Abstract
Vesicular stomatitis virus (VSV) shows promise as vaccine-vector and oncolytic virus. However, reports of neurotoxicity of VSV remain a concern. We compared 12 antiviral compounds to control infection of VSV-CT9-M51 and VSV-rp30 using murine and human brain cultures, and in vivo mouse models. Inhibition of replication, cytotoxicity and infectivity was strongest with ribavirin and IFN-α and to some extent with mycophenolic acid, chloroquine, and adenine 9-β-D-arabinofuranoside. To generate continuous IFN exposure, we made an adeno-associated virus vector expressing murine IFN; AAV-mIFN-β protected mouse brain cells from VSV, as did a combination of ribavirin and chloroquine. Intracranial AAV-mIFN-β protected the brain against VSV-CT9-M51. In SCID mice bearing human glioblastoma, AAV-mIFN-β moderately enhanced survival. VSV-CT9-M51 doubled median survival when administered after AAV-mIFN-β; some surviving mice showed complete tumor destruction. Together, these data suggest that AAV-IFN or IFN with ribavirin and chloroquine provide an optimal anti-virus combination against VSV in the brain.
Keywords: oncolytic virus, VSV, antiviral, brain, interferon, ribavirin, AAV
INTRODUCTION
The brain is a unique organ with respect to virus infection. The blood brain barrier restricts entry of many viruses; on the other hand, CNS neurons do not replicate, may not express MHC proteins, and antibodies and cells of the immune system may operate in restricted manners within the brain (Paul et al., 2007). The brain can show enhanced sensitivity to both viruses and antiviral drugs. Virus or drug-induced apoptosis may be much more detrimental in the brain than in other organs due to the general lack of neuron replenishment in most regions of the adult brain (Bechmann, 2005).
Vesicular stomatitis virus (VSV) has emerged as a promising candidate in two fields, as a broad-spectrum oncolytic virus and as a potent vaccine vector (Barber, 2004; Geisbert and Feldmann, 2011; Lichty et al., 2004; McKenna et al., 2003; Roberts et al., 1999; Rose et al., 2001). VSV combines a number of favorable characteristics for future potential therapeutic application, including broad cell and tissue tropism, absence of nuclear integration with risk of cell transformation, a small and well-studied genome accessible for genetic engineering, a largely non-pathogenic nature in humans, optional rapid or inactive replication cycle, and low pre-existing immunity in most of the population (Hastie and Grdzelishvili, 2012). VSV has shown efficacy in animal models of glioblastoma, with an ability to achieve selective infection within a brain tumor even after intravenous inoculation (Ozduman et al., 2008). However, potential neurotoxicity particularly after intranasal or intracranial application (Johnson et al., 2007; van den Pol, Dalton, and Rose, 2002) remains a potential complication. Application of VSV as an oncolytic agent outside the brain is currently being tested in a clinical trial (NCT 01628640) for liver cancer using an attenuated recombinant VSV-IFN-β designed to express interferon to boost an antiviral defense in normal cells.
VSV is a negative-strand RNA virus of the Rhabdovirus family primarily infecting cattle, horses, and other livestock and their corresponding insect vectors. It causes mostly mild disease with flu-like symptoms and blistering stomatitis. The viral 11 kb genome contains 5 genes, which encode 5 viral proteins: N,P,M,G, and L (Lyles and Rupprecht, 2007). Several attenuation strategies have been pursued in recent years resulting in a number of VSV variants with decreased neurotoxicity. For instance, VSV-M51 mutants reduce the virus’ ability to counter cellular antiviral responses (Stojdl et al., 2003); truncated G protein variants such as VSV-CT9 (Publicover, Ramsburg, and Rose, 2004) result in slower replication cycles as do genome shifting variants VSV-1′GFP or VSV-12′GFP (van den Pol and Davis, 2012; Wollmann et al., 2010) and genome shuffled variants (Clarke et al., 2007; Flanagan et al., 2001); variants pseudotyped with LCMV G protein in place of VSV G protein show reduced tropism to neurons (Muik et al., 2011), and G-protein deleted variants show effectiveness as a single round agent without consecutive spread (Publicover, Ramsburg, and Rose, 2005; van den Pol et al., 2009). The combination of two replication restricted gene-deleted VSVs to a semi-replication competent system reduced neurotoxicity (Muik et al., 2012). Additionally, a VSV engineered to express interferon, VSV-IFN, showed increased safety in preclinical models after peripheral application, although neurotoxicity was still possible when the virus gained access to the CNS (Yarde et al., 2013).
The generation and use of some attenuated VSV variants is based on the view that VSV infection can be attenuated by an innate immune response before the systemic immune response, generally in the form of neutralizing antibodies, clears the virus infection. A central mediator of the initial antiviral response to VSV is interferon (IFN) (Detje et al., 2009). The ability of brain cells to mount an IFN response differs in some aspects from cells outside the brain. For instance, neurons are responsive to IFN signaling (Wang and Campbell, 2005) but their contribution to local IFN production varies with several factors (Delhaye et al., 2006; Kallfass et al., 2012; Yin et al., 2009). Adaptive immunity can operate within the brain as well: we have shown that peripheral immunization effectively protects from VSV neurotoxicity after intracranial inoculation (Ozduman et al., 2009). This pre-treatment immunization strategy, however, may limit the efficacy of some therapeutic VSV applications, thus there is a continued interest in how VSV neurotoxicity could be abrogated in unvaccinated/VSV-naïve hosts. Therefore, we thought to address the question whether antiviral compounds could play a role in aiding existing viral attenuation strategies to prevent or limit the extent of VSV neurotoxicity.
We addressed the use of antiviral drugs both in vitro and in vivo regarding their potential to inhibit VSV infection and replication and VSV-associated morbidity. The 12 compounds tested were chosen based on their previously reported antiviral effects on peripheral or CNS infection of VSV or related viruses: IFN (Detje et al., 2009; Wollmann, Robek, and van den Pol, 2007), ribavirin (Toltzis and Huang, 1986; Willoughby et al., 2005), octyl gallate (Yamasaki et al., 2007), mycophenolic acid (MPA) (Ye et al., 2012), dansylcadaverine (Schlegel et al., 1982), rimantadine (Kolocouris et al., 1996), amantadine (Schlegel et al., 1982; Superti et al., 1985; Willoughby et al., 2005), adenine 9-β-D-arabinofuranoside (Ara-a) (Grant and Sabina, 1972), chloroquine (Dille and Johnson, 1982), acetylsalicylic acid (aspirin) (Chen, Warner, and Reiss, 2000), adenosine (Schnitzlein and Reichmann, 1980), and S-Nitroso-N-acetylpenicillamine (SNAP) (Bi and Reiss, 1995). These drugs act by a variety of mechanisms including interfering with RNA metabolism and replication via nucleoside analogues (ribavirin, Ara-A) or non-nucleoside inhibitors (mycophenolic acid), delaying of viral replication (octyl gallate), inhibition of virus internalization and uncoating (dansylcadaverine, amantadine, rimantadine), G-protein processing (chloroquine), and nitric oxide supply (SNAP). We tested these compounds for their in vitro protection of human and mouse brain cell cultures against two recombinant VSVs, the attenuated VSV-CT9-M51 (Wollmann et al., 2010) and the tumor-adapted variant VSV-rp30 (Wollmann et al., 2013) as well as its parent strain, VSV-G/GFP. In addition, the in vivo efficacy in protecting mouse brain from viral neurotoxicity was examined. Finally, we generated an IFN-expressing AAV vector for enhanced mouse IFN gene expression in mouse brain cells that gave effective protection from neurotoxicity of intracranial injections of VSV and also extended survival in VSV-CT9-M51-treated brain tumor mouse models.
MATERIALS AND METHODS
Cell culture
Primary cultures from adult human brain tissue were established from temporal lobectomy specimens removed for epilepsy solely for the benefit of the patient as previously described (Wollmann, Robek, and van den Pol, 2007). The use was approved by the Yale University Human Investigators Committee. Normal embryonic human astrocytes were obtained from Sciencell (San Diego, CA). Cells were confirmed as astrocytes using GFAP immunostaining as previously described (Ozduman et al., 2008). Murine brain cells were cultured from the brains of E18-P0 Swiss Webster mice as described previously (Gao and van den Pol, 2001). Glia cultures were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum and kept in a humidified atmosphere containing 5% CO2 at 37°C. Murine neuron-enriched cultures were maintained in neurobasal medium supplemented with B27 (Gibco/Invitrogen); this medium enhances neuronal survival and depresses glial cell division.
Drugs
Adenosine, amantadine, ara-A, aspirin, dansylcadaverine, human IFN-αA/D, mycophenolic acid, ribavirin, rimantadine and SNAP were obtained from Sigma-Aldrich (St. Louis, MO). Chloroquine was obtained from MP Biomedicals (Solon, OH). All drugs were diluted either in PBS, Ethanol or DMSO according to manufacturers’ specifications or, if unavailable, previously published methods.
VSVs
Recombinant VSV strain VSV-CT9-M51 was generated by Dr. J. Rose (Yale University, New Haven, CT) as previously described (Wollmann et al., 2010), VSV-rp30 was derived from recombinant VSV-G/GFP (Dalton and Rose, 2001) and generated by repetitive adaptive passage on human U87 glioblastoma cells (Wollmann, Tattersall, and van den Pol, 2005).
rAAVs
The adeno-associated virus AAV-2 based vector expressing mouse IFN was generated as follows: The pscAAV-CMV-tdTomato construct, used to generate AAV-tdTomato virus, was assembled by blunting the 1.96 kb SnaBI-AflII fragment of pCMV-tdTomato (Clontech) and cloning this into 4.2 Kb SnaBI-digested and phosphatased fragment of pscAAV-CMV-eGFP (a gift of Dr. R. Clark, Nationwide Children’s Hospital, Columbus, OH). The resulting construct contains the CMV promoter in front of the tdTomato ORF, and this gene cassette lies between the two AAV2-derived inverted terminal repeats (ITRs), one of which is mutated to allow each virion to package a self-complimentary (palindromic) 5 kb single-stranded DNA genome, allowing more efficient transduction (Gray et al., 2011). The pscAAV-CMV-mIFN-β construct, used to generate AAV-mIFN-β virus, was generated by cloning the 780 bp SgrAI-MfeI fragment of pORF-mIFN-β (InvivoGen. San Diego, CA) into the 4.8 kb AgeI-MfeI fragment of pscAAV-CMV-eGFP. The resulting construct encodes a self-complementary AAV in which the CMV ie1 promoter drives mIFN-β expression. Plasmid constructs were sent to the Viral Vector Core Laboratory at Nationwide Children’s Hospital in Columbus, OH for large-scale AAV vector recovery, purification, quality control testing, and accurate titration of DNAse-resistant particles (drp) per ml. Constructs were packaged in the AAV2 capsid.
