preferentially store fat in the gluteal–femoral region is not known; however, one hypothesis is that women accumulate energy reserves in the subcutaneous depot to prepare for adipose tissue mobilization required for lactation. Longitudinal studies of skin-fold thickness during pregnancy and lactation consistently show fat accumulation in the supra-iliac and mid-thigh regions (subcutaneous adipose tissue depots) during pregnancy, which is mobilized postpartum (Brewer et al., 1989; Kramer et al., 1993; Sohlstrom and Forsum, 1995). These findings have been confirmed in a longitudinal magnetic resonance imaging study of lactating women as well as in a cross-sectional study of subjects in the Third National Health and Nutrition Examination Survey, all studies supporting the fact that adipose tissues in the subcutaneous depot are mobilized preferentially during lactation (Lassek and Gaulin, 2006). Withdrawal of estrogens and progesterone is a prerequisite for lactogenesis, because these sex steroids inhibit the lactogenic effects of prolactin. The precipitous fall in estrogens following parturition is accompanied by a decline in lipoprotein lipase activity and increased sensitivity of the subcutaneous adipose tissues to the lipolytic effect of catecholamines (Rebuffe-Scrive et al., 1985).
In further support of a critical role for estrogens in modulating adipose tissues, with severe weight loss women develop amenorrhea and no longer ovulate. This may be an adaptive response to the insufficient amount of subcutaneous fat to support the requirements of pregnancy and lactation. This deficit is signaled to the brain byway of low circulating leptin. Leptin interacts with the hypothalamic pituitary axis leading to hypothalamic amenorrhea (Kopp et al., 1997). Female brains are more sensitive to the effects of leptin to regulate food intake and energy expenditure, indicating a strong synergy between the adiposity hormone leptin, and estrogens in the regulation of reproduction and energy homeostasis (Clegg et al., 2006).
Basal metabolic rate during the course of pregnancy progressively increases particularly in the third trimester. Interestingly though, this increase in metabolic rate is thought to be out of proportion to the increase in body fat suggesting the accumulated fat is more metabolically active (Lof et al., 2005). These findings lead to the speculation that increasing estrogens during the course of pregnancy may contribute to altering the metabolic rate of adipose tissues consistent with increased browning of white subcutaneous adipose tissues. Increased brown fat thermogenesis may be mediated by ANP and BNP which progressively rise during the course of pregnancy reaching a plateau during the last trimester (Yoshimura et al., 1994).
Newborn infants have the highest amount of brown adipose tissues. Natriuretic peptides are also elevated in the new born infant during the first several weeks after birth then fall (Koch and Singer, 2003; Mir et al., 2003). Estrogens mediate production of atrial natriuretic peptides by the placenta and this effect may account for the high levels of natriuretic peptides in the developing infant and contribute to the accumulation of brown fat typical of new born infants (Graham et al., 1996; Huang et al., 1992).
The decline in circulating estrogens during menopause leads to a shift in adipose tissue deposition favoring the visceral depot. It has been well characterized that reductions in circulating estrogens by more than 90% leads to symptoms such as hot flashes and an increased prevalence diseases associated with the metabolic syndrome. During menopause, adipose tissues become the primary source of estrogens. Androstenedione, produced by the adrenal gland, and small amounts of testosterone derived from the ovary is also converted to estrogens. Importantly, conversion of these estrogenic precursors to estrogens by aromatase occurs predominately in the visceral depot. From a teleologic standpoint, one could hypothesize shifts in fat distribution favoring the visceral depot in post menopausal women is due to the fact that this site is the primary source of estrogen and therefore visceral adipose tissues may represent a ‘third ovary’. The total estrogen produced after menopause, however, is far less than that produced during a woman’s reproductive years.
