Abstract
Objective
Multi-family psychoeducational psychotherapy (MF-PEP) is an efficacious treatment for children with mood disorders. Given comorbidity between disruptive behaviors and mood disorders, this study examined associations among disruptive behaviors and impairment, impact of MF-PEP on disruptive behaviors, and whether disruptive behaviors affected treatment response of mood symptoms.
Method
Secondary analyses examined the randomized controlled trial of MF-PEP versus waitlist control (N = 165 children ages 8–11 with mood disorders and their parents). Comorbid behavioral diagnoses occurred in 97% of children. All participants continued treatment as usual.
Results
Greater degree of disruptive behaviors was associated with worse mood symptoms and impairment. Between-group analyses examining outcome of disruptive behaviors were non-significant. Within-group analyses and between-group effect sizes suggest MF-PEP was associated with reductions in attention-deficit/hyperactivity disorder (d = 0.39), oppositional defiant disorder (d = 0.30), and overall disruptive behavior symptoms (d = 0.30), but not conduct disorder symptoms. Baseline severity of disruptive behaviors did not impact treatment response of mood symptoms to MF-PEP.
Conclusions
MF-PEP is an effective intervention for children with mood disorders and provides some benefit for disruptive behaviors. Given that disruptive behavior severity does not impact children’s ability to experience improved mood symptoms, MF-PEP may be an important early intervention for children with comorbid mood and disruptive behavior disorders. Subsequent intervention targeting behavioral symptoms after improvement in mood may be beneficial. Studies examining treatment sequencing for children with comorbid mood and disruptive behavior disorders are needed. Clinical trial registration information—Family psychoeducation for children with mood disorders; http://www.clinicaltrials.gov; NCT00050557.
Keywords: children, disruptive behavior, family therapy, mood disorders, psychoeducation
Introduction
Depressive and bipolar disorders are common in prepubertal children. Epidemiologic studies suggest that depressive disorders affect 2–5% of children.1 Although no epidemiological studies of bipolar disorders have focused exclusively on school-aged children, a recent meta-analysis, which examined studies of youth ages 7–21, suggests that bipolar disorders (i.e., bipolar I and II disorders, bipolar disorder not otherwise specified [NOS], cyclothymic disorder, mania, hypomania) affect 1.8% of youth (prevalence ranged from 0.1–6.3).2 Childhood mood disorders are associated with chronicity, impairment, and increased risk for development of other psychiatric disorders. Given the psychosocial complexity and psychiatric comorbidities amongst children with mood disorders, interventions are needed that address mood symptoms, family context, and coexisting difficulties.3–5
Childhood mood disorders are highly comorbid with disruptive behavior disorders (DBDs), including attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and conduct disorder (CD). Clinical samples of children with depression demonstrate rates of DBDs consistently greater than 60%.6,7 Clinical samples of children with bipolar disorder show even higher rates of DBDs.8 Epidemiologic studies suggest that ADHD co-occurs in 14–16% of children with depression, while ODD and/or CD co-occur in 14–30%.9 Similarly high rates of comorbid DBDs have been reported in epidemiological studies of children and adolescents with bipolar disorders; specifically, ADHD (6–29%), ODD and/or CD (6–50%).10,11
Despite high rates of comorbidity, the impact of childhood behavioral disturbance on psychosocial mood treatment and the effect of mood interventions on disruptive behaviors have received little research attention. Characteristics of youth with DBDs and their families may negatively impact treatment of mood disorders. However, treatments that target mood disorders may offer some benefit to children’s disruptive behaviors, such that subsequent behavioral interventions may be more likely to be successful. Thus, research in this area may elucidate treatment techniques and sequencing considerations.
Six randomized controlled trials (RCTs) of psychosocial interventions for adolescents with diagnosed depression evaluated impact of DBDs on mood outcome, three of which detected a signal. In the Adolescents Coping with Depression Course (CWD-A) versus Life Skills/Tutoring Control (LS) study (N = 114; ≥ 81.6% with DBDs), presence of ADHD predicted longer time to recovery (40 versus 14 weeks).12 In the Treatment for Adolescents with Depression Study (TADS; N = 439; 23.46% with DBDs), ADHD moderated depression treatment response; those with ADHD demonstrated similar improvements among all active treatments (fluoxetine, cognitive behavioral therapy [CBT], CBT+fluoxetine) compared with control (clinical management+pill placebo), whereas for those without ADHD only CBT+fluoxetine was superior to control.13 In the Treatment of Resistant Depression in Adolescents (TORDIA) study, which focused on second-step treatment strategies (12 weeks of medication switch and CBT or a medication switch alone) among 334 adolescents with selective serotonin reuptake inhibitor treatment-resistant depression (9.6% with ODD or CD; 16.6% with ADHD), ADHD marginally predicted increased response to combined CBT+SSRI intervention.14
However, three RCTs found DBDs did not impact mood outcome. An examination of CBT, systematic behavioral family therapy, and nondirective supportive therapy (N = 107; 20.6% with DBDs), found DBDs did not impact acute treatment response; however, DBDs predicted need for additional treatment during follow-up (31.1% versus 12.9%).15 Similarly, in an evaluation of CWD-A versus waitlist control (WLC; N = 151; 19.9% with DBDs), DBDs did not impact depression treatment response. DBDs were associated with more baseline impairment and predicted shorter time to depression recurrence post-treatment and higher likelihood of relapse over follow-up (36.4% versus 13.2%).16 Although ADHD moderated treatment response of depression in TADS, all DBDs combined did not affect depression treatment response.17 Finally, in the Adolescent Depression Antidepressants and Psychotherapy Trial (ADAPT), 208 youth (30.3% with DBDs) were treated with routine psychological care and psychopharmacology; half received CBT. CD was associated with higher suicidality, but there were no differences in treatment response.18
Four of the aforementioned RCTs also evaluated impact of mood treatment on DBD symptoms. All but 1 noted improvement in DBD symptoms, although not always to a significantly greater degree than control.19 CWD-A reduced oppositionality; however, improvements were nonsignificant when compared with WLC. Additional reductions in oppositionality were observed over 6-month follow-up, though this difference was not compared to control.20 In addition, booster sessions during follow-up significantly reduced oppositionality compared with assessment-only control.21 In the RCT of CWD-A versus LS, recovery of CD between interventions was not significant posttreatment (CWD-A = 9%; LS = 17%), 6-month follow-up (CWD-A = 54%; LS = 60%), or 12-month follow-up (CWD-A = 63%; LS = 63%). In addition, both CWD-A and LS showed significant reduction in oppositionality posttreatment and at 6-month follow-up, but not 12-month follow-up.22 Finally, in TADS, all active treatments significantly reduced oppositionality; however, those receiving fluoxetine or CBT+fluoxetine experienced significantly greater reduction in oppositionality posttreatment than those in CBT only or control.23
Only one nonrandomized trial including 58 children with diagnosed depression and/or anxiety (32.8% with ODD), examined the impact of psychosocial treatment for youth with internalizing disorders on DBD symptoms. Over 2-year follow-up, children receiving psychodynamic psychotherapy demonstrated significant improvement in externalizing symptoms compared with community services control.24 No RCTs of psychosocial interventions for children with depression have examined whether DBD severity influences mood outcome.
