Abstract
The “Industrial Heartland” of Alberta is Canada’s largest hydrocarbon processing center, with more than 40 major chemical, petrochemical, and oil and gas facilities. Emissions from these industries affect local air quality and human health. This paper characterizes ambient levels of 77 volatile organic compounds (VOCs) in the region using high-precision measurements collected in summer 2010. Remarkably strong enhancements of 43 VOCs were detected, and concentrations in the industrial plumes were often similar to or even higher than levels measured in some of the world’s largest cities and industrial regions. For example maximum levels of propene and i-pentane exceeded 100 ppbv, and 1,3-butadiene, a known carcinogen, reached 27 ppbv. Major VOC sources included propene fractionation, diluent separation and bitumen processing. Emissions of the measured VOCs increased the hydroxyl radical reactivity (kOH), a measure of the potential to form downwind ozone, from 3.4 s−1 in background air to 62 s−1 in the most concentrated plumes. The plume value was comparable to polluted megacity values, and acetaldehyde, propene and 1,3-butadiene contributed over half of the plume kOH. Based on a 13-year record (1994–2006) at the county level, the incidence of male hematopoietic cancers (leukemia and non-Hodgkin lymphoma) was higher in communities closest to the Industrial Heartland compared to neighboring counties. While a causal association between these cancers and exposure to industrial emissions cannot be confirmed, this pattern and the elevated VOC levels warrant actions to reduce emissions of known carcinogens, including benzene and 1,3-butadiene.
Keywords: Volatile organic compounds, Emissions, Industrial Heartland, Alberta, Hematopoietic cancer
1. Introduction
Volatile organic compounds (VOCs) are emitted from natural biogenic sources such as vegetation and biomass burning, and from anthropogenic sources such as the production, distribution and consumption of fossil fuels, including vehicular emissions (Guenther et al., 2000; Buzcu and Fraser, 2006). VOCs play key roles in the radiative forcing and chemistry and of the atmosphere, for example producing tropospheric ozone (O3) and secondary organic aerosol (SOA) (Sillman, 1999; Robinson et al., 2007). VOCs also control concentrations of the hydroxyl radical (OH) (Guenther et al., 1995), the principal oxidizing agent in the troposphere. Several halogenated VOCs are potent greenhouse gases and cause stratospheric ozone depletion, and are regulated under the Montreal Protocol and its Amendments (MPA) (UNEP, 2012).
In addition to their influence on air quality and climate, VOCs are of concern because of their potential health effects. As examples, benzene and 1,3-butadiene are known carcinogens (IARC, 2010). Biological evidence supports the causal linkage between certain pollutants and certain cancers, for example, between leukemia incidence/mortality and exposure to benzene (Snyder, 2002; Forrest et al., 2005) and 1,3-butadiene (Cheng et al., 2007; Kirman et al., 2010). Increased rates of leukemia, melanoma and genotoxic risk have been shown in petroleum workers and populations living downwind of petrochemical facilities such as oil refineries (Wong and Raabe, 2000; Whitworth et al., 2008; Barregard et al., 2009; Basso et al., 2011), although elevated rates and cancer mortality are not consistently observed (Tsai et al., 2004; Axelsson et al., 2010).
Established in the 1950s, the Industrial Heartland of Alberta is currently a large (582 km2) industrial area with more than 40 companies, including chemical, petrochemical, and oil and gas facilities (http://www.industrialheartland.com). It is situated about 30 km northeast of Edmonton (53°32′N, 113°30′W; population 812,000) and a few km northeast of Fort Saskatchewan (53°43′N, 113°13′W; population 19,000) in an otherwise rural farming area of Alberta (Fig. 1 and Fig. S1). The Industrial Heartland is the largest hydrocarbon processing region in Canada, and major land holdings include Shell Canada, Dow Chemical Canada, and Provident Energy & Williams Energy Canada (now Pembina Pipeline & Williams Energy Canada) (http://www.industrialheartland.com). Their products include ethane, propane, propene, butane, styrene, hexane, benzene, heavy aromatics, synthetic crude oil and condensate (AIHA, 2012). For example, Shell Scotford is the largest land holding in the Heartland and includes a chemical plant, a refinery, and an upgrader that separates diluent and processes bitumen from oil sands mined approximately 450 km to the north, with a current processing capacity of 255,000 barrels/day (AIHA, 2012).
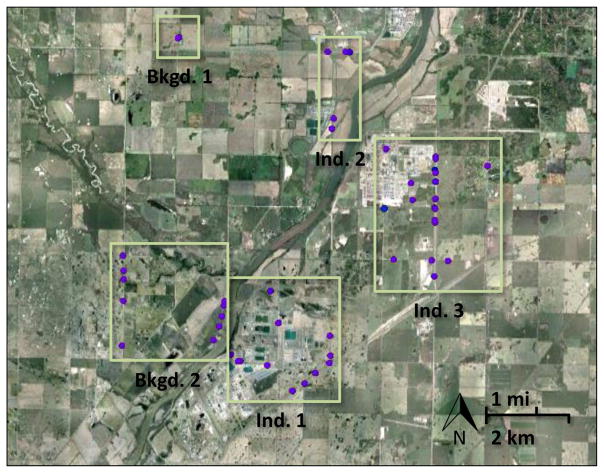
Fig. 1.