In men, the evolutionary pressure to deposit more fat in the visceral depot may be due to the fact that this fat depot is more readily mobilizable and can quickly act as an energy surfeit. Indeed, in obese men exercise with or without reduction in body weight leads to a preferential loss of visceral fat (Ross et al., 2000). Visceral fat in the omentum and mesentery allow for fatty acids to drain into the liver by way of the portal circulation where they can undergo β-oxidation generating large amounts of energy or be processed into lipoprotein particles such as LDL and VLDL. Lipoprotein particles provide a transport mechanism for delivery of fuel by way of transport of fatty acids to peripheral tissues. It has been hypothesized that prehistoric man surviving through hunting and quick escape would need an easily mobilizable energy source thus explaining the predilection for fat storage in the visceral depot in men.
Recent data show a sexual dimorphism exists in the way the brain responds to high fat diet. These findings demonstrated in men there are adverse metabolic affects which develop following consumption of diets high in fat which are not observed in women. These findings can further be described by the fact that storage of fat beyond that required for immediate energy expenditure may represent an evolutionary pressure discouraging high fat intake in men. Following consumption of a high fat diet, the brain tissue of males mirrored the fatty acid composition of the diet whereas in females, this did not occur (Morselli et al., 2014). The males had elevated levels of saturated fatty acids such as palmitic acid as well as elevations in sphingolipids and ceramides which were associated with elevated markers of inflammation when compared to female mice. Treatment of neuronal and astrocyte cell cultures with palmitic acid in an attempt to mimic the effects of high fat intake increases inflammation in the male cells and this does not occur in the female tissues (Morselli et al., 2014). In these studies there were reductions in ERα in neurons and astrocytes following palmitic acid treatment in male, but not female, mice. Restoration in the levels of ERα reduced markers of inflammation. Importantly, development of hypothalamic inflammation is associated with depressed myocardial function in male, but not female, mice (Morselli et al., 2014). These data are consistent with previous studies showing less development of left ventricular hypertrophy in female versus male mice in response to chronic high fat intake (Böhm et al., 2013). These important findings further demonstrate there are sexual dimorphisms with respect to metabolism and obesity emphasizing the importance of sex-based research.
8. Summary
In this review we have demonstrated that estrogens, directly or through activation of their receptors on adipocytes and in adipose tissues, facilitate adipose tissue deposition and function. Estrogens not only are protective in women, but a recent report by Finkelstein et al. further demonstrates that estrogens are necessary in men. Blocking conversion of androgens to estrogens resulted in reductions in insulin sensitivity and decrements in metabolism (Finkelstein et al., 2013). Evidence suggests that estrogens augment the sympathetic tone differentially to the adipose tissue depots favoring lipid accumulation in the subcutaneous depot in women and visceral fat deposition in men. At the level of adipocyte function, estrogens and their receptors influence the expandability of fat cells enhancing the expandability in the subcutaneous depot and inhibiting it in the visceral depot. Teleologically, the influences of sex hormones on adipose tissues may have provided a selection bias in that women needed to have adipose tissues deposited in the subcutaneous depot to support lactation and thus this required expandability of these adipocytes to take up more lipid prior to parturition to augment the energy demands of lactation. We suggest a potential new and novel role for the natriuretic peptides in facilitating these effects. In males, there was a teleological advantage to having a readily mobilizable deposition of adipose tissue to facilitate rapid bursts of energy required during hunting and gathering. Sex hormones clearly influence adipose tissue function and deposition, determining how to capture and utilize their function in a time of caloric surfeit requires more information. The key will be harnessing the beneficial effects of sex hormones in such a way as to provide ‘healthy’ adiposity.