Considerably less research, and no RCT, has examined the impact of psychosocial treatment for youth with bipolar disorder on DBDs. One open study of child and family focused CBT for children (N = 34; 73.5% with ADHD; 35% with ODD) found improvement in ADHD and aggression. One open study of family focused treatment for adolescents (N = 20; 40% with ADHD; 55% with ODD) found improvement in externalizing symptoms.25–27 Finally, no studies of psychosocial interventions for youth with bipolar disorder have evaluated whether DBD severity influences mood outcome.
In summary, despite high rates of comorbid DBDs in youth with mood disorders, little is known about their impact on mood outcome or the impact of mood treatment on DBD symptoms. Results from adolescent RCTs indicate DBDs did not impact depression treatment response; however, DBDs were associated with more impairment, depression recurrence, and need for additional treatment. Adolescent studies examining effect of depression treatments on DBDs yielded mixed results. Only 1 nonrandom clinical trial of children with internalizing disorders and 2 open trials of youth with bipolar disorders demonstrated improvement in DBDs. No RCT of psychosocial treatment for children with depression and bipolar disorders has examined these questions. Limited findings support the conclusion that two problem areas are worse than one and youth with comorbid mood and behavior disorders may require more extensive treatment to achieve improvement.
Multi-family psychoeducational psychotherapy (MF-PEP) is an efficacious psychosocial treatment for children with mood disorders.28 This adjunctive, manual-driven, group-based intervention combines psychoeducation, social support, and skills development. MF-PEP empowers children and families via education about mood disorders and effective treatment and improves symptom management through CBT, communication, and problem-solving skills. An RCT of 165 children found youth who immediately received MF-PEP plus treatment as usual (IMM+TAU) experienced significant reduction in mood symptoms compared to WLC+TAU over 1-year follow-up.29 Mediator analyses revealed MF-PEP helped parents become better consumers of mental health services, and accessing higher-quality services resulted in decreased mood symptom severity.29,30 Secondary moderator analyses demonstrated MF-PEP treatment response was not affected by children’s demographics, intelligence, anxiety disorders, and mood diagnoses, parents’ general psychiatric and mood symptoms, and familial expressed emotion.31,32 However, MF-PEP produced the greatest treatment response for children who were most functionally impaired.32 Thus, MF-PEP is an effective treatment for impaired children’s mood symptoms regardless of demographics, mood and anxiety disorder status, parental psychopathology, and familial environment.
The current study examined associations among DBD symptoms and impairment, impact of MF-PEP on severity of DBD symptoms, and whether DBDs affected response of mood symptoms to MF-PEP. Given previous findings, the following hypotheses were tested: 1) class and severity of disruptive behavior symptoms would be positively correlated with mood symptom severity and negatively correlated with global functioning; and 2) IMM+TAU would significantly reduce ADHD, ODD, CD, and overall DBD symptoms in children with mood disorders compared with WLC+TAU. Exploratory analyses examined whether class and severity of disruptive behavior symptoms impacted treatment response of mood symptoms to MF-PEP.
Method
Sample
Secondary analyses examined the MP-PEP RCT (the National Institute of Mental Health [NIMH]: R01MH061512), which included 165 children with mood disorders and their families.29 Participants were recruited from Midwestern rural and urban settings through a referral network of mental health professionals, presentations to professional and community-based groups, and media feature stories about the study. Children had to be ages 8–11 and have a mood disorder and IQ > 70.
The sample size was determined a priori based on a power calculation requiring 70% power to detect a medium effect size, including adjustment for multiple comparisons, in primary analyses.29, 33 Most children (70%, n = 115) had bipolar disorders (62, bipolar I; 22, bipolar II; 29, bipolar disorder NOS; 2, substance induced mood disorder); 30% (n = 50) had depressive disorders (38, major depressive disorder; 5, dysthymic disorder; 6, major depressive and dysthymic disorder; 1, mood disorder NOS). All had comorbid diagnoses (97%, behavior disorders; 68%, anxiety disorders). Children were 9.9 years on average (SD = 1.3); a majority were male (73%). Most were White, non-Hispanic (90.9%; 6.7%, African American; 1.8%, mixed race; 0.6%, White, Hispanic). Most parents had obtained some college education (2.4%, partial high school; 15.8%, graduated high school; 38.8%, partial college; 26.1%, college degree; 16.4%, graduate/professional education; 0.6%, missing information). The median family income range was $40,000 to $59,000 (11%, < $20,000; 20% ≥ $100,000). See Table 1 for baseline characteristics of the intent-to-treat (ITT) cohort.