Air sampling locations in the Industrial Heartland of Alberta (August 12–13, 2010; purple circles). ‘Bkgd.1’: farm background (n = 8); ‘Bkgd.2’: rural background closer to the industrial activity (n = 9); ‘Ind.1’: Fort Saskatchewan county (downwind of BP Canada, Dow Chemical or Keyera Energy; n = 21); ‘Ind.2’: Sturgeon county (downwind of Access Pipeline, Evonik Degussa or Provident/Williams; n = 9); ‘Ind.3’: Strathcona County (downwind of Shell Canada; n = 33). Fort Saskatchewan lies to the southwest of ‘Ind.1’; Edmonton is another 30 km southwest (Fig. S1). Industrial land holdings in the Heartland are shown at http://www.strathcona.ca/files/New-Files/AT-EDT-MA-HEARTLAND2010-1.pdf. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Industrial emissions in the Heartland affect the local air quality, for example causing intermittent odor episodes in the nearby community of Fort Saskatchewan. However, there have been very few independent, peer-reviewed analyses of air quality in the region. Thirty VOCs were measured in the Heartland from 2004 to 2006, and elevated VOC levels were attributed primarily to industry followed by vehicles (Mintz and McWhinney, 2008). Air quality is monitored locally by the Fort Air Partnership (FAP), a multi-stakeholder group with members from industry, government and the public (http://www.fortair.org). Though the FAP data have not been published in the peer-reviewed literature, they show several exceedances of Alberta Ambient Air Quality Objectives (AAAQO) in 2010 for PM2.5, SO2, NH3 and NO2 (FAP, 2010). There were no reported O3 exceedances in 2010 both for AAAQO standards (82 ppb in 1 h) and for Canada-Wide Standards (65 ppb in 8 h). The annual O3 average for 2010 was 22 ppb, and a maximum 1-h O3 value of 72 ppb was recorded in June (FAP, 2010).
Here we present concentrations of VOCs and carbon monoxide (CO) measured in the Industrial Heartland in August 2010, and we discuss potential impacts of industrial VOC emissions on air quality and on human health in the local population.
2. Methods
2.1. Ground-based air sampling
Previously our group identified VOC emission hot-spots within a 12 × 12 km region of the Industrial Heartland, during a grid study on April 10, 2008 (n = 58) as part of an Environmental Impact Assessment in the Heartland (unpublished data). For example, maximum levels of benzene, ethylbenzene and styrene downwind of the Shell Scotford complex were 1.6, 2.0 and 4.0 parts per billion by volume (ppbv, 10−9), respectively, or 19, 435 and 6070 times higher than local background concentrations measured on the same day. During the 2010 study the sampling strategy focused on these emission hotspots. Speciated VOC measurements were obtained by collecting whole air samples (WAS) into evacuated 2 L stainless steel canisters, followed by analysis at our University of California, Irvine (UC Irvine) laboratory using multi-column gas chromatography (see Supplementary material). Individual air samples were collected concurrently at an upwind farm and downwind of several Heartland industries throughout the day and evening of August 12 and 13, 2010 (n = 80; Fig. 1). In many but not all cases, strong odors were associated with samples collected downwind of industrial activity. Because the sampling campaign occurred over a limited 2-day time frame, the results are not intended to represent an assessment of conditions over longer time scales.
Based on climate data from 1990 to 2002, the predominant wind direction in the Fort Saskatchewan area (Strathcona County) is from the southwest (SW) quadrant in fall and winter, the northwest (NW) and southeast quadrants in spring, and NW in summer (McCallum et al., 2003). During this study most of the sampled air masses arrived from the NW—i.e., not from Edmonton to the SW—at a median wind speed of 15 km h−1 or a moderate breeze (Fig. S2). Therefore we do not expect emissions from Edmonton to be a confounding factor in this study. The temperature ranged from 14 to 21 °C (http://www.casadata.org/Reports/SelectCategory.asp) and conditions were overcast with occasional drizzle and rain—in other words not ideal for active in situ photochemistry.
2.2. Laboratory analysis
Each air sample was returned to UC Irvine and analyzed within 10 days for CO and 77 VOCs, including C1–C10 hydrocarbons, C1–C2 halocarbons, C1–C5 alkyl nitrates and C1–C2 sulfur compounds. Our analytical procedures and calibration protocols are described in the Supplementary material. The detection limit of our measurements varies by compound and ranges from 0.005 to 100 pptv (Tables S1–S3). The measurement precision and accuracy also vary by compound and are 3% and 5%, respectively, for alkanes, alkenes and aromatics. Rigorous sensitivity tests have shown that most measured VOCs are stable within our canisters, though oxygenated hydrocarbon levels can increase or decrease at a rate of a few percent per day, which is reflected by their more poorly constrained precision and accuracy (Tables S1–S3).
2.3. VOC data analysis
Trace gas concentrations typically vary with factors including season and latitude. During this study the background VOC concentrations showed little diurnal variability for most compounds (Fig. S3), and the upwind farm samples were used to calculate the average local background concentrations for this latitude and time of year (n = 8). Because the plume samples were collected outside the perimeter of the industrial facilities, perhaps 500 m or more downwind of the emission source, the extent to which the plumes had become mixed and diluted with background air before being sampled is unclear. As a result the industrial plume averages were calculated as the average of the top 10th percentile concentrations for each species (n = 8). We note that these industrial plume values will be less concentrated than stack samples.
2.4. Human health data analysis
To investigate potential impacts of exposure to industrial pollutants on human health, in particular cancer incidences, two memos, tables and figures were obtained from the Alberta Cancer Board (Chen, 2006, 2008) under the Canadian Freedom of Information and Protection of Privacy (FOIP) Act. These documents provide limited analyses of cancer incidences in the region, specifically comparing the three-county area of Fort Saskatchewan, Strathcona County and Sturgeon County (Fig. 1) to the rest of the Edmonton-area health region, and also to the rest of Alberta. Currently Fort Saskatchewan houses 18 major industries, Strathcona County has 16 industries, and Sturgeon County has 9 industries (AIHA, 2012).
Based on surveillance data from 1994 through 2006 (inclusive), Chen (2008) remarks that the age-standardized incidence rates for male hematopoietic cancer and male non-Hodgkin lymphoma in the three-county area are elevated with respect to the two comparison areas. We extended this analysis by computing the mean (±standard error) standardized incidence rate for male hematopoietic cancers in the three-county region using two five-year periods (1997–2001 and 2002–2006) that help to reduce the year-to-year fluctuations in cancer cases (since the population is relatively small).