Analysis of viral infection and cytopathic effects
Cultures of normal human adult astroglia were plated overnight in 24-well dishes at a density of 50,000 cells per well. Mouse neuronal cultures were plated at a density of 5–10 ×105 cells per well in a 12-well dish and given 5–7 days for maturation. Medium was replaced with drug containing solution and cultures were incubated for 6 hrs. 5 × 104 pfu VSV (5 × 105 for neuronal cultures) was added for a final concentration of MOI 1. Viral infection was analyzed based on ratio of cells expressing viral GFP reporter 48 hours post inoculation using an Olympus IX 71 fluorescence microscope. Cytopathic effects were monitored using phase contrast mode. A microscope mounted Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI) was used for image capturing. Photomicrographs were optimized for contrast and color using Adobe Photoshop.
MTT-Assay
Cell viability was assessed using the Vybrant MTT-Assay kits supplied by Invitrogen (Carlsbad, CA). Human astrocytes were plated in 96-well dishes at a density of 10,000 cells per dish in quadruplicates for each experimental condition. After incubation overnight, medium was exchanged with phenol-red free MEM containing the appropriate drug concentration. Virus was added 6 hours later at an MOI of 0.1. MTT Compound A was added at 48 h, followed by Compound B at 52 h post infection following manufacturer’s instruction. Optical density measurements were performed 12 h later at 570 nm using a Dynatech MR500 plate reader (Dynatech Lab Inc, Alexandria, VA).
Propagation Assay and Plaque-Assay
Human astrocytes were infected at an MOI 0.1 with VSV-rp30. Supernatants were harvested at 24 and 48 h after infection and frozen at −80°C until further analysis. Serial dilutions of these samples were plated on BHK-21 cells (ATCC, Manassas, VA) followed by a 0.5% agar overlay and fluorescent plaques were assessed 24 hours later.
AAV-mIFN-β VSV protection assay
25,000 murine glial cells per well were seeded in 24-well plates. Medium was exchanged to medium containing either AAV-tdTomato at 5,000 genomes (measured as DNAse resistant particles (drp))/cell, AAV-mIFN-β at 5,000 or 500 genomes/cell, or IFN-αA/D at 10 U/ml. 5 days later, 50% of medium in all wells was exchanged for fresh medium. After 4 additional days, plain growth medium in one set of wells was exchanged for medium containing IFN-αA/D at 10 U/ml. The next day, wells were infected with VSV-G/GFP at MOI of 5. 1 day, 2 days, and 3 days post-infection with VSV-G/GFP, triplicate wells were assessed for infection and cytopathic effects using the microscope and camera described above.
Immunocytochemistry for NeuN
20 hours after VSV infection (MOI 1) of mouse neuronal cultures, cells were fixed with 4% paraformaldehyde. After permeabilization with 0.4% Triton-X, cells were immunostained for murine neuron-specific nuclear protein NeuN using primary antibody MAB377 (Chemicon, Temecula, CA) diluted 1:200, followed by secondary staining with Alexa Fluor 568 anti-mouse IgG1 (Life Technologies, Grand Island, NY) diluted 1:200. Analysis of six microscopic fields for each condition allowed identification of red fluorescent NeuN-positive cells, each of which were then scored as GFP positive or negative.
Animal procedures
Swiss-Webster mice were used for experiments involving immunocompetent mice and CB17 SCID mice were used for xenograft brain tumor models. All mice were obtained from Taconic Farms Inc., Germantown, NY. Intracranial injections and postoperative animal care was conducted in accordance with the guidelines of the Yale University Animal Care and Use Committee. Mice were anesthetized using a combination of ketamine and xylazine (100 and 10 mg/kg, respectively) applied intraperitoneally. All intracranial injections were performed on the left side, 1.5 mm lateral and 2.0 mm rostral of the bregma at 2.0 mm depth using microsyringes from Hamilton (Reno, NV) controlled by a stereotactic injector from Stoelting Co (Wood Dale, IL). For testing efficacy of intracranially applied antiviral drugs, 2.5 μl of solution containing IFN-αA/D (1000 IU), ribavirin (7 mg/kg body weight), and chloroquine (30 mM) were injected over a duration of 2.5 minutes and the needle was kept in place for an additional 15 minutes before removal. Afterward, VSV-CT9-M51 was injected using the same coordinates at a concentration of 1.5 × 104 PFU per 250μl inoculum. For testing efficacy of repetitive intraperitoneal applications of antiviral compounds mice received an injection of 0.5 ml isotonic solution containing IFN-αA/D (2500 IU), ribavirin (40 mg/kg) (Jeulin et al., 2006), and chloroquine (50 mg/kg) every 48 hours for a total of 4 injections starting at the time of intracranial VSV-CT9-M51 application as detailed above. For testing efficacy of AAV-mIFN-β, 2 μl of a stock solution containing 1013 genomes per ml were stereotactically injected at the coordinates listed above. Control mice received injection of 2μl PBS. 14 days later, mice received stereotactic injection of 250μl of VSV-CT9-M51 containing 1.5 × 104 PFU per inoculum at the same striatal location. For treatment of an orthotopic brain tumor xenograft model human glioblastoma cells stably expressing red fluorescent reporter gene dsRed rU87 were used (Ozduman et al., 2008). Tumors were established by striatal injection of 1 μl of cell suspension containing 2 × 104 cells. 15 minutes before tumor cell injection, mice received 2 μl of either AAV-mIFN-β or PBS in the same location. 14 days later, all mice received a single intravenous bolus of 100 μl containing 3 × 107 PFU of VSV-CT9-M51. Mice were monitored daily for body weight, food and water consumption, motility, and presence of any neurological symptoms. Mice showing serious neurological dysfunction or dropping below 75% of starting body weight were given an anesthetic overdose and perfused transcardially with 4% paraformaldehyde.
Imaging
Serial sections of mouse brain were cut using a cryotome, mounted with DAPI-containing embedding medium, and analyzed for fluorescence signals using the same microscope and camera as above.
Statistics
Unless otherwise specified, data are expressed as mean and standard error of the mean. Statistical analysis was performed using t-test for pairwise comparison, or ANOVA for more than two groups, respectively, using Kaleidagraph (Synergy software, Reading, PA). Graph Pad Prism 6 (GraphPad software, San Diego, CA) was used for Kaplan-Meier survival curves and for log-rank test to analyze different groups for significance.
RESULTS
Here we compare 12 drugs and compounds for their potential to control the infection of VSV in human brain cultures and in mouse brain. The 12 compounds were selected based on previously reported antiviral properties against VSV, against related mononegaviridae members, or against other RNA viruses. 11 compounds were selected for comparison with interferon IFN-αA/D, a common agent used to control VSV infection: ribavirin, octyl gallate (OG), mycophenolic acid (MPA), dansylcadaverine (DC), rimantadine hydrochloride, amantadine hydrochloride, adenine 9-β-D-arabinofuranoside (Ara-A), chloroquine diphosphate, acetylsalicylic acid (ASA), adenosine, and S-nitroso-N-acetylpenicillamine (SNAP). Later, we also test a viral vector that expresses interferon. Both mouse and human brain cells are used in conjunction with recombinant VSVs.
Antiviral compound toxicity and dose response
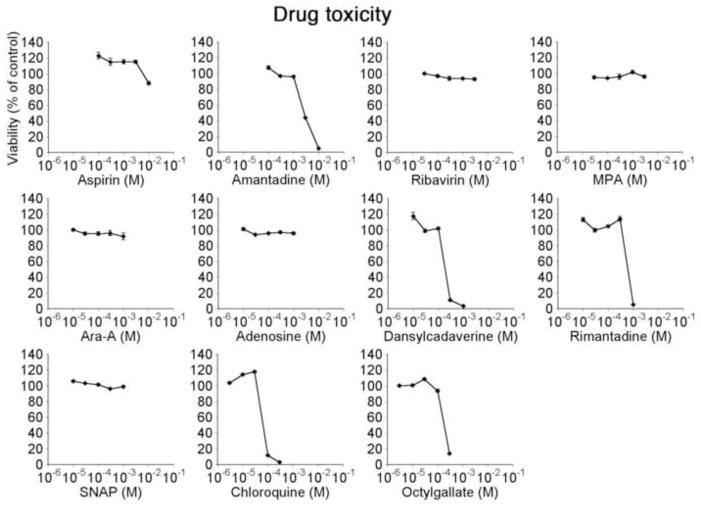
To establish dose limits and adverse drug effects on human brain cultures, we tested each drug at 5 drug concentrations in half log dilution steps. Concentration ranges were set based on factors including maximum solubility, maximum acceptable solvent concentration, and previously reported doses. Brain cultures consist of a number of cell types, with astrocytes being the most common. Using the MTT assay, we identified the thresholds of toxicity in these cultures after 48 hours of incubation (Figure 1). Of the 11 drugs tested, MPA, ribavirin, Ara-A, adenosine, and SNAP did not generate cellular toxicity throughout the concentration range tested. ASA showed a small decrease in viability at the highest concentration tested (10 mM). Amantadine, DC, rimantadine, chloroquine, and OG were toxic to the cells in the highest one or two concentrations. From these results, compound concentrations were selected to combine with viral inoculation. ‘High’ drug concentration was defined as the highest non-toxic dose; ‘low’ drug concentration was 1 log lower (equivalent to 2 steps down the tested concentration curve). In addition, IFN has been used in our lab in concentrations well above 1000 IU per ml without adverse effect in vitro. Here, we chose 100 IU per ml as a high dose and 10 IU per ml as a low drug concentration. We also tested IFN concentrations of 1 IU per ml independently to assess its potency on human glia at a concentration that is defined to reduce infection of VSV-permissive cells by 50% (Pestka, 1986). Table 1 shows each drug with its corresponding high and low dose.
Figure 1. Dose range and toxicity studies.