Table 1.
Baseline Demographic and Clinical Descriptive Statistics of Intent-to-Treat Cohort
| Variables | IMM+TAU (n = 78) |
WLC +TAU (n = 87) |
Tests of Difference X2 or t-test |
|---|---|---|---|
| Age, y, M (SD) | 10.0 (1.3) | 9.8 (11.2) | t(163) = −0.86 |
| Male, % | 76 | 71 | X2(1) = 0.40 |
| White, % | 94 | 89 | X2(3) = 1.77 |
| Annual Income, Mdn, $ | 40,000–59,000 | 40, 000–59, 000 | X2(5) = 3.37 |
| Parents’ Completing Partial College, % | 33 | 44 | X2(4) = 3.38 |
| Bipolar Spectrum Disorders, % | 70.5 | 69.0 | X2(1) = 0.05 |
| Disruptive Behavior Disorder Comorbidity, % | |||
| ADHD | 86 | 93 | X2(1) = 2.31 |
| ODD or CD | 97 | 97 | X2(1) = 0.11 |
| Symptom Severity and Functioning, M (SD) | |||
| Mood Severity Index | 32.5 (13.3) | 31.4 (16.1) | t(163) = −0.48 |
| ADHD Symptoms | 11.6 (5.1) | 11.4 (4.8) | t(163) = −0.21 |
| ODD Symptoms | 5.9 (2.0) | 5.7 (2.5) | t(163) = −0.57 |
| CD Symptoms | 2.0 (2.5) | 2.2 (2.6) | t(163) = 0.44 |
| Overall DBD Symptoms | 19.4 (7.8) | 19.3 (7.7) | t(163) = −0.16 |
| Current Global Functioning | 43.0 (8.0) | 44.4 (8.8) | t(163) = 1.09 |
Note: There were no significant differences between groups.
ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; DBD = disruptive behavior disorder; IMM = immediate treatment group; ODD = oppositional defiant disorder; TAU = treatment as usual; WLC = waitlist control group.
Measures
This study used a subset of measures from the MF-PEP RCT. Only baseline and 12-month follow-up assessments were used. The Children’s Interview for Psychiatric Syndromes –Child Form (ChIPS) and Parent Form (P-ChIPS) are structured diagnostic interviews assessing psychopathology using DSM-IV criteria in youth ages 6– 18. ChIPS and P-ChIPS assess 20 behavioral, anxiety, mood, and other syndromes and stressors.34,35 Adequate reliability and validity have been demonstrated in child and adolescent populations and in inpatient, outpatient, and community settings.36 In this study, ChIPS and P-ChIPS diagnoses had significant inter-rater reliability (k = 0.82 and 0.78, respectively). When making diagnoses, either informant's symptom endorsement was used unless there were clear reasons not to trust the informant. For current analyses only parent report was used. Covariates of type and severity of disruptive behavior were derived from number of ADHD, ODD, and CD items endorsed on P-ChIPS (range 0–18, 0–8, and 0–15, respectively). Overall DBD symptoms were computed by adding ADHD, ODD, and CD endorsed items (range 0–41).
The Children’s Depression Rating Scale–Revised (CDRS-R) is a semistructured interview assessing severity of 17 depressive symptoms. Total scores range from 17–113, with increasing severity.38 It has adequate inter-rater reliability (r = .86), test-retest reliability (r = .81), and validity.37 In this study, inter-rater reliability was substantial (k = 0.68).
The Mania Rating Scale (MRS) is an 11 item semi-structured interview assessing manic symptom severity.38 Total scores range from 0–60, with increasing severity. Validity and reliability are adequate for adults and children. 39,40 A study with children found significant internal consistency (α = 0.91) and a 1-factor solution from exploratory and confirmatory factor analyses with younger and older youth.40 In this study, inter-rater reliability was substantial (k = 0.71).
The Mood Severity Index (MSI) was computed from CDRS-R and MRS scores and utilized to provide a single outcome measure combining manic and depressive symptoms. An MSI score was calculated with the formula (CDRS-R score − 17 × 11/17) + MRS score. The formula adjusts for the greater number of CDRS-R items and the difference in minimum scores between measures. As irritability is rated on both scales, these items were down-weighted by half to avoid overweighting. MSI scores range from 0–116 with 4 severity categories: minimal (< 10); mild (11–20); moderate (21–35); severe (> 35).
The Children's Global Assessment Scale (CGAS) is a clinical rating scale used to assess children's functioning.41 Scores range from 1 (severe impairment) to 100 (superior functioning). Reliability and validity are adequate.41
Procedures
All procedures were approved by a University Medical Center’s Institutional Review Board. After determining potential eligibility through a phone screen, child and parent completed informed consent/assent and participated in baseline assessment. Subsequently, families were randomized into either a group immediately receiving MF-PEP (IMM+TAU) or a 1-year waitlist control (WLC+TAU). All families continued TAU throughout the study (i.e., medication management, school-based services, other therapies). If families requested or if a child was deemed severely impaired with no or inadequate treatment, additional treatment referrals were made after their baseline assessment. Stratified randomization was used after each set of 15 families completed baseline assessments to ensure equal distributions of mood disorders, comorbid disorders, and demographic variables. Project coordinators summarized these variables and the principal investigator completed randomization while masked to all other information. Follow-up assessments were completed by masked graduate research associates. Families participated in follow-up assessments at 6, 12, and 18 months. The IMM+TAU group participated in MF-PEP between baseline and 6-month assessments. The WLC+TAU group participated in MF-PEP between 12- and 18- month assessments. All assessments and 22 MF-PEP groups were conducted at a Midwestern university.