3. Results and discussion
3.1. VOC concentrations
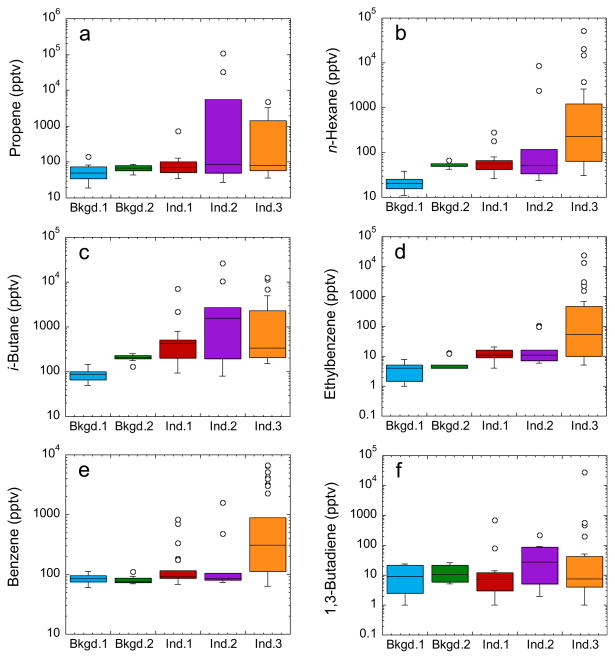
Complete results for the 2010 sampling campaign are summarized in Tables S1–S3. With the exception of methane (CH4), which is long-lived and relatively abundant in the atmosphere, background VOC levels ranged from sub- or low- parts per trillion by volume (pptv, 10−12) up to low ppbv levels. By comparison, concentrations of many VOCs were clearly elevated in the industrial plumes compared to background values (Tables S1 and S2). Of the 77 measured VOCs, 43 were very strongly enhanced in the plumes, with concentrations spanning roughly 1 to 4 orders of magnitude (Fig. 2a–f and Fig. S4a–c). These compounds include all 14 aromatics that were measured, 12 alkanes, 6 alkenes, 5 oxygenated compounds, 5 halocarbons and ethyne (Table S1). After CH4, the most abundant VOCs in the industrial plumes were, in descending order, propene (maximum of 107 ppbv), i-pentane (103 ppbv), n-pentane (97 ppbv), acetaldehyde (74 ppbv) and 2-methylpentane (62 ppbv). By comparison, their average background levels (±1σ) ranged from 0.031 ± 0.013 ppbv to 1.4 ± 0.8 ppbv, or factors of 55–1980 lower. The most strongly enhanced compounds were methyl tert-butyl ether (enhanced by up to a factor of 6194), ethylbenzene (6179×), 3-methylpentane (4414×), trans-2-butene (3609×) and 2,3-dimethylbutane (3048×).
Fig. 2.
Box-whisker plots of selected VOCs measured in the Industrial Heartland. Categories are described in Fig. 1. ‘Outliers’ (open circles) are values greater than the third quartile plus 1.5 times the interquartile distance (IQD), and lower than the first quartile minus 1.5 times the IQD.
An additional 15 compounds showed small-to-moderate, statistically significant enhancements (up to 1.06–2.8-fold) in the industrial plumes compared to background values (Table S2). These include CH4, two sulfur compounds (DMS, OCS), three methyl halides (CH3I, CH3Br, CH3Cl), three brominated compounds (CH3Br, CH2Br2, CHBr3), four long-lived halocarbons (9–26 years; HCFC-141b, HCFC-142b, HCFC-22, CCl4), and three short-lived solvents (1–5 months; acetone, methyl acetate, CHCl3) (Fig. S2d–f). With the exception of CH4, their plume averages remained below 1 ppbv (Table S2). Although carbon tetrachloride (CCl4) is restricted under the MPA, the precision of these measurements is 1% (about 0.8 pptv at the measured mixing ratios), and CCl4 shows clear and measurable enhancements in industrial plumes downwind of Dow and Shell compared to the background of 89.4 ± 0.4 pptv (Fig. S2f). We speculate that these elevated plume concentrations are due to emissions from pre-existing reservoirs.
Carbon monoxide and the remaining 19 of 77 measured VOCs showed similar concentration ranges in both background air and plumes, and were not appreciably impacted by industrial emissions (Fig. S3a–d). This group comprises a number of halocarbons (CFCs, halons, CH3CCl3, HFC-134a, 1,2-dichloroethene), biogenic compounds (isoprene, α-pinene and β-pinene) and alkyl nitrates (Table S3). Several of the halocarbons are restricted under the MPA, and their lack of industrial emission is not surprising (Fig. S3a). Although the pinenes have previously shown an unexpected association with industrial emissions from oil sands operations near Fort McMurray (Simpson et al., 2010), an industrial signature was not evident here (Fig. S3b). Carbon monoxide was not enhanced in the industrial plumes (Fig. S3c), showing that combustive sources (including vehicular emissions) did not significantly impact the measured plumes. Alkyl nitrate levels remained in the low pptv range (Fig. S3d), indicating little evidence of secondary photochemistry. This is most likely explained by a combination of unfavorable conditions for in situ photochemistry (Section 2.1) and the short travel time from plume emission to sample collection. For example, an emitted plume could reach the sampling sites in as little as a few minutes based on a wind speed of 10–20 km h−1 (Section 2.1) and a downwind sampling distance of 500 m.
3.2. Emission signatures
Based on linear correlations among the measured VOCs using least squares linear fits (Simpson et al., 2010), the emitted VOCs fell into at least five distinct correlating groups. First, the C3–C4 alkenes were strongly correlated (0.99 ≤ r2 ≤ 1.00), driven by high concentrations measured downwind of the Provident/Williams facility (Fig. 2a), which includes a natural gas liquids and propene fractionation project and produces C2–C4 alkanes and C3–C4 butenes (AIHA, 2012). Remarkably, the maximum propene level (107 ppbv) was almost double that measured in the Houston–Galveston Bay area (56 ppbv), even though Houston is both a much larger metropolitan area than Fort Saskatchewan and the largest petrochemical manufacturing center in the United States (Ryerson et al., 2003; Gilman et al., 2009).