11 compounds were tested for in vitro toxicity on human astrocyte monolayers using MTT viability assay. Dose ranges consisted of 5 dosage steps diluted in half-log increments, starting from either maximum solubility concentration or previously reported toxic values. For subsequent experiments, high drug concentration is defined as the highest non-toxic concentration, low drug concentration as a 10-fold dilution of the respective high concentration (equivalent to 2 dilution steps from dose range curves shown here).
Table 1. List of antiviral compounds and concentrations used.
High concentration was defined as the highest non-toxic dose tested based on dose/viability studies in Figure 1. Low concentration represents the value two dilution steps below respective high concentration. A possible mechanism of action for each compound is included; additional mechanisms may also exist.
| Compound | Concentrations | Mechanism |
|---|---|---|
|
| ||
| Adenosine | 1mM & 100μM | Inhibition of pyrimidine synthesis (Schnitzlein and Reichmann, 1980; Stollar and Malinoski, 1981) |
| Amantadine | 1mM & 100μM | Inhibition of endocytosis (Schlegel et al., 1982; Superti et al., 1985) |
| Ara-A | 1mM & 100μM | Inhibition of RNA synthesis (Grant and Sabina, 1972) |
| Aspirin | 3mM & 300μM | Promotion of NO synthesis (Chen et al., 2000) |
| Chloroquine | 30μM & 3μM | Inhibition of endosomal escape (Coombs et al., 1981; Dille and Johnson, 1982; Pérez and Carrasco, 1994) |
| Dansylcadaverine | 100μM & 10μM | Inhibition of endocytosis (Schlegel et al., 1982) |
| Interferon | 100U/mL & 10U/mL | Induction of antiviral genes (Detje et al., 2009; Wollmann et al., 2007) |
| Mycophenolic acid | 300μM & 30μM | Depletion of GTP (Ye et al., 2012) |
| Octyl-gallate | 100μM & 10μM | Unknown. Inhibits viral replication. (Uozaki et al., 2007; Yamasaki et al., 2007) |
| Ribavirin | 3mM & 300μM | Pleiotropic effects. Guanosine analog. (Toltzis and Huang, 1986; Hong and Cameron, 2002; Shah et al., 2010) |
| Rimantadine | 300μM & 30μM | Inhibition of endocytosis (Kolocouris et al., 1996; Mato et al., 1983; Schlegel et al., 1982) |
| SNAP | 1mM & 100μM | Organic NO donor (Bi and Reiss, 1995) |
Abbreviations: Ara-A, adenine 9-β-D-arabinofuranoside; SNAP S-Nitroso-N-acetylpenicillamine; NO, Nitric Oxide.
Antiviral effects of drug preincubation on human brain cultures
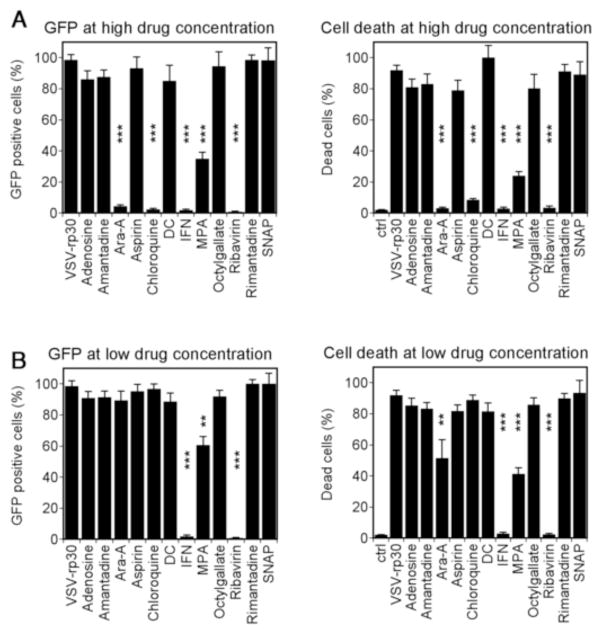
Normal human adult brain cultures at 80% confluency were treated with antiviral drug compounds 6 hours prior to addition of VSV-rp30 at an MOI of 1 PFU/cell. 48 hours post infection cultures were analyzed for viral infection and cytopathic effects using phase contrast and fluorescent microscope imaging. Figure 2A shows VSV-rp30 infection rates and infection induced cell toxicity in the presence of high drug concentration, Figure 2B shows results for low drug concentration. In high drug conditions, Ara-A, chloroquine, and ribavirin provided protection from infection and cell death comparable to 100 IU of IFN-α. High concentrations of MPA provided significant but incomplete protection from VSV infection (60–70%). With low drug concentrations, ribavirin and IFN showed protection comparable to that seen in high drug conditions, whereas MPA showed less but still significant protection when compared to high drug concentration. When we tested pre-incubation with as little as 1 IU of IFN, we still found about a 50% protection from infection (56 ± 9.6% GFP positive cells). Chloroquine and Ara-A had little protective effect in low drug conditions. Adenosine, amantidine, ASA, DC, OG, rimantadine, and SNAP showed little or nor protection from VSV-rp30 infection at high or low doses.
Figure 2. Effect of antiviral compounds on VSV infectivity and cytotoxicity on normal human glia cultures.
Primary cultures of normal adult human glia tissue were infected with VSV-rp30 at an MOI of 1 after 6 hr preincubation with 12 antiviral compounds at high (A) or low (B) concentration. Infectivity was assessed through virus-mediated GFP expression (left graphs), cytotoxicity through cellular cytopathic effects (rounding, blebbing) (right graphs). *** indicates p<0.001, ** indicates p<0.01 significance (n=10; ANOVA with Bonferroni Post-Hoc Analysis).
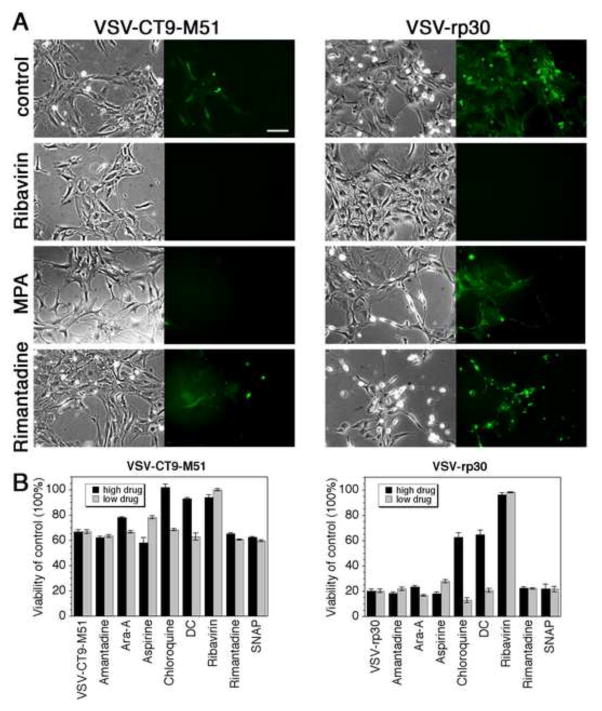
Representative micrographs of human brain cultures treated 6 h in advance with the respective high concentrations of highly protective drug (ribavirin), a moderately effective drug (MPA) and a non-effective drug (rimantadine) and infected with VSV-rp30 for 48 hours are displayed in Figure 3A. In addition to VSV-rp30, we also tested a double attenuated viral mutant VSV-CT-M51 for GFP-reported infectivity rates and virus induced cytopathic effects (representative figures shown in Figure 3A, left panels). Infectivity rates were generally lower with VSV-CT9-M51 than for tumor-adapted VSV-rp30. As with VSV-rp30, ribavirin and IFN provided complete protection at both low and high drug concentration for VSVCT9-M51.
Figure 3. Antiviral effects of compounds on attenuated VSV-CT9-M51 and tumor-adapted VSV-rp30.
A) Representative fluorescence and phase contrast photomicrographs of primary adult human glia cultures infected with VSV-CT9-M51 (left panel) or VSV-rp30 (right panel). Ribavirin preincubation completely protected the cultures from viral infection. MPA protected from VSV-CT9-M51 infection with little effect on VSV-rp30 infection. Rimantadine had no protective effect. Scale bar 100 μm. B) Graphs displaying viability rates of normal embryonic human astrocytes infected with VSV-CT9-M51 (left) or VSV-rp30 (right) at MOI 0.1 using MTT viability after incubation with compounds at low or high drug concentration respectively.
Embryonic astrocytes may be more susceptible to viral infection compared to adult astrocytes in part because the IFN system appears to mature with cell differentiation (Harada et al., 1990). In parallel experiments we tested a selection of antiviral drugs for their inhibitory effects on infection of normal human embryonic astrocytes by VSV-CT9-M51 and VSV-rp30. We used an MTT assay to assess viability of confluent astrocyte cultures infected at an MOI of 0.1 after 48 hpi. Figure 3B summarizes triplicate tests run with 8 drugs at both high and low concentration. Similar to primary cultures from adult human glia shown above, ribavirin again provided significant protection even at a low dose, and chloroquine was again protective only at a high dose. Dansylcadaverine (DC) was protective (at a high dose). Ara-A and amantadine were ineffective (even at a high dose). Similar findings were observed for both VSV-rp30 and VSV-CT9-M51.
Antiviral drug effects on human glia cultures post viral inoculation
The set of experiments above focused on the screening of 12 agents for antiviral effects to protect human brain cells against infection with VSV-CT9-M51 and VSV-rp30. In those experiments drugs were applied 6 hrs before virus was added. To test the effect of the three most effective drugs, IFN, ribavirin, and MPA, to interfere with an ongoing infection, we infected normal adult human glial cells with VSV-rp30 at an MOI of 0.1 and added drugs in their respective high concentrations at 0, 2, 4, and 6 hours post virus inoculation (Figure 4). Cell viability was assessed using the MTT assay at 48 hpi. IFN was effective in completely protecting viability of the cultures when added up to 4 hrs post virus inoculation, with significant protection from VSV even after adding at 6 hpi. Though not complete, ribavirin also provided significant protection with over 80% viability when added up to 6 hours after virus application. In contrast, whereas MPA showed a protective effect (>50% viability) when co-administered with the virus, its efficacy decreased substantially when added 2 hpi, and had almost no effect at later time points.
Figure 4. Time course of antiviral effects of compounds added after virus inoculation of normal human glia cultures.