Multi-Family Psychoeducational Psychotherapy (MF-PEP)
MF-PEP consists of eight 90-minute sessions with concurrent parent and child groups. Sessions briefly begin and end with parents and children together. Initial parent and child sessions review mood symptoms, comorbid conditions, medications, and how to differentiate children from their symptoms. Subsequently, parents learn about: mental health, school, and community-based treatment; how to attain optimal services for children and work effectively with service providers; communication skills; problem-solving; and symptom management. Children learn about: CBT strategies (i.e., behavioral coping skills, cognitive restructuring); problem-solving; and verbal/nonverbal communication. Families have weekly projects and breathing exercises. MF-PEP ends with a review and graduation. Additional MF-PEP information can be found at www.moodychildtherapy.com.
Analyses
Only baseline and 12-month data were used for analyses, as these were time points that provided DBD data. Differences in baseline symptom severity and functional impairment were compared via two-tailed, independent t-tests or chi-square analyses between IMM+TAU versus WLC+TAU and treatment completers versus noncompleters. Pearson correlation coefficients were calculated for the entire baseline sample to assess strength of relationship between DBD symptoms, mood symptoms, and functional impairment. The effect of MF-PEP on class and severity of DBD symptoms was analyzed using analysis of covariance (ANCOVA), assessing differences in number of behavior symptoms at 12-months between each group, covarying severity of baseline behavior symptoms. As tests were underpowered, two-tailed paired t-tests examined within group change over time. ANCOVA was used to test effect of behavior symptoms on mood treatment response in MF-PEP. Twelve-month MSI score was the dependent variable; baseline MSI and the different behavior scores were covariates. Cohen’s d was calculated for ANCOVA and t-test analyses.
Results
Baseline Data
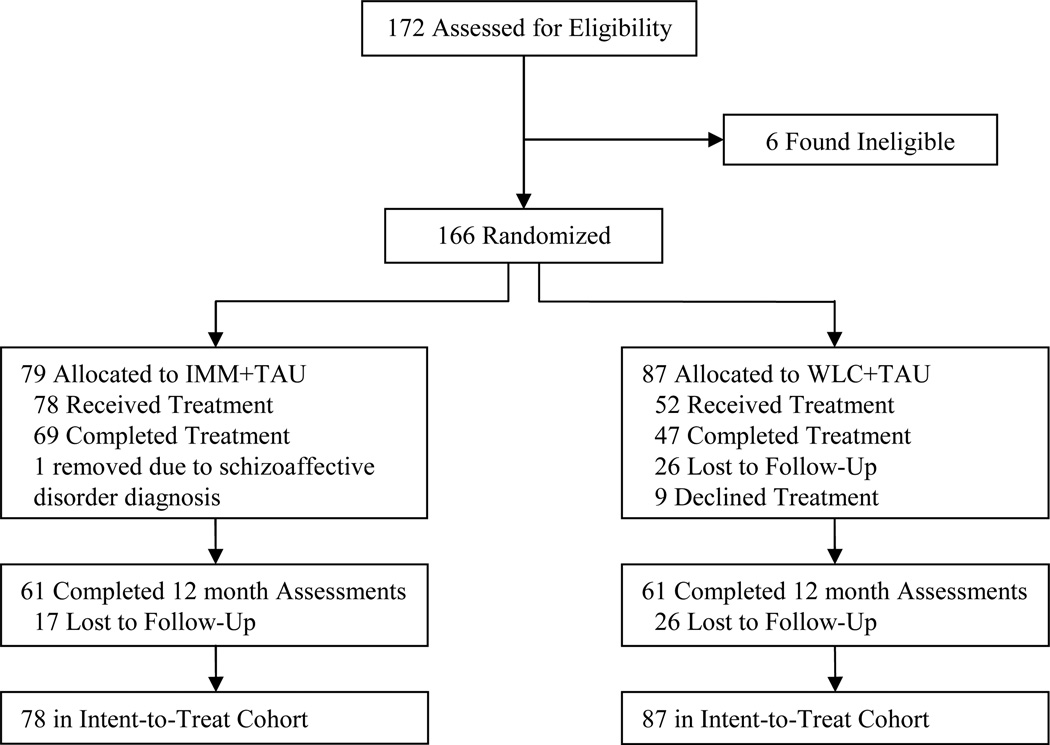
Study recruitment and assessments were conducted from 2001 to 2005. Figure 1 summarizes participant flow. Analyses included all participants for whom 12-month follow-up data were available, except for Pearson correlations, which used the ITT cohort. By 12-month follow-up, 18 families had dropped out of IMM+TAU and 26 dropped out of WLC+TAU. Families not completing treatment (n = 49 who did not complete ≥ 6 sessions) did not differ from completers on baseline severity of ADHD (t[163] = 1.72, p = .09), MSI (t[163] = 1.04, p = .30), or CGAS (t[163] = −1.84, p = .07); however, ODD (t[163] = 2.38, p = .02), CD (t[163] = 2.64, p = .01), and overall DBDs (t[163] = 2.67, p = .01) were significantly more severe in non-completers. As previously reported, while the treatment effect of MF-PEP in IMM+TAU compared with WLC+TAU was greater in treatment completers (8.17 MSI points) than in the ITT sample (6.48 MSI points), both were significant effects.29
Figure 1.
CONSORT randomized trial flow diagram. Completed Treatment = completed ≥ 6 sessions; IMM = immediate treatment group; TAU = treatment as usual; WLC = waitlist control group;
Correlations of Disruptive Behavior Symptoms, Mood Symptom Severity, and Functional Impairment
Baseline Pearson correlation coefficients indicated symptoms of ADHD, ODD, CD, and overall DBDs were significantly positively correlated with mood symptom severity and significantly negatively correlated with current global functioning (Table 2).
Table 2.