Second, the C5–C7 alkanes and methacrolein were highly correlated (0.81 ≤ r2 ≤ 1.00), with largest concentrations downwind of Shell Scotford, which separates diluent and processes bitumen (Section 1), and Access Pipeline, which produces diluent and blended bitumen (Fig. 2 band Fig. S4a). The maximum n-hexane level (52 ppbv) was 2.5–17 times higher than maximum values measured in some of the world’s megacities (Beijing, Mexico City, and Tokyo) (Parrish et al., 2009), although lower than the maximum levels measured during a ship-based study in Houston/ Galveston Bay (81 ppbv) (Gilman et al., 2009). Simpson et al. (2010) associated elevated levels of C4–C9 alkanes with emissions from oil sands and its products and/or diluent, and this second group of VOCs is consistent with a diluent/bitumen signature. Even though methacrolein and methyl vinyl ketone are both major isoprene oxidation products (Montzka et al., 1993) they were uncorrelated during this study (r2 < 0.01). Because the maximum methacrolein level (20 ppbv) far exceeds the amount that isoprene oxidation chemistry can explain, its excess concentrations are attributed to industrial emissions.
Third, acetaldehyde (Fig. S4b), i-butane (Fig. 2c) and n-butane were correlated strongly with one another (0.88 ≤ r2 ≤ 0.98) and somewhat with the C3–C4 alkenes (0.58 ≤ r2 ≤ 0.68). Maximum levels of all three compounds (26–74 ppbv) were measured downwind of Provident/Williams, which produces C2–C4 alkanes (AIHA, 2012); Shell Scotford, which lists C3–C4 mix as a product; and Access Pipeline. Surprisingly, the maximum butane levels were comparable to those in central Mexico City during the mid-1990s when liquefied petroleum gas (LPG) was a major source of butanes and contributed to poor air quality (Blake and Rowland, 1995). The characteristic emission ratio of i-butane/n-butane is 0.2–0.3 for vehicular exhaust, 0.46 for LPG, and 0.6–1.0 for natural gas (Russo et al., 2010 and references therein). Here the average (±1σ) ratio in the top 10% of plumes (based on the highest i-butane and n-butane concentrations) was 0.47 ± 0.18, similar to that for LPG and to that measured downwind of the oil sands industry (0.42 ± 0.03) (Simpson et al., 2010), suggesting that the i-butane/n-butane ratio for various petrochemical processes resembles that for LPG. The main global source of acetaldehyde is photochemical hydrocarbon oxidation, with a relatively small industrial source (Singh et al., 2004; Millet et al., 2010). Here, however, the very high acetaldehyde levels cannot be explained by secondary photochemical production (Section 3.1) and they are attributed to direct industrial emission from various facilities. For example, the Shell Scotford chemical plant reportedly released 3.9 tonnes of acetaldehyde in 2010 (NPRI, 2012).
Fourth, toluene and the xylenes correlated strongly with one another (0.79 ≤ r2 ≤ 0.98) and with the second group of compounds (0.60 ≤ r2 ≤ 0.89). The highest levels of toluene and the xylenes (2.7 ppbv and 0.65–3.4 ppbv, respectively) were measured downwind of the Shell Scotford complex (Fig. S4c), which lists heavy aromatics among its products. The maximum toluene level was 69 times higher than background (Table S1), but lower than maximum values in megacities such as Mexico City, Tokyo and Beijing (~10 ppbv) and near major petrochemical complexes in Texas and Spain (16–77 ppbv) (Gilman et al., 2009; Ras et al., 2009).
Fifth, n-octane and the C9 aromatics (ethylbenzene, trimethylbenzenes, n-propylbenzene) correlated strongly (0.74 ≤ r2 ≤ 1.00), and with highest concentrations downwind of the Shell Scotford complex. The maximum ethylbenzene mixing ratio (23 ppbv; Fig. 2d) was much larger than for other compounds in this group (0.22–0.83 ppbv), indicating clear emissions of this possible carcinogen. The Shell Scotford refinery manufactures a range of products including gasoline, diesel and jet fuel, and reportedly released 0.562 tonnes of ethylbenzene in 2010 (NPRI, 2012).
Other chemicals were clearly emitted but did not necessarily correlate strongly with other VOCs. Ethane and propane were moderately correlated (r2 = 0.62), with highest levels measured downwind of Keyera and Provident/Williams (ethane and propane) and Dow Chemical (ethane only). The maximum propane mixing ratio (45 ppbv) was lower than in Houston/Galveston Bay (347 ppbv) (Gilman et al., 2009). Benzene showed some correlation with ethylbenzene (r2 = 0.58) and the highest benzene level (6.6 ppbv; Fig. 2e) was measured downwind of Shell Scotford, which produces benzene and reportedly released 2.5 tonnes of benzene from its refinery in 2010 (NPRI, 2012). The highest 1,3-butadiene level was also measured downwind of the Shell facility (27 ppbv; Fig. 2f), though 1,3-butadiene is not listed in the National Pollutant Release Inventory (NPRI) for Shell. The combustion tracers ethene and ethyne were only weakly correlated (r2 = 0.52) and their highest concentrations were measured downwind of Dow, which produces ethene. Ethene/ethyne ratios of 1–3 and 10–30 are characteristic of tailpipe emissions and petrochemical facilities, respectively (Ryerson et al., 2003). Here the ethene/ethyne ratio was 9.7 ± 1.0, which confirms the industrial rather than vehicular nature of the observed plumes.
3.3. Air quality impacts
The contribution of individual VOCs to O3 formation is a function of their concentration and their reactivity towards OH, and can be expressed as the total OH reactivity (kOH) (Kovacs et al., 2003; Mao et al., 2010; Kim et al., 2011):
| (1) |
Here kOH is used to evaluate the relative contributions of CO and the measured VOCs to downwind photochemistry. Because we did not measure nitrogen oxides (NOx), which can contribute 15–50% to kOH in cities such as Houston, Mexico City and New York (Mao et al., 2010), the reactivity reported here is likely underestimated and is understood to be only for the measured species, rather than total OH reactivity.