References
- Abildgarrd JPA, Green CJ, Harder-Lauridsen NM, Solomon TP, Thomsen C, Juul A, et al. Menopause is associated with decreased whole body fat oxidation during exercise. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1227–E1236. doi: 10.1152/ajpendo.00492.2012. [DOI] [PubMed] [Google Scholar]
- Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J. Neurosci. 2012;32:15913–15921. doi: 10.1523/JNEUROSCI.2591-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P, Lithell H, Wahrenberg H, Bronnegard M. Expression of lipoprotein lipase in different human subcutaneous adipose tissue regions. J. Lipid Res. 1991;32:423–429. [PubMed] [Google Scholar]
- Asarian L, Geary N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am. J. Clin. Nutr. 1995;61:39–43. doi: 10.1093/ajcn/61.1.39. [DOI] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm C1, Benz V, Clemenz M, Sprang C, Höft B, Kintscher U, et al. Sexual dimorphism in obesity-mediated left ventricular hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H211–H218. doi: 10.1152/ajpheart.00593.2012. [DOI] [PubMed] [Google Scholar]
- Brewer MM, Bates MR, Vannoy LP. Postpartum changes in maternal weight and body fat depots in lactating vs nonlactating women. Am. J. Clin. Nutr. 1989;49:259–265. doi: 10.1093/ajcn/49.2.259. [DOI] [PubMed] [Google Scholar]
- Buffenstein R, Poppitt SD, McDevitt RM, Prentice AM. Food intake and the menstrual cycle: a retrospective analysis, with implications for appetite research. Physiol. Behav. 1995;58:1067–1077. doi: 10.1016/0031-9384(95)02003-9. [DOI] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BA, Elahi D, Epstein FH. The influence of gender, age, and the menstrual cycle on plasma atrial natriuretic peptide. J. Clin. Endocrinol. Metab. 1990;70:349–352. doi: 10.1210/jcem-70-2-349. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- Collins S. A heart-adipose tissue connection in the regulation of energy metabolism. Nat. Rev. Endocrinol. 2014;10:157–163. doi: 10.1038/nrendo.2013.234. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsen L, Vistisen B, Astrup A. Impact of the menstrual cycle on determinants of energy balance: a putative role in weight loss attempts. Int. J. Obes. 2007;31:1777–1785. doi: 10.1038/sj.ijo.0803699. [DOI] [PubMed] [Google Scholar]
- Davis KD, Neinast M, Sun KM, Skiles WD, Bills JA, Zehr J, et al. The sexually dimorphic role of adipose and adipocyte estrogen receptors in modulating adipose tissue expansion, inflammation, and fibrosis. Mol. Metab. 2013;2:227–242. doi: 10.1016/j.molmet.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, et al. Gonadal steroids and body composition, strength, and sexual function in men. N. Engl. J. Med. 2013;369:1011–1022. doi: 10.1056/NEJMoa1206168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. J. Am. Med. Assoc. 2014;312:1151–1153. doi: 10.1001/jama.2014.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002;51:2951–2958. doi: 10.2337/diabetes.51.10.2951. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, et al. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J. Clin. Endocrinol. Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- Gavin KM, Cooper EE, Hickner RC. Estrogen receptor protein content is different in abdominal than gluteal subcutaneous adipose tissue of overweight-to-obese premenopausal women. Metabolism. 2013;62:1180–1188. doi: 10.1016/j.metabol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Gavin KM, Cooper EE, Raymer DK, Hickner RC. Estradiol effects on subcutaneous adipose tissue lipolysis in premenopausal women are adipose tissue depot specific and treatment dependent. Am. J. Physiol. Endocrinol. Metab. 2013;304:E1167–E1174. doi: 10.1152/ajpendo.00023.2013. [DOI] [PubMed] [Google Scholar]
- Gealekman O1, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/s0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- Graham CH, Watson JD, Blumenfeld AJ, Pang SC. Expression of atrial natriuretic peptide by third-trimester placental cytotrophoblasts in women. Biol. Reprod. 1996;54:834–840. doi: 10.1095/biolreprod54.4.834. [DOI] [PubMed] [Google Scholar]