Pearson Correlations of Disruptive Behavior Symptoms, Mood Symptom Severity, and Current Global Functioning at Baseline
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. ADHD Symptoms | — | .433** | 375** | .885** | .197* | −.358** |
| 2. ODD Symptoms | — | .451** | .718** | .289** | −.292** | |
| 3. CD Symptoms | — | .696** | .185* | −.386** | ||
| 4. Overall DBD Symptoms | — | .271** | −.439** | |||
| 5. Mood Severity Index | — | −.467** | ||||
| 6. Current Global Functioning | — |
Note: ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; DBD = disruptive behavior disorder; ODD = oppositional defiant disorder.
p < .05;
p < .01.
Impact of MF-PEP on Disruptive Behavior Symptoms
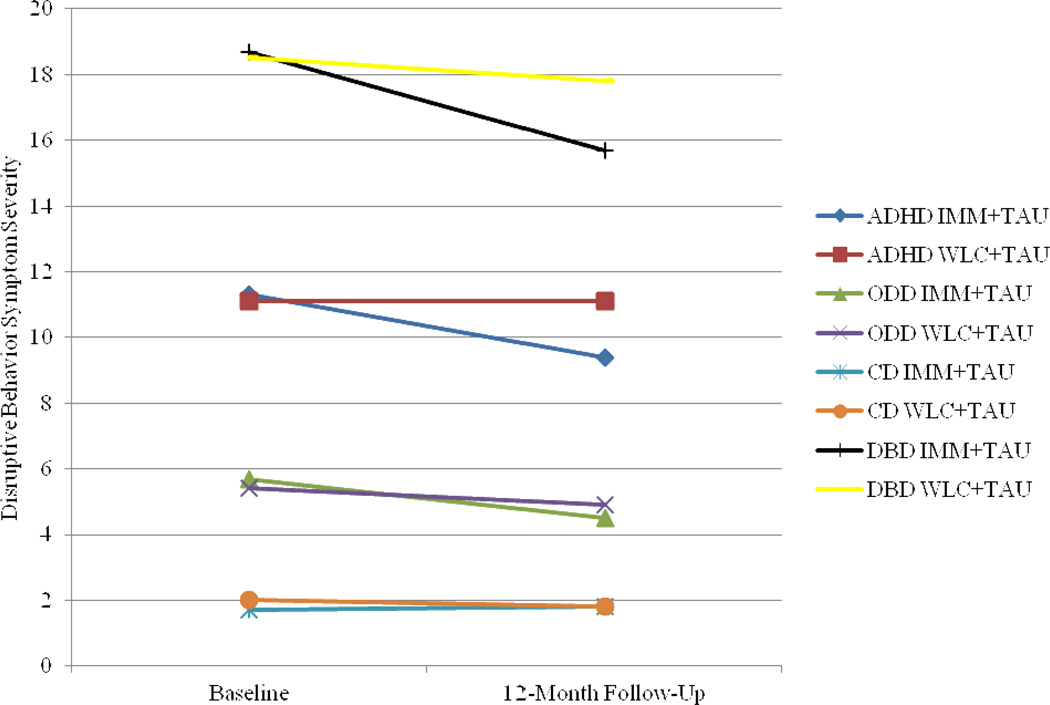
ANCOVA analyses indicated a trend approaching significance for differences between IMM+TAU and WLC+TAU ADHD symptoms at 12-month follow-up after covarying baseline ADHD severity, F(1,119) = 3.82, p = .05, d = 0.39 (observed power = 0.49). Paired t-tests between baseline and 12-month assessments for both groups suggest that MF-PEP is associated with a small but significant reduction in ADHD symptoms (Table 3).
Table 3.
Paired t-tests Comparing Baseline and 12-Month Follow-Up Means of Disruptive Behavior Symptoms
| Variables | Baseline M (SD) | 12-Month Follow-Up M (SD) | t (60) | d |
|---|---|---|---|---|
| ADHD Symptoms | ||||
| IMM+TAU | 11.3 (5.0) | 9.4 (5.4) | 2.10* | 0.36 |
| WLC+TAU | 11.1 (4.7) | 11.1 (4.8) | 0.06 | 0.01 |
| ODD Symptoms | ||||
| IMM+TAU | 5.7 (2.1) | 4.5 (2.6) | 3.17** | 0.53 |
| WLC+TAU | 5.4 (2.6) | 4.9 (2.7) | 1.29 | 0.18 |
| CD Symptoms | ||||
| IMM+TAU | 1.7 (2.4) | 1.8 (2.4) | −0.26 | −0.05 |
| WLC+TAU | 2.0 (2.5) | 1.8 (2.4) | 0.75 | 0.10 |
| Overall DBD Symptoms | ||||
| IMM+TAU | 18.7 (7.8) | 15.7 (8.5) | 2.05* | 0.37 |
| WLC+TAU | 18.5 (7.6) | 17.8 (8.0) | 0.75 | 0.09 |
Note. ADHD = attention-deficit/hyperactivity disorder; CD = conduct disorder; DBD = disruptive behavior disorder; IMM = immediate treatment group; ODD = oppositional defiant disorder; TAU = treatment as usual; WLC = waitlist control group.
p < .05;
p < .01.
Regarding ODD symptoms, ANCOVA analyses revealed no significant differences between IMM+TAU and WLC+TAU at 12-month follow-up after covarying baseline ODD severity, F(1,119) = 1.38, p = .24, d = 0.30 (observed power = 0.21). However, paired t-tests suggest that MF-PEP is associated with a significant, medium reduction in ODD symptoms (Table 3).
Similarly, ANCOVA analyses indicated no significant differences between IMM+TAU and WLC+TAU CD symptoms at 12-month follow-up after covarying baseline CD severity, F(1,119) = 0.071, p = .79, d = −0.12 (observed power = .06). There were no significant differences in average CD symptoms between baseline and 12- month assessments (Table 3).