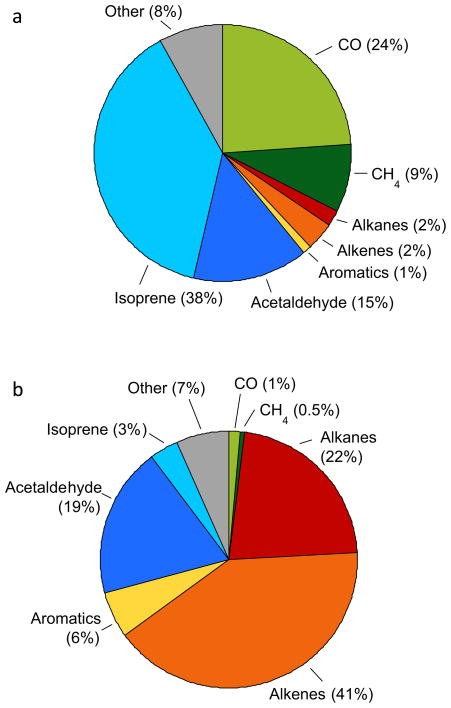
The OH reactivity in background air was 3.4 s−1, similar to clean air values of 1–3 s−1 (Kim et al., 2011; Lou et al., 2010). Not surprisingly, isoprene was the primary contributor to kOH in background air, followed by CO, acetaldehyde and CH4 (Fig. 3a). By contrast, kOH in the top 10th percentile of data with highest VOC loadings was 62 s−1, or 18 times larger than background. Even though we have missing reactivity, this plume kOH value is already comparable to levels in polluted megacities such as Mexico City, Tokyo and Hong Kong/Guangzhou, which typically range from 10 to 100 s−1 (Lou et al., 2010 and references therein). Because of their abundance and reactivity, propene, acetaldehyde and 1,3-butadiene were responsible for more than 50% of kOH in the plumes, while alkanes contributed another 23% (Fig. 3b). These results show some similarity to airborne studies in the greater Houston area, where propene and ethene were identified as the two VOCs primarily responsible for rapid O3 formation (Ryerson et al., 2003; deGouw et al., 2009) and alkene emissions from petrochemical facilities are the primary source of formaldehyde, also an O3 precursor (Parrish et al., 2012).
Fig. 3.
Relative contributions of CO and 77 measured VOCs to OH reactivity in (a) background air and (b) pollution plumes measured in the Heartland.
Despite the abundance of VOC precursors and strong OH reactivity in the industrial plumes, no O3 exceedances were measured in the Fort Saskatchewan region in 2010 (Section 1). In general, the highest monthly O3 averages occur during spring, and the highest 1-h O3 averages occur during hot summer afternoons when wind speeds are low (FAP, 2010). Ozone levels are lower within the center of the Heartland airshed, likely due to the presence of NOx which lower O3 concentrations through titration (FAP, 2010). Simpson et al. (2010) also found relatively low levels of O3 downwind of the Alberta oil sands because titration with NO exceeded O3 production on the short time-scale since precursor emission. Overall, it appears that industrial VOC sources in the Fort Saskatchewan area are emitted into a relatively clean background for O3, and local O3 exceedances are not common.
3.4. Gaps in VOC emission reporting
Although 43 of 77 measured VOCs were strongly elevated in the industrial plumes compared to local background concentrations, only 16 were quantified in the 2010 NPRI for the industries discussed in this paper (ethene, propene, 1,3-butadiene, 1,2-dichloroethane, n-hexane, benzene, toluene, ethylbenzene, total xylenes, styrene, 1,2,4-trimethylbenzene, acetaldehyde, carbonyl sulfide, chloroform, trichloroethene, HCFC-22; NPRI, 2012), with individual companies reporting 0–10 VOCs. As a first example, while strongly elevated levels of at least a dozen C2–C8 alkanes were detected downwind of several Industrial Heartland facilities (Table S1, Fig. 2b–c and Fig. S4a), only n-hexane is included in the NPRI. The VOCs reported in the NPRI include light alkenes and are weighted towards aromatic species, yet our study shows that alkanes are a leading contributor to kOH in the Heartland (Fig. 3b). Second, while 1,3-butadiene is a known carcinogen, emissions of this VOC are reported by only one of the companies considered here.
Even when emission rates are reported, they require verification to ensure that the reporting is accurate. For example, recent NPRI listings of VOC emission rates (including benzene) from an unnamed Canadian refinery were found to be underestimated by 15–18-fold (Chambers et al., 2008). In addition to improved reporting of speciated VOCs in the NPRI or other publically available inventories, especially 1,3-butadiene and light alkanes, we recommend independent air quality monitoring and VOC emission estimates in the Heartland region so that emitted compounds can be externally identified, quantified and reported in the peer-reviewed literature.
3.5. Human health impacts
Of the 77 VOCs measured here, at least 10 are either known human carcinogens (Group 1: benzene, 1,3-butadiene), probable carcinogens (Group 2A: trichloroethene, tetrachloroethene), or possible carcinogens (Group 2B: carbon tetrachloride, chloroform, 1,2-dichloroethane, dichloromethane, ethylbenzene, isoprene, styrene) (IARC, 2010). Of these, 1,3-butadiene and ethylbenzene were the most abundant in the industrial plumes, with maximum levels of 23–27 ppbv, or 3–4 orders of magnitude larger than their background values (Table S1).