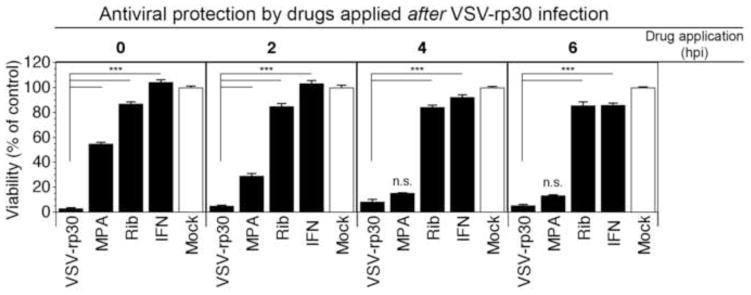
To test the potential of antiviral compounds to attenuate an ongoing infection of normal adult human glia cultures with VSV-rp30, drugs were applied at indicated time points after viral inoculation (MOI 0.1). Results are shown as viability rates compared to non-infected control using MTT assay. Both interferon (IFN) and Ribavirin (Rib) strongly attenuate VSV infection when added up to 6 hpi. MPA shows some protection only if given at the same time or shortly after VSV-rp30 application. *** indicates p<0.001 significance; n.s. = not significant (n=4; ANOVA with Bonferroni Post-Hoc Analysis).
Antiviral drug effects on virus replication
In addition to infection rates and viability, we tested the effect of 5 antiviral drugs on viral replication rates using a standard plaque assay. Human astrocyte cultures were treated with respective high and low drug concentrations 6 hrs before addition of VSV-rp30 at an MOI of 0.1. Supernatants were collected at 24 and 48 hpi, respectively, serially diluted, and plated onto a BHK cell monolayer. Untreated, viral replication reached 3.65 × 106 and 4.0 × 106 PFU/ml for 24 hpi and 48 hpi, respectively (Figure 5). Dansylcadaverine had little effect on viral replication in both its low and high concentration and at both time points. Adenosine was minimally effective at low concentrations but showed a mildly attenuating effect on viral replication at high concentration by reducing the titer 4-fold at 24 hpi and 10-fold at 48 hpi. MPA showed a strong reduction of viral titer by nearly three orders of magnitude in both concentrations at 24 hpi. Interestingly, MPA had a far more modest effect on viral titers at 48 hours, with titers reaching 5–10 × 105 PFU/ml, suggesting an effect that is transient and overcome by the virus after two days. Both IFN (not shown) and ribavirin showed strong and lasting suppression of VSV replication in both conditions with viral titers in the range of 1–8 × 103 PFU/ml, similar to the range of initially applied VSV.
Figure 5. Suppression of VSV-rp30 replication in normal embryonic human astrocytes by selected antiviral compounds.
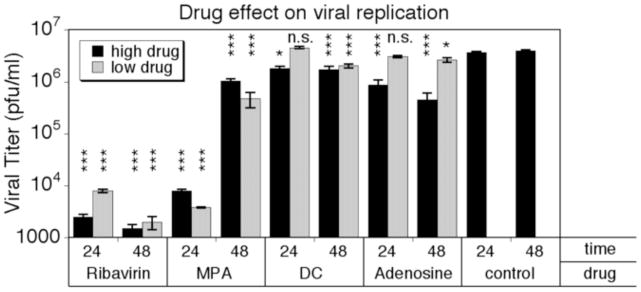
The effect of selected antiviral compounds on VSV-rp30 replication rates on normal embryonic human astrocytes was tested using standard plaque assay. Ribavirin incubation strongly inhibited viral replication. MPA effectively halted viral replication during the first 24 hrs, but had less impact on replication after 48 hrs. Adenosine mildly attenuated viral replication at high concentration only. *** indicates p<0.001 significance; * = p<0.05; n.s. = not significant (n=4; ANOVA with Bonferroni Post-Hoc Analysis).
AAV-IFN vector protects mouse glia and neurons from VSV
Given the efficacy of IFN at protecting human glia and neurons from VSV, and the potential utility of AAV as an ongoing IFN delivery vector, we asked whether a viral vector that expressed IFN could protect mouse glia and neurons from VSV infection. We generated AAV-mIFN-β, an AAV2-based self-complimentary vector in which murine IFN-β expression is driven by a CMV ie1 promoter. To serve as a control for any effects of AAV-vector infection on subsequent VSV infection, we also constructed self-complimentary AAV-tdTomato in which the same promoter drives expression of the red fluorescent reporter tdTomato.
First, murine glial cultures were treated with AAV and incubated for 10 days to allow transgene expression. By 3 days post-inoculation with VSV-G/GFP, virus had infected and killed most control glial brain cells (Fig. 6A–C, No Pretreatment). Control cells not infected by VSV-G/GFP grew and replicated normally, as seen in Fig. 6B and in the top panel of Fig. 6C. Cells pretreated with high MOI AAV-mIFN-β (5,000 genomes/cell) were only very minimally affected by VSV-infection; cell density 3 dpi did not differ from that of uninfected control cells (Fig. 6B), and the percentage of cells infected never exceeded 4%, and was less than 1% at 3 dpi (Fig. 6A). Low MOI AAV-mIFN-β (500 genomes/cell) had a weaker but still highly protective effect: cell density 3 dpi was 80% of that of uninfected cells, and the percentage of cells infected was 3.1%. Under phase contrast, AAV-mIFN-β-protected cell monolayers appeared healthy and indistinguishable from untreated monolayers (Fig 6C).
Figure 6. AAV-mIFN-β protects mouse glia from VSV infection.
After each pretreatment, as described in the text, mouse glia cultures were infected with VSV-G/GFP and assessed daily for density of living cells (B) and percentage of cells infected (GFP+, shown here as percentage of the value for the control group that received no antiviral pretreatment, 3dpi) (A). Micrographs taken 3 dpi are shown in C. Error bars, SEM.
The control AAV vector not expressing IFN, AAV-tdTomato, showed no efficacy in protection from VSV; by 3 dpi after VSV inoculation, the majority of cells (>90%) were detached or showed significant cytopathic effects (CPE) (Fig. 6B), and most of the cells were GFP-positive corroborating viral infection (Fig. 6A). IFN-βA/D was effective at protecting glia when added 1 day before VSV-infection, showing a degree of protection similar to AAV-mIFN, however when added 10 days before VSV infection, the same concentration of IFN only moderately protected cells, which decreased to less than 30% of their original density 3dpi, showed marked cytopathic effects, and were 20% infected by VSV-G/GFP. Together, these findings support the view that a single application of AAV-mIFN-β protected brain cells from VSV-G/GFP, and that this protection was substantially more effective and longer lasting than a single application of exogenous IFN.
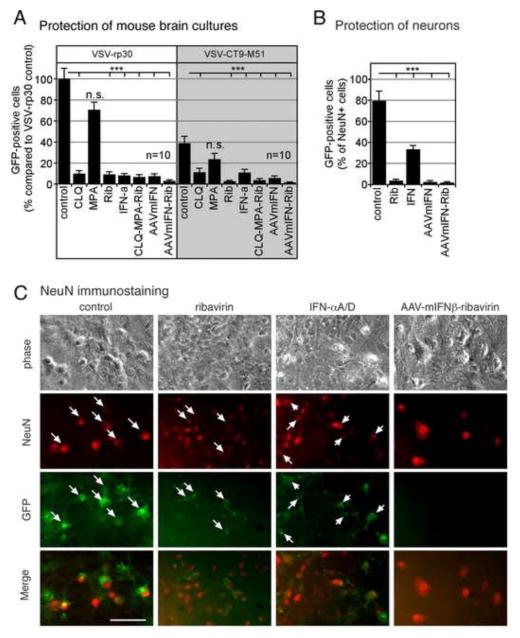
Mouse neuron cultures grown in Neurobasal medium that favors neuronal survival over glia were pretreated with selected antiviral compounds for 6 hours; cultures treated with AAV-mIFN-β were incubated for 10 days with the vector to allow sufficient time for gene expression. Treatment with chloroquine (CLQ; 30μM), ribavirin (Rib; 300 μM), and IFN-αA/D (100 IU/ml) increased resistance of neuronal cultures to infection of VSV-rp30 and VSV-CT9-M51, respectively, at an MOI of 1 (Figure 7A). In contrast, pretreatment with MPA (300 μM) showed little effect on VSV infection. A triple drug combination (CLQ-MPA-Rib) was comparable to ribavirin alone. Cultures pretreated with AAV-IFN-β (5,000 genomes per cell) for 10 days showed robust resistance to VSV infection, which could be enhanced to near full protection with consecutive 6 hour pretreatment with ribavirin (300 μM). Thus a combination of IFN, particularly based on AAV-IFN, together with ribavirin appeared to be the most effective combination in blocking VSV infection of neurons.
Figure 7. Effect of antiviral compounds and AAV-mIFN-β on infection of neuronal cultures.
(A) Mouse brain neuron cultures were pretreated for 6 hrs with either chloroquine (CLQ; 30 μM), MPA (300 μM), ribavirin (Rib; 300 μM), or IFN (100 IU/ml) and combinations thereof (CLQ-MPA-Rib). Cultures pretreated with AAV-mIFN-β were incubated for 10 days with a vector concentration of 5,000 genomes per cell. Cultures were infected with an MOI of 1 of VSV-rp30 or VSV-CT9-M51, respectively. At 36 hpi, infected cells were counted via fluorescence microscopy. Bars indicate analysis of ten microscopic fields, error bars expressed as SEM. (B) Neuronal marker NeuN was used to identify and quantify infection rates in neurons. The percentage of mouse neurons (NeuN+ cells) in murine brain cultures infected with VSV was determined 20hpi for unprotected cells, and for cells protected by IFN-αA/D (100 IU/ml), ribavirin (Rib; 300 μM), AAV-mIFN-β (5,000 genomes per cell), or AAV-mIFN-β + ribavirin. Error Bars, SEM. (C) Image panel of representative micrographs showing corresponding phase contrast, NeuN immunostaining, and GFP images. Merged images show co-localization of VSV infection on neurons (arrows) in control conditions (no pretreatment) and at much reduced rates in the treated cultures. *** indicates p<0.001 significance; n.s. = not significant (n=10; ANOVA with Bonferroni Post-Hoc Analysis). Scale bar 50 μm.