- Hernandez TL, Kittelson JM, Law CK, Ketch LL, Stob NR, Lindstrom RC, et al. Fat redistribution following suction lipectomy: defense of body fat and patterns of restoration. Obesity. 2011;19:1388–1395. doi: 10.1038/oby.2011.64. [DOI] [PubMed] [Google Scholar]
- Hong M, Yan Q, Tao B, et al. Estradiol, progesterone and testosterone exposures affect the atrial natriuretic peptide gene expression in vivo in rats. Biol. Chem. Hoppe-Seyler. 1992;373:213–218. doi: 10.1515/bchm3.1992.373.1.213. [DOI] [PubMed] [Google Scholar]
- Huang W, Lee D, Yang Z, Casley D, Throsby M, Copolov DL, et al. Evidence for atrial natriuretic peptide-(5–28) production by rat placental cytotrophoblasts. Endocrin. 1992;131:919–924. doi: 10.1210/endo.131.2.1386304. [DOI] [PubMed] [Google Scholar]
- Jankowski M, Rachelska G, Donghao W, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc. Natl. Acad. Sci. U.S.A. 2001;98:11765–11770. doi: 10.1073/pnas.201394198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Neinast MD, Frank AP, Sun K, Park J, Zehr JA, et al. ERα upregulates Phd3 to ameliorate HIF-1 induced fibrosis and inflammation in adipose tissue. Mol. Metab. 2014;3:642–651. doi: 10.1016/j.molmet.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N. Engl. J. Med. 2004;350:2549–2557. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–878. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp W, Blum WF, von Prittwitz S, Ziegler A, Lubbert H, Emons G, et al. Low leptin levels predict amenorrhea in underweight and eating disordered females. Mol. Psychiatry. 1997;2:335–340. doi: 10.1038/sj.mp.4000287. [DOI] [PubMed] [Google Scholar]
- Kramer FM, Stunkard AJ, Marshall KA, McKinney S, Liebschutz J. Breast-feeding reduces maternal lower-body fat. J. Am. Diet. Assoc. 1993;93:429–433. doi: 10.1016/0002-8223(93)92289-a. [DOI] [PubMed] [Google Scholar]
- Lassek WD, Gaulin SJ. Changes in body fat distribution in relation to parity in American women: a covert form of maternal depletion. Am. J. Phys. Anthropol. 2006;131:295–302. doi: 10.1002/ajpa.20394. [DOI] [PubMed] [Google Scholar]
- Lazzarini SJ, Wade GN. Role of sympathetic nerves in effects of estradiol on rat white adipose tissue. Am. J. Physiol. 1991;260:R47–R51. doi: 10.1152/ajpregu.1991.260.1.R47. [DOI] [PubMed] [Google Scholar]
- Lindberg MK, Weihua Z, Andersson N, Moverare S, Gao H, Vidal O, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J. Endocrinol. 2002;174:167–178. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- Lissner L, Stevens J, Levitsky DA, Rasmussen KM, Strupp BJ. Variation in energy intake during the menstrual cycle: implications for food-intake research. Am. J. Clin. Nutr. 1988;48:956–962. doi: 10.1093/ajcn/48.4.956. [DOI] [PubMed] [Google Scholar]
- Lof M, Olausson H, Bostrom K, Janerot-Sjoberg B, Sohlstrom A, Forsum E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am. J. Clin. Nutr. 2005;81:678–685. doi: 10.1093/ajcn/81.3.678. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina MM, Boersma G, Overton JM, Eckel LA. Estradiol decreases the orexigenic effect of melanin-concentrating hormone in ovariectomized rats. Physiol. Behav. 2006;88:523–528. doi: 10.1016/j.physbeh.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Mir TS, Laux R, Hellwege HH, et al. Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: marked and rapid increase after birth. Pediatrics. 2003;112:896–899. doi: 10.1542/peds.112.4.896. [DOI] [PubMed] [Google Scholar]
- Morselli E, Fuente-Martin E, Finan B, Kim M, Frank A, Garcia-Caceres C, et al. Hypothalamic PGC-1α protects against high-fat diet exposure by regulating ERα. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.09.025. (Epub before press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S1, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, et al. Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor alpha (ERalpha): a potential role for estrogen receptor beta (ERbeta) Horm. Metab. Res. 2002;34:758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- Nookaew I, Svensson PA, Jacobson P, et al. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. J. Clin. Endocrinol. Metab. 2013;98:E370–E378. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer BF, Clegg DJ. Oxygen sensing and metabolic homeostasis. Mol. Cell. Endocrinol. 