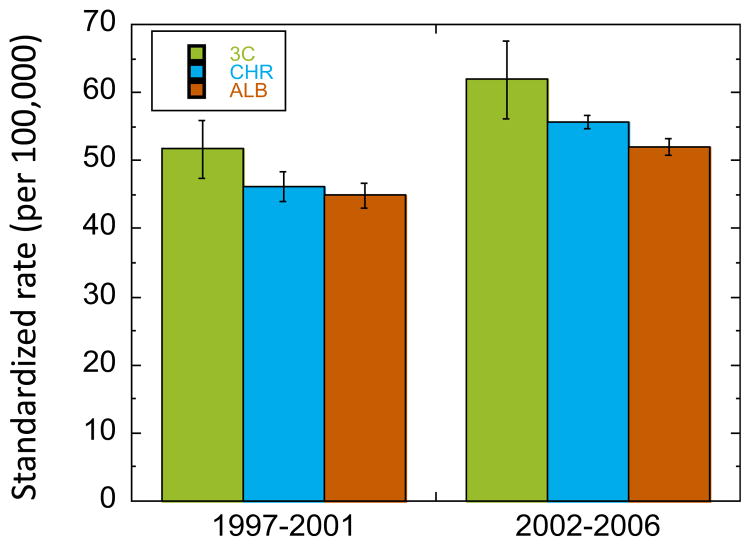
An analysis of cancer incidences in the Industrial Heartland shows elevated incidence rates of male hematopoietic cancers in the three-county area where the industries are located (Fort Saskatchewan, Strathcona County and Sturgeon County) compared to neighboring regions for both 1997–2001 and 2002–2006, although the error bars are large due to small sample sizes (Fig. 4). Several steps would help to confirm such trends and possibly provide a more direct link between these cancers and emissions of toxic VOCs in the Heartland: improved estimates of VOC emissions and exposure estimates that included more detail and historical data; better cancer surveillance that included regular evaluations, breakdown by cancer type (e.g., myelogenous, monocytic and lymphocytic leukemias) and geocoding of cases; collection of potential covariates and confounders (e.g., residence and work history); and use of statistical and epidemiological techniques to investigate spatial, temporal and exposure-related patterns of disease in the community.
Fig. 4.
Age standardized incidence rates (±1 standard error) of invasive hematopoietic cancer among males in the Heartland region for 1997–2001 and 2002–2006. “3C”: three-county industrial area of Fort Saskatchewan, Strathcona and Sturgeon counties; “CHR”: former Capital Health Region of Edmonton and surrounding region, excluding the three-county area; “ALB”: rest of Alberta, excluding the CHR.
Elevated risk of hematopoietic cancers has also been found in other populations living downwind of industrial facilities, even at relatively low VOC exposures. For example, leukemia incidence an exposed population living near a large Swedish oil refinery known to emit benzene and other VOCs was significantly elevated (33 cases vs. 22 expected cases) compared to local controls (50 cases vs. 56 expected), despite an estimated refinery contribution to annual average VOC concentrations of only 0.63 ppb for benzene and 0.23 ppb for 1,3-butadiene (Barregard et al., 2009). The authors note that risk estimates extrapolated from high-level exposure would not predict an increase of leukemia at low VOC exposures, and they suggest that risk estimates using standard carcinogenic unit risk or slope factors do not adequately represent true risks from much lower exposures. As a second example of a population-based study, higher exposure to benzene and 1,3-butadiene in 886 census tracts surrounding Houston, Texas was associated with increased incidence of childhood lymphohematopoietic cancers (Whitworth et al., 2008). Some of the highest exposures occurred in the Houston Ship Channel area, which contains a large number of petroleum and chemical industries.
Recommended exposure limits and risk-based criteria evolve as our understanding of the chemical toxicity of carcinogens improves. Using benzene as an example, the recommended exposure limit relevant for occupational settings has decreased from 100 ppm in 1947 to 1 ppm (Wong et al., 1999; McHale et al., 2010; Smith, 2010); the 1-h average ambient air quality guideline in Alberta is 9 ppb (Chambers et al., 2008). However, adverse health outcomes, including hematological changes and gene perturbations, have been reported at exposure levels below 1 ppm (McHale et al., 2010; Qu et al., 2002; Lan et al., 2004; Xing et al., 2010). Indeed, recent literature suggests that there is probably no safe exposure level to benzene because it does not appear to have a functional low-dose threshold, and because the effects of exposure appear to be additive in a linear or supralinear fashion (Smith, 2010). Further, in environmental settings (as compared to work-place), exposure to compound mixtures rather than a single compound at a time is common, and simultaneous exposure to complex mixtures, including multiple carcinogens, may involve interactions and possibly synergistic effects on target organs or systems at low exposure (Basso et al., 2011). Although VOC levels were significantly elevated above concurrent local background values in the Heartland, concentrations remained below existing guidelines for short-term exposure. Guidelines for long-term exposures generally use a risk-based approach, and there is considerable uncertainty regarding the unit risk factors that describe the toxicity of a chemical (or mixture) for the public and susceptible individuals, as well as debate over what is acceptable or protective. (A number of U.S. state and federal rules use individual lifetime cancer risks in the range of 1 in 10,000 to 1 in 1,000,000.)
The elevated incidence of cancers within the Industrial Heartland that are known to be linked to VOCs released in the region raises questions regarding whether ambient levels, emission controls, and risk calculations are adequately protective of public health. In addition, on-site workers may be at increased risk because of their closer proximity to emission sources. While several factors might well explain an observation of increased cancer rates, e.g., variability of a population’s genetic makeup, differences in dietary or lifestyle factors, and statistical variability, it is also important and responsible to improve health surveillance and VOC exposure measurements, to utilize epidemiological studies that can better link environmental factors to disease, and to reduce exposures to pollutants that might plausibly be related to adverse health impacts.
4. Conclusions
Ambient monitoring in the Industrial Heartland of Alberta, the largest hydrocarbon processing region in Canada, showed remarkable enhancements in VOC concentrations. Even though the Heartland is situated within a generally rural area, many maximum concentrations were comparable to those measured in the world’s largest cities. Thirty VOCs were present at levels above 1 ppbv, and maximum propene and i-pentane levels exceeded 100 ppbv. Some of the largest VOC excesses were measured in samples designated as “no smell”, showing that absence of odor does not necessarily indicate good air quality. The industrial plumes showed distinct chemical signatures that varied not only between facilities but also within individual facilities. An analysis of OH reactivity in the plumes suggests that propene, acetaldehyde and 1,3-butadiene have the greatest potential to form downwind O3.