Finally, ANCOVA analyses indicated no significant differences between IMM+TAU and WLC+TAU overall DBD symptoms at 12-month follow-up after covarying baseline overall DBD severity, F(1,119) = 2.08, p = .15, d = 0.30 (observed power = .30). However, paired t-tests suggest that MF-PEP is associated with a small but significant reduction in overall DBD symptoms. Table 3 displays results from paired t-tests comparing baseline and 12-month assessment means of DBDs for IMM+TAU and WLC+TAU. Figure 2 graphically displays results.
Figure 2.
Baseline and 12-month follow-up means of disruptive behavior symptoms. ADHD = attention-deficit/ hyperactivity disorder; CD = conduct disorder; DBD = disruptive behavior disorder; IMM = immediate treatment group; ODD = oppositional defiant disorder; TAU = treatment as usual; WLC = waitlist control group.
Impact of Disruptive Behavior Symptoms on Mood Treatment Outcome
There was a significant difference in mood symptoms at 12-month follow-up between IMM+TAU (M = 21.73, SD = 16.40) and WLC+TAU (27.84, SD = 16.70) after covarying baseline MSI severity, F(1,113) = 6.32, p = .01, d = 0.58. Consistent with hypotheses, severity of DBDs at baseline did not impact treatment response of MSI symptoms, which remained significant after covarying baseline severity of ADHD (F[1,112] = 6.29, p = .01), ODD (F[1,112] = 6.54, p = .01), CD (F[1,112] = 5.83, p = .02), and overall DBDs (F[1,112] = 6.25, p = .01).
Discussion
Children with mood disorders commonly present with comorbid DBDs. Little is known about the impact of psychosocial mood interventions on DBD symptoms and the impact of DBDs on mood symptom treatment response. This secondary analysis of MF-PEP, an efficacious psychoeducational treatment for children with mood disorders, revealed that disruptive behaviors were associated with more severe mood symptoms and worse global functioning at baseline. Between-group analyses examining outcomes of disruptive behavior symptoms were nonsignificant. However, within-group analyses indicated MF-PEP resulted in small to medium improvements in ADHD, ODD, and overall DBD symptoms, but not CD symptoms. Findings and implications should be interpreted with caution, as secondary analyses exhibited low statistical power to detect significant results.
DBD symptom severity did not impact mood outcome for children who received MF-PEP. These preliminary findings suggest that MF-PEP may be an important first line psychosocial treatment for children with comorbid mood and behavioral problems, as both symptom areas demonstrated some improvement and severity of children’s disruptive behaviors did not impact the treatment effect of MF-PEP on mood symptoms. However, as findings are based on preliminary and underpowered analyses, definitive conclusions about disruptive behavior outcomes and moderating effects in MF-PEP cannot be drawn.
As expected, disruptive behaviors were associated with more severe mood symptoms and worse global functioning. These results are consistent with previous RCTs of psychosocial treatments for adolescents with depression, which found DBDs were associated with more impairment, suicidality, depression recurrence, and need for additional treatment.12,15,16,18 This is the first RCT of a psychosocial treatment for children with depressive and bipolar disorders examining the effect of a psychosocial mood treatment on DBD symptoms and the impact of DBDs on mood outcome. Results are consistent with findings from RCTs of adolescents with depression, which indicated DBDs did not impact treatment response.15–18 Although previous studies in youth reported mixed findings for effect of mood treatments on DBD symptoms, the current study suggests MF-PEP may offer some benefit for disruptive behaviors.19–27 Results also offer preliminary empirical support for treatment guidelines of mood disorders, which recommend mood stabilization or treatment of most impairing disorder prior to addressing comorbidities.3–5
Although improvements in ADHD, ODD, and overall DBD symptoms were noted, comparisons with WLC+TAU were non-significant, within-group effect sizes were small to medium, and no effect was found on CD symptoms. The need for ongoing support for both mood and behavioral symptoms after acute phase CBT has been shown, particularly for youth with comorbid DBDs.15,21 Thus, it may be that additional, targeted behavioral treatment following MF-PEP would be helpful for children with comorbid DBDs. Mediator analyses demonstrated MF-PEP helped parents become better consumers of mental health services, and accessing higher-quality services resulted in decreased mood symptom severity.29,30 However, psychoeducation regarding effective treatment focused primarily on mood interventions. Thus, additional focus on effective interventions of DBDs during the psychoeducational component of MF-PEP may improve parents’ knowledge and ability to access higher-quality services for children’s mood and behavioral symptoms and result in subsequent improvement in both areas.
Preliminary findings suggest MF-PEP may offer some improvement for children’s comorbid DBD symptoms. A sequential approach to treatment might be beneficial. Sensitizing parents and children to strategies to ameliorate dysregulated mood may be an important precursor to improving management of behavioral symptoms, for which evidence-based treatments have been developed. The mechanism by which MF-PEP may lead to improved mood and behavioral symptoms is unknown, though several theories are suggested. First, MF-PEP may provide parents with knowledge and skills to implement effective coping strategies and access more appropriate services for children, subsequently resulting in improved mood and behavioral symptoms. Second, it may be that once children’s mood is stabilized, implementation of parent management strategies for disruptive behaviors are more effective. Third, as children benefit from a non-blaming treatment for their mood disorder, it may be that their tendency to misbehave declines. Lastly, common symptoms of these disorders, such as irritability and negative emotionality, may respond similarly to MF-PEP. Replication, sequencing, and dismantling studies are needed to confirm whether MF-PEP leads to meaningful improvement in DBDs, evaluate the effective components of MF-PEP, and determine which elements are associated with improvement in mood and which may lead to benefit in DBDs.