In order to assess the effect of antiviral drugs specifically on identified neurons we used immunostainng for the selective neuronal marker NeuN which is expressed only by neurons. Mouse brain cultures enriched with neurons were infected with VSV at an MOI 1 either in untreated control condition or after pretreatment with AAV-mIFN-β (5,000 genomes/cell for 10 days), ribavirin (300 μM for 6 hours), universal IFN-αA/D (100 IU/ml for 6 hours), or AAV-mIFN-β combined with ribavirin. 20 hours post-infection with VSV-G/GFP, cells were fixed and immunostained for NeuN (Fig 7C). Only NeuN-positive cells (neurons) were scored for GFP positivity, indicating VSV-infection. Results are shown in Fig 7B. 80% of untreated control neurons were infected with VSV under these conditions; in contrast, only 3.6% of ribavirin protected cells were infected. Similarly, VSV infected only 2.5% of neurons protected by AAVmIFN-β, and only 1.8% of neurons protected by a combination of AAVmIFN-β and ribavirin. Short-term IFN-exposed neurons were partially protected with 33% showing infection.
Antiviral compounds and AAV-mIFN-β protect brain from intracranial VSV-CT9-M51 infection
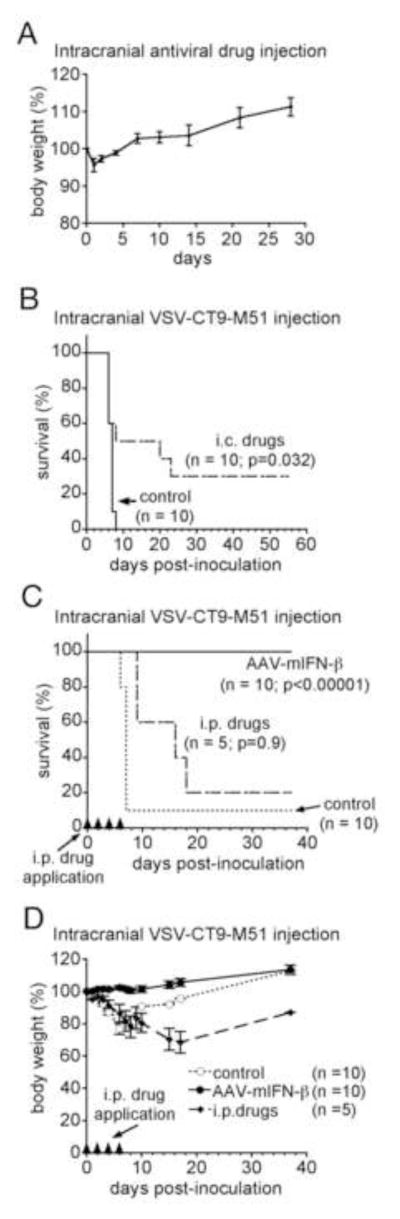
We next tested the efficacy of VSV-protective compounds and AAV-mIFN-β in vivo, specifically their ability to protect animals from the neurotoxicity of intracranial VSV-CT9-M51. In 10 adult mice, a mixture of three of the most effective anti-VSV compounds was injected into the striatum followed 15 minutes later by intracranial VSV-CT9-M51 challenge (1.5×104 pfu in 250 nl). The antiviral drug cocktail applied directly into the mouse brain was well tolerated in control experiments and evoked no adverse effects (n=5, Fig. 8A). 2.5 μl drug solution was injected containing IFN-αA/D (1000 IU) + ribavirin (7 mg/kg body weight) + chloroquine (30 mM); 10 control mice received PBS injection before being injected with VSV-CT9-M51; survival results are shown in Fig. 8B. The antiviral drug administration significantly extended survival of mice challenged with intracranial VSV from a median of 6 days in controls to 13 days in drug treated mice (p=0.032; Log-rank test). In controls, VSV was 100% lethal by 8 dpi; 3 of 10 treated mice (30%) survived long-term until the experiment was ended 55 dpi. Given the relative ease of systemic administration clinically, we next tested if repeated intraperitoneal (i.p.) drug administration could achieve a degree of protection comparable to that conferred by intracranial administration. 5 mice challenged i.c. with VSV-CT9-M51 (1.5×104 pfu in 250 nl) were treated i.p. with 500 μl of the drug mixture containing IFN-αA/D (2500 IU) + ribavirin (40 mg/kg) + chloroquine (50 mg/kg) on days 0, 2, 4, and 6 post infection; survival results are shown in Fig. 8C. 1 of 5 treated mice (20%) survived long term, not statistically different from the 30% long-term survival seen after intracranial drug administration (p=0.9; Log-rank test). 1 of 10 control mice (10%) survived long term in this experiment. Although the rate of long-term survival was greater in drug-treated mice, this was not statistically significant in this experiment (p>0.05; Log-rank test); however, the median survival was noticeably extended in drug-treated mice to 15 days compared to 6 days in non-treated mice.
Figure 8. AAV-mIFN-β protects from VSV-CT9-M51 neurotoxicity in vivo.
Direct injection of antiviral drugs into the brain of mice (n=5) resulted in no observable toxicity or mortality (28 days observation) (A). Intracranial injection of VSV-CT9-M51 resulted in mortality within 10 days; simultaneous intracranial (i.c.) application of a mix of antiviral compounds that showed efficacy in vitro improved survival (B). Multiple intraperitoneal (i.p.) injections of the same compound mix increased time to death but did not impact long-term survival (C, dashed line). In contrast, intracranial injection of AAV-mIFN 10 days prior to intracranial VSV-CT9-M51 challenge provides full protection from mortality (C, full line). Body weight chart illustrates stable weight development in mice protected with AAV-mIFN compared to control and i.p. drug receiving mice (only survivor weights included) (D). Black triangles on X-axis indicate time points for i.p. drug application. Error bars, SEM.
In this same experiment, we also tested the ability of intracranial AAV-mIFN-β to protect mice from i.c. VSV-CT9-M51 when AAV is administered 14 days prior to VSV challenge, giving transduced cells time to express the mIFN-β transgene. 2 μl (containing 2 × 1010 genomes) of AAV-mIFN-β were injected into the striatum of 10 adult mice. 14 days later, these mice were given the same intracranial VSV-CT9-M51 challenge as the other groups; survival data in Fig. 8C show that all ten of the AAV-treated mice survived long-term until the experiment was concluded 40 days after VSV inoculation. This complete protection stood in clear contrast to the near 100% lethality of VSV in unprotected animals (p<0.0001). No neurological symptoms or obvious signs of distress were noted in mice having received AAV-mIFN-β. In addition, as shown in Fig. 8D, AAV-protected mice had no weight loss following VSV challenge, another indication of VSV neurotoxicity being greatly diminished; both i.p. drug-treated animals and control animals demonstrated significant weight loss immediately after VSV injection.
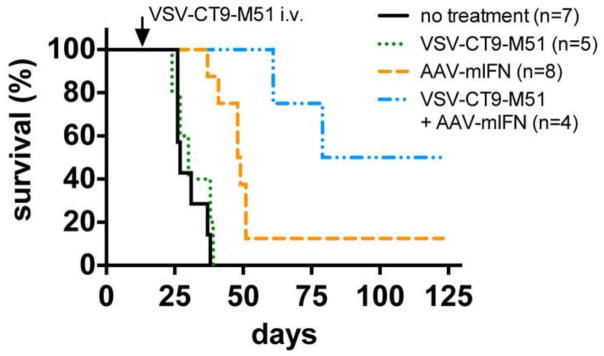
Survival of glioma-bearing mice treated with systemic VSV-CT9-M51 is enhanced by intracranial AAV-mIFN-β
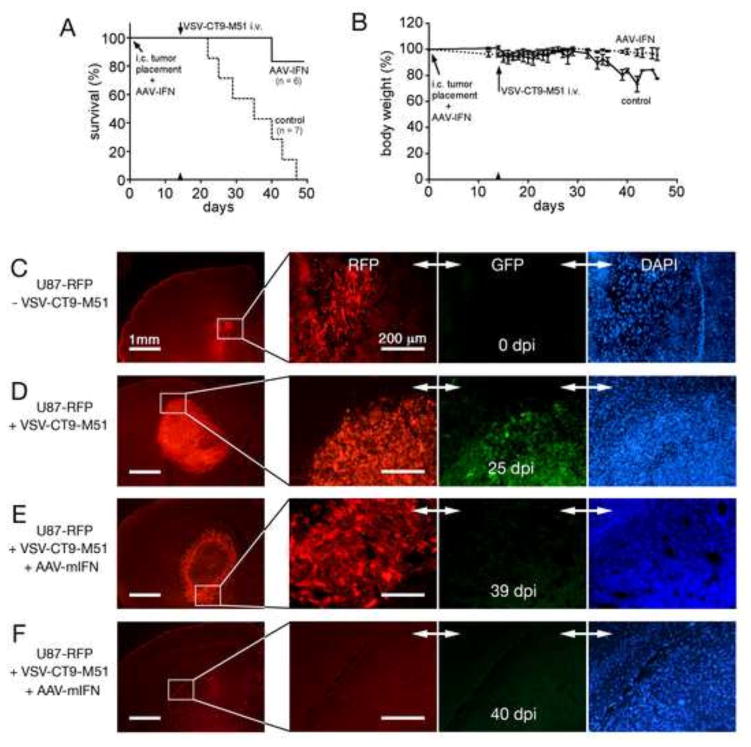
Orthotopic human gliomas in SCID mice are infected selectively after systemic application of VSV (Ozduman et al., 2008). Although in short term experiments lasting a few days, VSV initially infects only the tumor and spreads selectively throughout the tumor, the concern remains that over a longer period virus may spread into normal tissue. Here we sought to mitigate this spread by pre-application of AAV-mIFN-β, creating a zone of VSV-resistance in the vicinity of the tumor. 15 mice received intracranial human glioblastoma rU87 expressing red fluorescent protein as a reporter molecule; eight were given 2μl containing 2 × 1010 drp of AAV-mIFN-β 15 minutes before tumor grafting at the same injection site; the remaining seven control mice received PBS only. 14 days later, two AAV-treated mice were euthanized with anesthetic overdose to corroborate successful tumor grafts (Fig 9C) and 13 mice were administered 3 × 107 pfu of VSV-CT9-M51 via tail vein injection; survival results are shown in Figure 9A. Mice showing substantial weight loss (>25% body weight reduction), or any neurological symptom including limb paralysis were euthanized, and for the sake of this analysis, were included in the adverse lethal response group.
Figure 9. AAV-mIFN-β increases survival in VSV-CT9-M51 treated human glioblastoma-bearing SCID mice.