2014:S303–S7207. doi: 10.1016/j.mce.2014.08.001. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- Ramirez ME, McMurry MP, Wiebke GA, Felten KJ, Ren K, Meikle AW, et al. Evidence for sex steroid inhibition of lipoprotein lipase in men: comparison of abdominal and femoral adipose tissue. Metabolism. 1997;46:179–185. doi: 10.1016/s0026-0495(97)90299-7. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Enk L, Crona N, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J. Clin. Invest. 1985;75:1973–1976. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelsen B. Increased alpha 2- but similar beta-adrenergic receptor activities in subcutaneous gluteal adipocytes from females compared with males. Eur. J. Clin. Invest. 1986;16:302–309. doi: 10.1111/j.1365-2362.1986.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Richelsen B, Pedersen SB, Moller-Pedersen T, Bak JF. Regional differences in triglyceride breakdown in human adipose tissue: effects of catecholamines, insulin, and prostaglandin E2. Metabolism. 1991;40:990–996. doi: 10.1016/0026-0495(91)90078-b. [DOI] [PubMed] [Google Scholar]
- Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R, et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann. Intern. Med. 2000;133:92–103. doi: 10.7326/0003-4819-133-2-200007180-00008. [DOI] [PubMed] [Google Scholar]
- Shen L, Wang DQ, Lo CM, Tso P, Davidson WS, Woods SC, et al. Estradiol increases the anorectic effect of central apolipoprotein A-IV. Endocrinology. 2010;151:3163–3168. doi: 10.1210/en.2010-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014;371:1131–1141. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- Sohlstrom A, Forsum E. Changes in adipose tissue volume and distribution during reproduction in Swedish women as assessed by magnetic resonance imaging. Am. J. Clin. Nutr. 1995;61:287–295. doi: 10.1093/ajcn/61.2.287. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J. Neurosci. 2002;22:2650–2659. doi: 10.1523/JNEUROSCI.22-07-02650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbins RE, Holcomb VB, Hong J, Nunez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012;51:861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- Tanko LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Central and peripheral fat mass have contrasting effect on the progression of aortic calcification in postmenopausal women. Eur. Heart J. 2003;24:1531–1537. doi: 10.1016/s0195-668x(03)00319-1. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol. Behav. 1971;7:847–852. doi: 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Koutsari C, Votruba SB, Tchkonia T, Giorgadze N, Thomou T, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010;18:1875–1880. doi: 10.1038/oby.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne A, Lonnqvist F, Apelman J, Hellers G, Arner P. A pilot study of long-term effects of a novel obesity treatment: omentectomy in connection with adjustable gastric banding. J. Intern. Assoc. Stud. Obes. 2002;26:193–199. doi: 10.1038/sj.ijo.0801871. [DOI] [PubMed] [Google Scholar]
- Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. American journal of physiology. Endocrinol. Metab. 2002;282:E1023–E1028. doi: 10.1152/ajpendo.00467.2001. [DOI] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013;19:1338–1344. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am. J. Cardiol. 2002;90:254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- Weber RV, Buckley MC, Fried SK, Kral JG. Subcutaneous lipectomy causes a metabolic syndrome in hamsters. Am. J. Physiol. Regul. Integr. Compar. Physiol. 2000;279:R936–R943. doi: 10.1152/ajpregu.2000.279.3.R936. [DOI] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Yoshimura M, Yasue H, et al. Plasma concentration of atrial natriuretic peptide and brain natriuretic peptide during normal human pregnancy and the postpartum period. J. Endocrinol. 1994;140:393–397. doi: 10.1677/joe.0.1400393. [DOI] [PubMed] [Google Scholar]
- Zhu Z, Liu X, Senthil Kumar SP, Zhang J, Shi H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm. Behav. 2013;63:533–542. doi: 10.1016/j.yhbeh.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]