Several limitations should be noted. This sample lacked diversity, and therefore, may not be representative of the broader population of children with mood disorders and DBDs. Secondary analyses exhibited severely low power to detect significant between-group differences. Effect sizes were small to medium; thus, we cannot be confident in the stability of these effects. Also, the high frequency of DBD diagnoses limited the ability to examine moderation. In addition, outcome measures reflected parents’ perspective of symptom change. Parents are acceptably reliable informants of both mood and behavioral symptoms in children. However, because parents are informants of both outcomes, shared method variance effects cannot be examined. In addition, MF-PEP+TAU was compared to WLC+TAU rather than placebo or active treatment; thus, conclusions cannot be drawn about specific components of MF-PEP compared to other interventions. Further, the study’s primary aim was to examine efficacy of MF-PEP in reducing children’s mood symptoms; thus, questionnaires or treatment components specifically targeting disruptive behaviors were not incorporated. Instead, P-ChIPS symptom tallies were used as a proxy for symptom severity, as endorsement of more symptoms in a diagnostic category likely reflects greater severity. Also, nonsignificant results for DBD outcomes, particularly CD, may have been due to a floor effect. Finally, behavioral measures were only administered at baseline and 12-month follow-up, further restricting analyses. Given these limitations, especially low statistical power, findings are preliminary and must be interpreted with caution. Nevertheless, results are informative regarding psychosocial treatment of children with mood disorders and comorbid DBDs.
In conclusion, MF-PEP is an efficacious intervention for children with mood disorders and also provides some benefit for ADHD, ODD, and overall DBD symptoms, but not CD symptoms. Though comorbid DBDs were associated with worse mood symptoms and functional impairment, DBDs did not impact MF-PEP’s treatment effect on mood symptoms. Thus, preliminary results suggest MF-PEP may be an important first line psychosocial treatment for children presenting with comorbid mood and disruptive behavior disorders, as comorbidity does not affect mood outcome and may result in some improvement in DBD symptoms. Families of children with comorbid mood and disruptive behavior disorders may especially benefit from psychoeducation about symptoms, management, and treatment of both disorders and how to access and coordinate appropriate services. Nevertheless, after mood stabilization, children likely will still require targeted behavioral treatment to address remaining DBD symptoms. Improvement in mood symptoms following MF-PEP may make subsequent treatment targeting DBD symptoms more effective. Thus, despite study limitations and low statistical power, preliminary results suggest MF-PEP may be an important intervention for youth with comorbid mood and disruptive behavior disorders.
Acknowledgments
Dr. Boylan served as the statistical expert for this research.
Dr. Fristad has received royalties for a treatment manual published by Guilford Press, Inc., and multifamily psychoeducational psychotherapy (MF-PEP) workbooks (www.moodychildtherapy.com). She has also received funding from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Dr. Boylan and Ms. MacPherson report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Khrista Boylan, McMaster University
Heather A. MacPherson, Ohio State University
Mary A. Fristad, Ohio State University
References
- 1.Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- 2.Van Meter AR, Moreira ALR, Youngstrom EA. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiatry. 2011;72:1250–1256. doi: 10.4088/JCP.10m06290. [DOI] [PubMed] [Google Scholar]
- 3.Birmaher B, Brent D, Bernet W, et al. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- 4.Kowatch RA, Fristad M, Birmaher B, et al. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 5.McClellan J, Kowatch R, Findling RL Work Group on QI. Practice parameter for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:107–125. doi: 10.1097/01.chi.0000242240.69678.c4. [DOI] [PubMed] [Google Scholar]
- 6.Vostanis P, Feehan C, Grattan E, Bickerton W. Treatment for children and adolescents with depression: Lessons from a controlled trial. Clin Child Psychol Psychiatry. 1996;1:199–212. doi: 10.1016/0165-0327(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 7.Weisz JR, Southam-Gerow M, Gordis EB, et al. Cognitive–behavioral therapy versus usual clinical care for youth depression: An initial test of transportability to community clinics and clinicians. J Consult Clin Psychol. 2009;77:383–396. doi: 10.1037/a0013877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowatch RA. Definitions. In: Kowatch RA, Fristad MA, Findling RL, Post RM, editors. A Clinical Manual for the Management of Bipolar Disorder in Children and Adolescents. Arlington, VA: American Psychiatric Press; 2008. pp. 43–70. [Google Scholar]
- 9.Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40:57–87. [PubMed] [Google Scholar]
- 10.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorders in a community sample of older adolescents: Prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- 11.Stringaris A, Santosh P, Leibenluft E, Goodman R. Youth meeting symptom and impairment criteria for mania-like episodes lasting less than four days: An epidemiological enquiry. J Child Psychol Psychiatry. 2010;51:31–38. doi: 10.1111/j.1469-7610.2009.02129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohde P, Seeley JR, Kaufman NK, Clarke GN, Stice E. Predicting time to recovery among depressed adolescents treated in two psychosocial group interventions. J Consult Clin Psychol. 2006;74:80–88. doi: 10.1037/0022-006X.74.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratochvil CJ, May DE, Silva SG, et al. Treatment response in depressed adolescents with and without comorbid attention-deficit/hyperactivity disorder in the treatment for adolescents with depression study. J Child Adolesc Psychopharmacol. 2009;19:519–527. doi: 10.1089/cap.2008.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asarnow JR, Emslie G, Clarke G, et al. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: Predictors and moderators of treatment response. J Am Acad Child Adolesc Psychiatry. 2009;48:330–339. doi: 10.1097/CHI.0b013e3181977476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brent DA, Kolko DJ, Birmaher B, Baugher M, Bridge J. A clinical trial for adolescent depression: Predictors of additional treatment in the acute and follow-up phases of the trial. J Am Acad Child Adolesc Psychiatry. 1999;38:263–271. doi: 10.1097/00004583-199903000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Rohde P, Clarke GN, Lewinsohn PM, Seeley JR, Kaufman NK. Impact of comorbidity on a cognitive-behavioral group treatment for adolescent depression. J Am Acad Child Adolesc Psychiatry. 2001;40:795–802. doi: 10.1097/00004583-200107000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Curry J, Rohde P, Simons A, et al. Predictors and moderators of acute outcome in the treatment for adolescents with depression study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45:1427–1439. doi: 10.1097/01.chi.0000240838.78984.e2. [DOI] [PubMed] [Google Scholar]
- 18.Dubicka B. The clinical implications of comorbid behaviour disorder and adolescent depression in ADAPT. Paper presented at: 58th Annual Meeting of the American Academy of Child and Adolescent Psychiatry; October 2011; Toronto, ON, Canada. [Google Scholar]
- 19.Kolko DJ, Brent DA, Baugher M, Bridge J, Birmaher B. Cognitive and family therapies for adolescent depression: Treatment specificity, mediation, and moderation. J Consult Clin Psychol. 2000;68:603–614. [PubMed] [Google Scholar]
- 20.Lewinsohn PM, Clarke GN, Hops H, Andrews JA. Cognitive-behavioral treatment for depressed adolescents. Behav Ther. 1990;21:385–401. [Google Scholar]
- 21.Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive-behavioral treatment of adolescent depression: Efficacy of acute group treatment and booster sessions. J Am Acad Child Adolesc Psychiatry. 1999;38:272–279. doi: 10.1097/00004583-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Rohde P, Clarke GN, Mace DE, Jorgensen JS, Seeley JR. An Efficacy/Effectiveness study of cognitive-behavioral treatment for adolescents with comorbid major depression and conduct disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:660–668. doi: 10.1097/01.chi.0000121067.29744.41. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs RH, Becker-Weidman E, Reinecke MA, et al. Treating depression and oppositional behavior in adolescents. J Clin Child Adol Psychol. 2010;39:559–567. doi: 10.1080/15374416.2010.486318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muratori F, Picchi L, Bruni G, Patarnello M, Romagnoli G. A two-year follow-up of psychodynamic psychotherapy for internalizing disorders in children. J Am Acad Child Adolesc Psychiatry. 2003;42:331–339. doi: 10.1097/00004583-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Miklowitz DJ, George EL, Axelson DA, et al. Family-focused treatment for adolescents with bipolar disorder. J Affect Disord. 2004;82:S113–S128. doi: 10.1016/j.jad.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavuluri MN, Graczyk PA, Henry DB, Carbray JA, Heidenreich J, Miklowitz DJ. Child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: Development and preliminary results. J Am Acad Child Adolesc Psychiatry. 2004;43:528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 27.West AE, Henry DB, Pavuluri MN. Maintenance model of integrated psychosocial treatment in pediatric bipolar disorder: A pilot feasibility study. J Am Acad Child Adolesc Psychiatry. 2007;46:205–212. doi: 10.1097/01.chi.0000246068.85577.d7. [DOI] [PubMed] [Google Scholar]
- 28.Fristad MA, Goldberg Arnold JS, Leffler JM. Psychotherapy for Children with Bipolar and Depressive Disorders. New York, NY: Guilford Press; 2011. [Google Scholar]
- 29.Fristad MA, Verducci JS, Walters K, Young ME. Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Arch Gen Psychiatry. 2009;66:1013–1020. doi: 10.1001/archgenpsychiatry.2009.112. [DOI] [PubMed] [Google Scholar]
- 30.Mendenhall AN, Fristad MA, Early TJ. Factors influencing service utilization and mood symptom severity in children with mood disorders: Effects of multifamily psychoeducation groups (MFPGs) J Consult Clin Psychol. 2009;77:463–473. doi: 10.1037/a0014527. [DOI] [PubMed] [Google Scholar]
- 31.Cummings CM, Fristad MA. Anxiety in children with mood disorders: A treatment help or hindrance? J Abnorm Child Psychol. 2012;40:339–351. doi: 10.1007/s10802-011-9568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacPherson HA, Algorta GP, Mendenhall AN, Fields BW, Fristad MA. Predictors and moderators in the randomized trial of multi-family psychoeducational psychotherapy for childhood mood disorders. J Clin Child Adolesc Psychol. doi: 10.1080/15374416.2013.807735. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1988. [Google Scholar]
- 34.Weller EB, Weller RA, Rooney MT, Fristad MA. Children's Interview for Psychiatric Syndromes (ChIPS) Washington, DC: American Psychiatric Press, Inc; 1999. [DOI] [PubMed] [Google Scholar]
- 35.Weller EB, Weller RA, Rooney MT, Fristad MA. Children's Interview for Psychiatric Syndromes, Parent Version (P-ChIPS) Washington, DC: American Psychiatric Press, Inc; 1999. [Google Scholar]
- 36.Weller EB, Weller RA, Fristad MA, Rooney MT, Schecter J. Children's interview for psychiatric syndromes (ChIPS) J Am Acad Child Adolesc Psychiatry. 2000;39:76–84. doi: 10.1097/00004583-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 37.Poznanski EO, al e. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 39.Fristad MA, Weller RA, Weller EB. The mania rating scale (MRS): Further reliability and validity studies with children. Ann Clin Psychiatry. 1995;7:127–132. doi: 10.3109/10401239509149039. [DOI] [PubMed] [Google Scholar]
- 40.Youngstrom EA, Danielson CK, Findling RL, Gracious BL, Calabrese JR. Factor structure of the young mania rating scale for use with youths ages 5 to 17 years. J Clin Child Adolesc Psychol. 2002;31:567–572. doi: 10.1207/S15374424JCCP3104_15. [DOI] [PubMed] [Google Scholar]
- 41.Shaffer D, Gould MS, Brasic J, Ambrosini P, Bird H, Aluwahlia S. A children's global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]