Injection of AAV-mIFN-β or 3 μl of PBS (control) into the striatum was accompanied by placement of human U87 glioblastoma xenografts at the same site. 14 days later, all mice received VSV-CT9-M51 via tail vein. Tumor targeting and subsequent viral replication in the intracranial tumor xenograft result in progressive neurotoxicity and mortality in control mice (A, dashed line). In contrast, mice pretreated with AAV-mIFN-β showed significantly improved survival (A, solid line). There was a transient weight loss in AAV-mIFN-β pretreated mice compared to progressive weight loss in the control group (B). Representative histological mouse brain sections are shown for tumor graft control (C), tumor graft treated with systemic VSV-CT9- M51 at 25 dpi (D); tumor graft treated with intracranial AAV-mIFN-β and systemic VSV-CT9-M51 at 39 dpi (E), and site of previous tumor graft placement after intracranial AAV-mIFN and systemic VSV-CT9-M51 application 40 dpi (F).
Whereas mice not protected by AAV-mIFN-β began to die within 8 days of VSV-infection, no AAV-protected mice died until at least 26 days after VSV infection. Similarly, whereas all 7 unprotected mice died within 33 days (median survival 20 days), all but one of the 6 AAV-protected mice remained alive to the end of the experiment 40 days after VSV infection; thus a marked and statistically significant (p=0.002; Log-rank test) survival advantage was conferred by AAV-mIFN-β. Body weight measurements showed substantially less body weight loss in AAV-protected mice as well (P<0.001; paired t-test), beginning 5 days after VSV infection and continuing throughout the experiment as shown in Fig. 9B. At the time of death (7-33 dpi for the VSV-only group) or at the end of the experimental period at 40 dpi for AAV-mIFN-treated groups, brains of mice were harvested and analyzed for tumor expansion and signs of VSV infection. All 7 mice in the VSV-only group showed large solid tumors (ranging 2–4 mm widest diameter) with widespread VSV-CT9-M51 in the tumor (Fig 9D). In contrast, the group treated with AAV-mIFN-β before application of VSV-CT9-M51 and sacrificed at the end of the experimental period showed tumors in only two mice (2.2 and 2.5 mm diameter; Fig 9E); three mice had no trace of tumors (Fig 9F). One mouse died and its brain was not available for assessment. Assessing VSV infection using GFP expression, we found consistent VSV infection in all tumors in the control group, but found little to no VSV-associated GFP in mice treated with AAV-mIFN at the end of the observation period, suggesting AAV-mIFN can prevent neurotoxicity-associated VSV spread without impairing the oncolytic activity of VSV on the tumor tissue.
Previous reports indicate little cross-reactivity between human and mouse IFN (Berger et el,2011; Tedeschi et al, 2011) suggesting the mouse IFN used here is selective for mice and therefore may not exert a direct effect on the human tumor), an antitumor effect of AAV-mIFN-β in this experiment using human tumors could still be due to effects of IFN on the host cells, for instance on tumor vascularization or on local immune system activation. Therefore, the effect of AAV-mIFN-β on survival of U87 tumor bearing mice was assessed. Compared to untreated mice with U87 xenografts (n=7), mice treated with AAV-mIFN-β alone (n=8) showed a significant survival benefit (median survival 27 days vs 48 days, p=0.0006, Wilcoxon test), indicating an antitumor effect of AAV-mIFN-β on U87 xenografts (Fig 10). Mice not protected by AAV-mIFN-β, but treated with VSV-CT9-M51 (n=5) had no significant survival benefit over control animals. In contrast, in an additional group (n=4) of U87 bearing mice treated systemically with VSV-CT9-M51 after local CNS application of AAV-mIFN-β, median survival was increased substantially from 48 days to 101 days by the VSV-CT9-M51 application, a significant survival benefit over AAV-mIFN-β alone, (p<0.05, Wilcoxon test) (Fig 10). Together, these data indicate that treatment of VSV-CT9-M51 together with AAV-mIFN-β provides a potential therapeutic effect on mouse survival when bearing human glioblastoma xenografts.
Figure 10. Long term control experiment assessing the effect of AAV-mIFN-β on survival of U87 tumor bearing mice.
SCID mice were stereotactically injected with human U87 glioma cells into the right striatum. At the time of tumor placement, two groups received AAV-mIFN-β injection into the same brain location. Two groups received sham injection. 14 days later, VSV-CT9-M51 was injected via tail vein into one group each, AAV-mIFN-β pretreated and mock treated. Compared to untreated controls bearing tumors, AAV-mIFN-β treatment increased survival of tumor bearing mice. Survival was significantly improved with the additional treatment with VSV-CT9-M51 in mice already injected with AAV-mIFN-β, compared to tumor bearing mice treated only with AAV-mIFN-β.
DISCUSSION
The brain can be particularly sensitive to both virus infection and to antiviral drugs. In the present study we compared the potential of a series of agents to limit VSV-associated neurotoxicity. We selected compounds reported to display antiviral activity against rhabdoviruses (Bi and Reiss, 1995; Detje et al., 2009; Grant and Sabina, 1972; Schlegel et al., 1982; Schnitzlein and Reichmann, 1980; Toltzis and Huang, 1986; Yamasaki et al., 2007) or against other RNA viruses (Kolocouris et al., 1996; Ye et al., 2012). Of 12 drugs tested in vitro, IFN and ribavirin were most effective at blocking viral infection, replication, and at abrogating cell death.
Chloroquine, MPA, and Ara-A showed moderate efficacy. Aspirin, adenosine, amantadine, rimantadine, SNAP, and octylgallate showed relatively little efficacy. Whereas IFN and ribavirin were highly effective in all tests, we found the ability of the moderately protective drugs to inhibit VSV varied as a function of dose, timing of drug administration, brain cell type, and/or VSV fitness; these studies serve to characterize these complex relationships between drug, virus, and cell. The most effective compounds in vitro showed a protective effect in vivo as well. Finally, we generated a potentially useful vector, AAV expressing IFN, which was highly effective in protecting brain from VSV-mediated toxicity in vitro and in vivo, and allowing a strong survival advantage for tumor-bearing SCID mice treated with oncolytic VSV.
Antiviral agents reduce VSV neurotoxicity
Ribavirin exerts its antiviral effects by multiple mechanisms, including inhibition of viral RNA synthesis and mutagenesis of viral RNA genomes; the different mechanisms may vary in their importance depending on the virus and the cell type (Hong and Cameron, 2002). Given that we see a near 100% suppression of the VSV GFP reporter gene expression by low dose ribavirin even for non-attenuated VSV-rp30 at 1 pfu/cell (Fig. 2A, B), as well as an abrogation of viral replication (Fig. 5), we conclude that ribavirin has potent anti-VSV activity that operates early in the viral replication cycle. Ribavirin efficacy against VSV can vary depending on cell type (Shah, Sunderland, and Grdzelishvili, 2010). Thus our finding of efficacy in human brain cells is significant and supports ribavirin’s possible utility in enhancing the safety of therapeutic VSV applications in the brain. An additional benefit of ribavirin/IFN is that VSV did not develop mutants resistant to co-treatment of IFN and ribavirin as long as therapeutic levels of the drugs were maintained (Cuevas et al., 2005).
Besides IFN and ribavirin, several other compounds showed a degree of efficacy in different contexts. Pre-administration of Ara-A and chloroquine to human brain cultures were nearly 100% protective against VSV-rp30 at high dose, but showed little to no effect at low dose. These results suggest that although these drugs can have anti VSV activity, they both require a dose close to their toxic concentration, thereby limiting their clinical efficacy. MPA showed only partial efficacy in protecting human glial cultures from tumor-adapted VSV-rp30, but was effective at low dose in protecting from attenuated VSV-CT9-M51. MPA had only a transient effect on VSV replication, blocking progeny formation at 24 but not 48hpi in human astrocytes.
Prolonged IFN activity via vector delivery significantly reduces VSV neurotoxicity
Type 1 IFNs upregulate a large cohort of cellular antiviral genes and play a key role both peripherally and in the CNS in attenuating VSV infection (Fensterl et al., 2012; Gresser, Tovey, and Bourali-Maury, 1975). IFN may be considered as one standard for VSV control. IFN action is generally restricted to the species of origin (Maher et al., 2007). In order to test the efficacy of the same type 1 IFN on both human and mouse brain cultures we used a recombinant IFN-αA/D that acts on both mouse and human IFN receptors with comparable potency (Sigma I-4410 product sheet). The efficacy of IFN for protection of brain against VSV operated in a variety of contexts, thereby supporting its potential utility for controlling VSV infection in the brain. Because peripherally administered IFN may not readily cross the blood brain barrier, alternative methods of administration, for example intranasally, have been explored (Ross et al., 2004).
Because IFN injections may have a short half-life, we generated a recombinant AAV-based vector expressing mouse IFN-β; a single application of this vector resulted in sustained protection of mouse brain cultures from VSV infection for days and weeks. With some reports indicating that IFN falls below detection threshold in the serum 24 hours after injection (Maher et al., 2007) the continuous delivery of type 1 IFN via AAV vectors may itself serve as an antitumor gene therapy agent (He et al., 2009; Meijer et al., 2009; Streck et al., 2006) or as an antiviral agent in experimental models of hepatitis virus infection (Berraondo et al., 2005). The potential for gene-delivered IFN expression to control or prevent infection of the brain has not been addressed before. The AAV-mIFN-β mediated protection from direct intracranial VSV was substantially stronger and longer lasting than direct IFN use. All mice receiving AAV-mIFN-β and intracranial VSV survived, compared to only 20% in both i.c. and i.p. drug application settings, respectively. The VSV-IFN-β currently in clinical trial for liver cancer has been reported to display neurotoxicity in cases in which it had gained access to the CNS via infection of meningeal metastases in a rat model of multiple myeloma (Yarde et al., 2013). In contrast, intracerebral application of AAV-mIFN-β provided complete protection from VSV-CT9-M51. IFN may act at some distance from its site of release within the CNS (van den Pol et al, 2014), suggestion potential long distance actions within the brain after local synthesis mediated by the AAV vector. Although beyond the scope of our present study, this raises the question if AAV-mIFN-β might also have potential for other rhabdovirus infections affecting the brain, for instance Chandipura or the VSV-related rabies virus. Although a case of successful rabies treatment has been reported (Willoughby et al., 2005), the majority of cases and fulminant rabies are still considered untreatable (Jackson, 2009). In such a scenario, the application of a safe vector expressing IFN in the brain might hold some promise.