Excess numbers of hematopoietic cancers were observed in the same region that emits substantial quantities of complex mixtures of industrial pollutants, including several VOCs that are known to cause these cancers. While there are many factors that preclude a causal linkage, including a lack of exposure history for the local population and uncertainties associated with the health impacts of low exposures to multiple compounds, we suggest that immediate reductions in emissions of known carcinogens such as benzene and 1,3-butadiene are warranted and prudent.
Supplementary Material
Acknowledgments
Laboratory analysis was performed by Brent Love and Gloria Liu Weitz. Barbara Chisholm provided logistical support. The authors thank Jo-Yu Chin for her comments, and Verona Goodwin and two local residents for their assistance during sampling. Field work and laboratory analysis was funded by the Tides Foundation.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atmosenv.2013.09.017.
References
- AIHA (Alberta’s Industrial Heartland Association) Industry and Organization Profiles. 2012 Jul; Available from: www.industrialheartland.com/images/stories/industry/aiha_industry_information_july_2012.pdf.
- Axelsson G, Barregard L, Holmberg E, Sallsten G. Cancer incidence in a petrochemical industry area in Sweden. Sci Total Environ. 2010;408:4482–4487. doi: 10.1016/j.scitotenv.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Barregard L, Holmberg E, Sallsten G. Leukaemia incidence in people living close to an oil refinery. Environ Res. 2009;109 (8):985–990. doi: 10.1016/j.envres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Basso E, et al. Cytogenetic biomonitoring on a group of petroleum refinery workers. Environ Mol Mutagen. 2011;52:440–447. doi: 10.1002/em.20641. [DOI] [PubMed] [Google Scholar]
- Blake DR, Rowland FS. Urban leakage of liquefied petroleum gas and its impact on Mexico City air quality. Science. 1995;269:953–956. doi: 10.1126/science.269.5226.953. [DOI] [PubMed] [Google Scholar]
- Buzcu B, Fraser MP. Source identification and apportionment of volatile organic compounds in Houston, TX. Atmos Environ. 2006;40:2385–2400. [Google Scholar]
- Chambers AK, Strosher M, Wootton T, et al. Direct measurement of fugitive emissions of hydrocarbons from a refinery. J Air Waste Manag Assoc. 2008;58:1047–1056. doi: 10.3155/1047-3289.58.8.1047. [DOI] [PubMed] [Google Scholar]
- Chen Y. Ten Year (1994–2003) Cancer Incidence in Fort Saskatchewan, Strathcona and Sturgeon Counties. Division of Population Health and Information, Alberta Cancer Board; Edmonton Alberta, Canada: 2006. Memo, tables and figures, dated June 26, 2006. [Google Scholar]
- Chen Y. Ten Year (1994–2006) Cancer Incidence in Fort Saskatchewan, Strathcona and Sturgeon Counties. Division of Population Health and Information, Alberta Cancer Board; Edmonton Alberta, Canada: 2008. Memo, tables and figures, dated Aug 22, 2008. [Google Scholar]
- Cheng H, Sathiakumar N, Graff J, et al. 1,3-Butadiene and leukemia among synthetic rubber industry workers: exposure–response relationships. Chem Biol Interact. 2007;166:15–24. doi: 10.1016/j.cbi.2006.10.004. [DOI] [PubMed] [Google Scholar]
- deGouw JA, Te Lintel Hekkert S, Mellqvist J, et al. Airborne measurements of ethene from industrial sources using laser photo-acoustic spectroscopy. Environ Sci Technol. 2009;43:2437–2442. doi: 10.1021/es802701a. [DOI] [PubMed] [Google Scholar]
- Forrest MS, Lan Q, Hubbard AE, et al. Discovery of novel biomarkers by microarray analysis of peripheral blood mononuclear cell gene expression in benzene-exposed workers. Environ Health Perspect. 2005;113 (6):801–807. doi: 10.1289/ehp.7635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAP (Fort Air Partnership) Fort Air Partnership Ambient Air Monitoring Network 2010 Annual Technical Report Network and Data Summary. 2010 Available from: http://www.fortair.org/wp-content/uploads/downloads/2012/08/reports/TechnicalReport-2010.pdf.
- Gilman JB, Kuster WC, Goldan PD, et al. Measurements of volatile organic compounds during the 2006 TexAQS/GoMACCS campaign: Industrial influences, regional characteristics, and diurnal dependencies of the OH reactivity. J Geophys Res. 2009;114:D00F06. http://dx.doi.org/10.1029/2008JD011525. [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, et al. A global model of natural volatile organic compound emissions. J Geophys Res. 1995;100 (D5):8873–8892. [Google Scholar]
- Guenther A, Geron C, Pierce T, et al. Natural emissions of non-methane volatile organic compounds, carbon monoxide, and oxides of nitrogen from North America. Atmos Environ. 2000;34:2205–2230. [Google Scholar]
- IARC (International Agency for Research on Cancer) IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2010 Available from: http://monographs.iarc.fr/ENG/Classification/index.php. [PMC free article] [PubMed]
- Kim S, Guenther A, Karl T, Greenberg J. Contributions of primary and secondary biogenic VOC to total OH reactivity during the CABINEX (Community Atmosphere-Biosphere Interactions Experiments)–09 field campaign. Atmos Chem Phys. 2011;11:8613–8623. [Google Scholar]
- Kirman CR, Albertini RA, Gargas ML. 1,3-Butadiene: III. Assessing carcinogenic modes of action. Crit Rev Toxicol. 2010;40:74–92. doi: 10.3109/10408444.2010.507183. [DOI] [PubMed] [Google Scholar]
- Kovacs TA, Brune WB, Harder H, et al. Direct measurements of urban OH reactivity during Nashville SOS in summer 1999. J Environ Monit. 2003;5:68–74. doi: 10.1039/b204339d. [DOI] [PubMed] [Google Scholar]
- Lan Q, Zhang L, Li G, et al. Hematotoxicity in workers exposed to low levels of benzene. Science. 2004;306:1774–1776. doi: 10.1126/science.1102443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S, Holland F, Rohrer F, et al. Atmospheric OH reactivities in the Pearl River Delta–China in summer 2006: measurement and model results. Atmos Chem Phys. 2010;10:11243–11260. [Google Scholar]
- Mao J, Ren X, Chen S, et al. Atmospheric oxidation capacity in the summer of Houston 2006: comparison with summer measurements in other metropolitan studies. Atmos Environ. 2010;44:4107–4115. [Google Scholar]
- McCallum K, Scotten R, Hasham F. Historical Prevailing Winds: Strathcona County. 2003 Available from: http://www.strathcona.ab.ca/files/Attachment-EEPS-prevailingwinds.pdf.