Alternatively, in the field of oncology an IFN expressing vector might offer the benefit of strengthening the non-tumor tissue front around the tumor mass. Humans undergoing cancer therapy often maintain an immune-suppressed state and additional safety measures need to be considered before oncolytic viruses can be applied, particularly with CNS tumors. Such a scenario was mimicked in our in vivo glioblastoma xenograft model employing immune compromised SCID mice. We have previously shown successful targeting, infection, and destruction of intracranial human brain tumors implanted in the brain of mice via systemically applied VSV-rp30 or VSV-CT9-M51, respectively (Ozduman et al., 2009; Ozduman et al., 2008). But so far the use of SCID mice limited the use of these models for survival studies, as the systemic immune system is required for VSV clearance from brain (Bi et al., 1995; Ozduman et al., 2009). Our finding that glioblastoma-bearing SCID mice treated with systemically applied VSV-CT9-M51 survived longer when co-treated with AAV-mIFN-β compared to control mice indicates that topically and continuously released IFN has the potential to protect the brain even from a wave of new viral progeny being produced and released from infected and lysed tumor cells. IFN might also act as an antitumor agent (Yoshida, Mizuno, and Wakabayashi, 2004). Human IFN-expressing AAV suppressed the growth of human brain tumor cells (Meijer et al., 2009). Because IFN receptor activation is selective for its species-specific IFN (Tedeschi et al, 1986; Berger et al, 2011), the mouse IFN in our study would not be expected to exert a direct action on the human tumor graft. However, our control experiments with AAV-mIFN-β treatment of human glioblastoma xenograft mouse models revealed an increase in survival compared to tumor bearing mice not treated with AAV-IFN-β. This could be due to effects of mouse IFN on vascularization, or some indirect effect on local innate immunity. AAV expressing human IFN-β can significantly inhibit human tumor xenografts in the periphery (Streck et al. 2006) and in the brain of immunocompromised mice (Maguire 2008, Meijer 2009). In these studies with human IFN-β, the causes of tumor inhibition were not delineated; however, an inhibition of angiogenesis was observed, and was postulated to result from either direct inhibition of the tumor’s ability to promote angiogenesis, or from a hypothetical cross-species effect on the murine vasculature at high concentrations of IFN. In the AAV vector used here, IFN was constitutively expressed under control of a cytomegalovirus ie1 promoter. In a clinical context, as long term IFN exposure may exert side effects within the brain, the use of an inducible promoter driving AAV- IFN expression may merit consideration.
To our knowledge, a post infection effect of IFN on VSV infected brain cultures had not been reported before. In vivo, application of IFN to mice 4 days after intranasal inoculation with VSV resulted in a survival benefit (Gresser, Tovey, and Bourali-Maury, 1975). Our data indicate that IFN applied after initiation of VSV infection not only limits virus spread to other cells but might have a protective effect even on cells already infected. In light of the plethora of cellular responses upon IFN signaling, including inhibition of VSV replication cycle at early and late stages (Stark et al., 1998), a protective effect of IFN after initiation of infection is conceivable. Ribavirin, which has direct antiviral properties as a nucleoside analogue disrupting viral replication was also effective in protecting cultures up to 6 hours after VSV application, confirming the view that ribavirin suppresses VSV progeny production (Toltzis and Huang, 1986).
In summary, we found that selected antiviral compounds can protect glia and neuronal cultures from VSV mediated cell death. IFN’s protection of brain cells from VSV can be substantially enhanced by prolonged IFN presence via AAV vector-delivered transgene. A combination of viral vector expressed IFN together with ribavirin gave the highest level of anti-virus protection to the brain.
Research Highlights.
A panel of compounds was compared for blocking toxicity and spread of VSV in brain.
VSV block in mouse and human brain cells in vitro and mouse brain in vivo was studied
AAV vector expressing IFN-β blocked VSV in vitro and in vivo.
The strongest antiviral actions were seen with ribavirin and AAV-mIFN-β.
AAV-mIFNβ prolonged survival in tumor-bearing mice treated with attenuated VSV.
Acknowledgments
We thank Yang Yang and Vitaliy Rogulin for technical facilitation and John N. Davis for helpful suggestions on the manuscript. Support provided by NIH CA124737, CA161048, and CA175577.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barber GN. Vesicular stomatitis virus as an oncolytic vector. Viral Immunol. 2004;17(4):516–27. doi: 10.1089/vim.2004.17.516. [DOI] [PubMed] [Google Scholar]
- Bechmann I. Failed central nervous system regeneration: a downside of immune privilege? Neuromolecular Med. 2005;7(3):217–28. doi: 10.1385/NMM:7:3:217. [DOI] [PubMed] [Google Scholar]
- Bekisz J, Schmeisser H, Hernandez J, Goldman ND, Zoon KC. Human interferons alpha, beta and omega. Growth Factors. 2004;22(4):243–51. doi: 10.1080/08977190400000833. [DOI] [PubMed] [Google Scholar]
- Berger Rentsch M, Zimmer G. A vesicular stomatitis virus replicon-based bioassay for the rapid and sensitive determination of multi-species type I interferon. PLoS ONE. 2011;6:e25858. doi: 10.1371/journal.pone.0025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berraondo P, Ochoa L, Crettaz J, Rotellar F, Vales A, Martinez-Anso E, Zaratiegui M, Ruiz J, Gonzalez-Aseguinolaza G, Prieto J. IFN-alpha gene therapy for woodchuck hepatitis with adeno-associated virus: differences in duration of gene expression and antiviral activity using intraportal or intramuscular routes. Mol Ther. 2005;12(1):68–76. doi: 10.1016/j.ymthe.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69(10):6466–72. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69(4):2208–13. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Warner JL, Reiss CS. NSAID treatment suppresses VSV propagation in mouse CNS. Virology. 2000;276(1):44–51. doi: 10.1006/viro.2000.0562. [DOI] [PubMed] [Google Scholar]
- Clarke DK, Nasar F, Lee M, Johnson JE, Wright K, Calderon P, Guo M, Natuk R, Cooper D, Hendry RM, Udem SA. Synergistic attenuation of vesicular stomatitis virus by combination of specific G gene truncations and N gene translocations. J Virol. 2007;81(4):2056–64. doi: 10.1128/JVI.01911-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K, Mann E, Edwards J, Brown DT. Effects of chloroquine and cytochalasin B on the infection of cells by Sindbis virus and vesicular stomatitis virus. J Virol. 1981;37:1060–1065. doi: 10.1128/jvi.37.3.1060-1065.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas JM, Sanjuan R, Moya A, Elena SF. Mode of selection and experimental evolution of antiviral drugs resistance in vesicular stomatitis virus. Infect Genet Evol. 2005;5(1):55–65. doi: 10.1016/j.meegid.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Dalton KP, Rose JK. Vesicular stomatitis virus glycoprotein containing the entire green fluorescent protein on its cytoplasmic domain is incorporated efficiently into virus particles. Virology. 2001;279(2):414–21. doi: 10.1006/viro.2000.0736. [DOI] [PubMed] [Google Scholar]
- Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103(20):7835–40. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detje CN, Meyer T, Schmidt H, Kreuz D, Rose JK, Bechmann I, Prinz M, Kalinke U. Local type I IFN receptor signaling protects against virus spread within the central nervous system. J Immunol. 2009;182(4):2297–304. doi: 10.4049/jimmunol.0800596. [DOI] [PubMed] [Google Scholar]
- Dille BJ, Johnson TC. Inhibition of vesicular stomatitis virus glycoprotein expression by chloroquine. J Gen Virol. 1982;62 (Pt 1):91–103. doi: 10.1099/0022-1317-62-1-91. [DOI] [PubMed] [Google Scholar]
- Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, Bergmann CC, Diamond MS, Virgin HW, Sen GC. Interferon-induced Ifit2/ISG54 protects mice from lethal VSV neuropathogenesis. PLoS Pathog. 2012;8(5):e1002712. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan EB, Zamparo JM, Ball LA, Rodriguez LL, Wertz GW. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J Virol. 2001;75(13):6107–14. doi: 10.1128/JVI.75.13.6107-6114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533(Pt 1):237–52. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Feldmann H. Recombinant vesicular stomatitis virus-based vaccines against Ebola and Marburg virus infections. J Infect Dis. 2011;204(Suppl 3):S1075–81. doi: 10.1093/infdis/jir349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JA, Sabina LR. Inhibition of vesicular stomatitis virus replication by 9-beta-D-arabinofuranosyladenine. Antimicrob Agents Chemother. 1972;2(3):201–5. doi: 10.1128/aac.2.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SJ, Foti SB, Schwartz JW, Bachaboina L, Taylor-Blake B, Coleman J, Ehlers MD, Zylka MJ, McCown TJ, Samulski RJ. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Gene Ther. 2011;22(9):1143–53. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I, Tovey MG, Bourali-Maury C. Efficacy of exogenous interferon treatment initiated after onset of multiplication of vesicular stomatitis virus in the brains of mice. J Gen Virol. 1975;27(3):395–8. doi: 10.1099/0022-1317-27-3-395. [DOI] [PubMed] [Google Scholar]
- Harada H, Willison K, Sakakibara J, Miyamoto M, Fujita T, Taniguchi T. Absence of the type I IFN system in EC cells: transcriptional activator (IRF-1) and repressor (IRF-2) genes are developmentally regulated. Cell. 1990;63(2):303–12. doi: 10.1016/0092-8674(90)90163-9. [DOI] [PubMed] [Google Scholar]
- Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93(Pt 12):2529–45. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LF, Wang YG, Xiao T, Zhang KJ, Li GC, Gu JF, Chu L, Tang WH, Tan WS, Liu XY. Suppression of cancer growth in mice by adeno-associated virus vector-mediated IFN-beta expression driven by hTERT promoter. Cancer Lett. 2009;286(2):196–205. doi: 10.1016/j.canlet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Hong Z, Cameron CE. Pleiotropic mechanisms of ribavirin antiviral activities. Prog Drug Res. 2002;59:41–69. doi: 10.1007/978-3-0348-8171-5_2. [DOI] [PubMed] [Google Scholar]
- Jackson AC. Therapy of rabies encephalitis. Biomedica. 2009;29(2):169–76. [PubMed] [Google Scholar]