- McHale CM, Zhang L, Lan Q, et al. Global gene expression profiling of a population exposed to a range of benzene levels. Environ Health Perspect. 2010;119 (5):628–634. doi: 10.1289/ehp.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet DB, Guenther A, Siegel DA, et al. Global atmospheric budget of acetaldehyde: 3-D model analysis and constraints from in-situ and satellite observations. Atmos Chem Phys. 2010;10:3405–3425. [Google Scholar]
- Mintz R, McWhinney RD. Characterization of volatile organic compound emission sources in Fort Saskatchewan, Alberta using principal component analysis. J Atmos Chem. 2008;60:83–101. [Google Scholar]
- Montzka S, Trainer M, Goldan PD, et al. Isoprene and its oxidation products, methyl vinyl ketone and methacrolein, in the rural troposphere. J Geophys Res. 1993;98 (D1):1101–1111. [Google Scholar]
- NPRI (National Pollutant Release Inventory) National Pollutant Release Inventory Online Data Search. 2012 Available from: http://www.ec.gc.ca/pdb/websol/querysite/query_e.cfm.
- Parrish DD, Kuster WC, Min S, et al. Comparison of air pollutant emissions among mega-cities. Atmos Environ. 2009;43:6435–6441. [Google Scholar]
- Parrish DD, Ryerson TB, Mellqvist J, et al. Primary and secondary sources of formaldehyde in urban atmospheres: Houston Texas region. Atmos Chem Phys. 2012;12:3273–3288. [Google Scholar]
- Qu Q, Shore R, Li G, et al. Hematological changes among Chinese workers with a broad range of benzene exposures. Am J Ind Med. 2002;42 (4):275–285. doi: 10.1002/ajim.10121. [DOI] [PubMed] [Google Scholar]
- Ras MR, Marcé RM, Borrull F. Characterization of ozone precursor volatile organic compounds in urban atmospheres and around the petrochemical industry in the Tarragona region. Sci Total Environ. 2009;407:4312–4319. doi: 10.1016/j.scitotenv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Robinson AL, Donahue NM, Shrivastava MK, et al. Rethinking organic aerosols: semivolatile emissions and photochemical aging. Science. 2007;315:1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- Russo RS, Zhou Y, White ML, et al. Multi-year (2004–2008) record of nonmethane hydrocarbons and halocarbons in New England: seasonal variations and regional sources. Atmos Chem Phys. 2010;10:4909–4929. [Google Scholar]
- Ryerson TB, Trainer M, Angevine WM, et al. Effect of petrochemical industrial emissions of reactive alkenes and NOx on tropospheric ozone formation in Houston, Texas. J Geophys Res. 2003;108(D8) http://dx.doi.org/10.1029/2002JD003070. [Google Scholar]
- Sillman S. The relation between ozone, NOxand hydrocarbons in urban and polluted rural environments. Atmos Environ. 1999;33:1821–1845. [Google Scholar]
- Simpson IJ, Blake NJ, Barletta B, et al. Characterization of trace gases measured over Alberta oil sands mining operations: 76 speciated C2-C10 volatile organic compounds (VOCs), CO2, CH4, CO, NO, NO2, NOy, O3 and SO2. Atmos Chem Phys. 2010;10:11931–11954. [Google Scholar]
- Snyder R. Benzene and leukemia. Crit Rev Toxicol. 2002;32 (3):155–210. doi: 10.1080/20024091064219. [DOI] [PubMed] [Google Scholar]
- Singh HB, Salas LJ, Chatfield RB, et al. Analysis of the atmospheric distribution, sources, and sinks of oxygenated volatile organic chemicals based on measurements over the Pacific during TRACE-P. J Geophys Res. 2004;109:D15S07. http://dx.doi.org/10.1029/2003JD003883. [Google Scholar]
- Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Publ Health. 2010;31 (1):133–148. doi: 10.1146/annurev.publhealth.012809.103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SP, Cardarelli KM, Wendt JK, Fraser AE. Mortality patterns among residents in Louisiana’s industrial corridor, USA 1970–99. Occup Environ Med. 2004;61:295–304. doi: 10.1136/oem.2003.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP (United Nations Environment Programme) Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer. (9) 2012 Available from: http://ozone.unep.org/Publications/MP_Handbook/MP-Handbook-2012.pdf.
- Whitworth KW, Symanski E, Coker AL. Childhood lymphohematopoietic cancer incidence and hazardous air pollutants in southeast Texas, 1995–2004. Environ Health Perspect. 2008;116 (11):1576–1580. doi: 10.1289/ehp.11593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong O, Trent L, Harris F. Nested case-control study of leukaemia, multiple myeloma, and kidney cancer in a cohort of petroleum workers exposed to gasoline. Occup Environ Med. 1999;56:217–221. doi: 10.1136/oem.56.4.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong O, Raabe GK. A critical review of cancer epidemiology in the petroleum industry, with a meta-analysis of a combined database of more than 350,000 workers. Regul Toxicol Pharmacol. 2000;32:78–98. doi: 10.1006/rtph.2000.1410. [DOI] [PubMed] [Google Scholar]
- Xing C, Marchetti F, Li G, et al. Benzene exposure near the U.S. permissible limit is associated with sperm aneuploidy. Environ Health Perspect. 2010;118 (6):833–839. doi: 10.1289/ehp.0901531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.