- Jeulin H, Grancher N, Kedzierewicz F, Le Faou AE, Venard V. Evaluation by Q-RTPCR of the efficacy of ribavirin complexed with beta-cyclodextrin against measles virus in a mouse encephalitis model. Pathol Biol (Paris) 2006;54(10):541–4. doi: 10.1016/j.patbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Nasar F, Coleman JW, Price RE, Javadian A, Draper K, Lee M, Reilly PA, Clarke DK, Hendry RM, Udem SA. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360(1):36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallfass C, Ackerman A, Lienenklaus S, Weiss S, Heimrich B, Staeheli P. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J Virol. 2012;86(20):11223–30. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolocouris N, Kolocouris A, Foscolos GB, Fytas G, Neyts J, Padalko E, Balzarini J, Snoeck R, Andrei G, De Clercq E. Synthesis and antiviral activity evaluation of some new aminoadamantane derivatives. 2. J Med Chem. 1996;39(17):3307–18. doi: 10.1021/jm950891z. [DOI] [PubMed] [Google Scholar]
- Lichty BD, Power AT, Stojdl DF, Bell JC. Vesicular stomatitis virus: re-inventing the bullet. Trends Mol Med. 2004;10(5):210–6. doi: 10.1016/j.molmed.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lyles DS, Rupprecht CE. Rhabdoviridae. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Lippincott, Williams and Wilkins; Philadelphia: 2007. [Google Scholar]
- Maguire CA, Meijer DH, LeRoy SG, Tierney LA, Broekman MLD, Costa FF, Breakefield XO, Stemmer-Rachamimov A, Sena-Esteves M. Preventing growth of brain tumors by creating a zone of resistance. Mol Ther. 2008;16:1695–1702. doi: 10.1038/mt.2008.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher SG, Romero-Weaver AL, Scarzello AJ, Gamero AM. Interferon: cellular executioner or white knight? Curr Med Chem. 2007;14(12):1279–89. doi: 10.2174/092986707780597907. [DOI] [PubMed] [Google Scholar]
- Mato JM, Pencev D, Vasanthakumar G, Schiffmann E, Pastan I. Inhibitors of endocytosis perturb phospholipid metabolism in rabbit neutrophils and other cells. Proc Natl Acad Sci USA. 1983;80:1929–1932. doi: 10.1073/pnas.80.7.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna PM, McGettigan JP, Pomerantz RJ, Dietzschold B, Schnell MJ. Recombinant rhabdoviruses as potential vaccines for HIV-1 and other diseases. Curr HIV Res. 2003;1(2):229–37. doi: 10.2174/1570162033485320. [DOI] [PubMed] [Google Scholar]
- Meijer DH, Maguire CA, LeRoy SG, Sena-Esteves M. Controlling brain tumor growth by intraventricular administration of an AAV vector encoding IFN-beta. Cancer Gene Ther. 2009;16(8):664–71. doi: 10.1038/cgt.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A, Kneiske I, Werbizki M, Wilflingseder D, Giroglou T, Ebert O, Kraft A, Dietrich U, Zimmer G, Momma S, von Laer D. Pseudotyping vesicular stomatitis virus with lymphocytic choriomeningitis virus glycoproteins enhances infectivity for glioma cells and minimizes neurotropism. J Virol. 2011;85(11):5679–84. doi: 10.1128/JVI.02511-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muik A, Dold C, Geiß Y, Volk A, Werbizki M, Dietrich U, von Laer D. Semireplication-competent vesicular stomatitis virus as a novel platform for oncolytic virotherapy. J Mol Med. 2012;90(8):959–70. doi: 10.1007/s00109-012-0863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozduman K, Wollmann G, Ahmadi SA, van den Pol AN. Peripheral immunization blocks lethal actions of vesicular stomatitis virus within the brain. J Virol. 2009;83(22):11540–9. doi: 10.1128/JVI.02558-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozduman K, Wollmann G, Piepmeier JM, van den Pol AN. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J Neurosci. 2008;28(8):1882–93. doi: 10.1523/JNEUROSCI.4905-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89(6–7):770–8. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pérez L, Carrasco L. Involvement of the vacuolar H(+)-ATPase in animal virus entry. J Gen Virol. 1994;75 (Pt 10):2595–2606. doi: 10.1099/0022-1317-75-10-2595. [DOI] [PubMed] [Google Scholar]
- Pestka S. Interferon standards and general abbreviations. Methods Enzymol. 1986;119:14–23. doi: 10.1016/0076-6879(86)19004-5. [DOI] [PubMed] [Google Scholar]
- Publicover J, Ramsburg E, Rose JK. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J Virol. 2004;78(17):9317–24. doi: 10.1128/JVI.78.17.9317-9324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Publicover J, Ramsburg E, Rose JK. A single-cycle vaccine vector based on vesicular stomatitis virus can induce immune responses comparable to those generated by a replication-competent vector. J Virol. 2005;79(21):13231–8. doi: 10.1128/JVI.79.21.13231-13238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73(5):3723–32. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, Montefiori D, Roberts A, Buonocore L, Rose JK. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–49. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH., 2nd Intranasal administration of interferon beta bypasses the blood-brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151(1–2):66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Schlegel R, Dickson RB, Willingham MC, Pastan IH. Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin. Proc Natl Acad Sci U S A. 1982;79(7):2291–5. doi: 10.1073/pnas.79.7.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzlein WM, Reichmann ME. Inhibition of vesicular stomatitis virus replication by adenosine. Virology. 1980;103(1):123–37. doi: 10.1016/0042-6822(80)90131-2. [DOI] [PubMed] [Google Scholar]
- Shah NR, Sunderland A, Grdzelishvili VZ. Cell type mediated resistance of vesicular stomatitis virus and Sendai virus to ribavirin. PLoS One. 2010;5(6):e11265. doi: 10.1371/journal.pone.0011265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, Marius R, Reynard J, Poliquin L, Atkins H, Brown EG, Durbin RK, Durbin JE, Hiscott J, Bell JC. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Stollar V, Malinoski F. The effects of adenosine and guanosine on the replication of Sindbis and vesicular stomatitis viruses in Aedes albopictus cells. Virology. 1981;115:57–66. doi: 10.1016/0042-6822(81)90088-x. [DOI] [PubMed] [Google Scholar]
- Streck CJ, Dickson PV, Ng CY, Zhou J, Hall MM, Gray JT, Nathwani AC, Davidoff AM. Antitumor efficacy of AAV-mediated systemic delivery of interferon-beta. Cancer Gene Ther. 2006;13(1):99–106. doi: 10.1038/sj.cgt.7700878. [DOI] [PubMed] [Google Scholar]
- Superti F, Seganti L, Pana A, Orsi N. Effect of amantadine on rhabdovirus infection. Drugs Exp Clin Res. 1985;11(1):69–74. [PubMed] [Google Scholar]
- Tedeschi B, Barrett JN, Keane RW. Astrocytes produce interferon that enhances the expression of H-2 antigens on a subpopulation of brain cells. J Cell Biol. 1986;102(6):2244–53. doi: 10.1083/jcb.102.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toltzis P, Huang AS. Effect of ribavirin on macromolecular synthesis in vesicular stomatitis virus-infected cells. Antimicrob Agents Chemother. 1986;29(6):1010–6. doi: 10.1128/aac.29.6.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uozaki M, Yamasaki H, Katsuyama Y, Higuchi M, Higuti T, Koyama AH. Antiviral effect of octyl gallate against DNA and RNA viruses. Antiviral Res. 2007;73:85–91. doi: 10.1016/j.antiviral.2006.07.010. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Dalton KP, Rose JK. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J Virol. 2002;76(3):1309–27. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Davis JN. Highly-attenuated recombinant vesicular stomatitis virus VSV-12′ GFP displays immunogenic and oncolytic activity. J Virol. 2012 doi: 10.1128/JVI.01106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Ozduman K, Wollmann G, Ho WS, Simon I, Yao Y, Rose JK, Ghosh P. Viral strategies for studying the brain, including a replication-restricted self-amplifying delta-G vesicular stomatis virus that rapidly expresses transgenes in brain and can generate a multicolor golgi-like expression. J Comp Neurol. 2009;516(6):456–81. doi: 10.1002/cne.22131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Ding S, Robek MD. Long distance interferon signaling within the brain blocks virus spread. J Virol. 2014;88:3695–3704. doi: 10.1128/JVI.03509-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Campbell IL. Innate STAT1-dependent genomic response of neurons to the antiviral cytokine alpha interferon. J Virol. 2005;79(13):8295–302. doi: 10.1128/JVI.79.13.8295-8302.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby RE, Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352(24):2508–14. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- Wollmann G, Davis JN, Bosenberg MW, van den Pol AN. Vesicular stomatitis virus variants selectively infect and kill human melanoma but not normal melanocytes. J Virol. 2013 doi: 10.1128/JVI.03311-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Robek MD, van den Pol AN. Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells. J Virol. 2007;81(3):1479–91. doi: 10.1128/JVI.01861-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Rogulin V, Simon I, Rose JK, van den Pol AN. Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells. J Virol. 2010;84(3):1563–73. doi: 10.1128/JVI.02040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Tattersall P, van den Pol AN. Targeting human glioblastoma cells: comparison of nine viruses with oncolytic potential. J Virol. 2005;79(10):6005–22. doi: 10.1128/JVI.79.10.6005-6022.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Uozaki M, Katsuyama Y, Utsunomiya H, Arakawa T, Higuchi M, Higuti T, Koyama AH. Antiviral effect of octyl gallate against influenza and other RNA viruses. Int J Mol Med. 2007;19(4):685–8. [PubMed] [Google Scholar]
- Yarde DN, Naik S, Nace RA, Peng KW, Federspiel MJ, Russell SJ. Meningeal myeloma deposits adversely impact the therapeutic index of an oncolytic VSV. Cancer Gene Ther. 2013;20(11):616–621. doi: 10.1038/cgt.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Li J, Zhang T, Wang X, Wang Y, Zhou Y, Liu J, Parekh HK, Ho W. Mycophenolate mofetil inhibits hepatitis C virus replication in human hepatic cells. Virus Res. 2012;168(1–2):33–40. doi: 10.1016/j.virusres.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Gardner CL, Burke CW, Ryman KD, Klimstra WB. Similarities and differences in antagonism of neuron alpha/beta interferon responses by Venezuelan equine encephalitis and Sindbis alphaviruses. J Virol. 2009;83(19):10036–47. doi: 10.1128/JVI.01209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida J, Mizuno M, Wakabayashi T. Interferon-beta gene therapy for cancer: basic research to clinical application. Cancer Sci. 2004;95(11):858–65. doi: 10.1111/j.1349-7006.2004.tb02194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]