Abstract
Thrombosis and infection are two common problems associated with blood-contacting medical devices such as catheters. Nitric oxide (NO) is known to be a potent antimicrobial agent as well as an inhibitor of platelet activation and adhesion. Healthy endothelial cells that line the inner walls of all blood vessels exhibit a NO flux of 0.5~4×10−10 mol cm−2 min−1 that helps prevent thrombosis. Materials with a NO flux that is equivalent to this level are expected to exhibit similar anti-thrombotic properties. In this study, NO-releasing catheters were fabricated by incorporating S-nitroso-N-acetylpenicillamine (SNAP) in the Elast-eon E2As polymer. The SNAP/E2As catheters release physiological levels of NO for up to 20 d, as measured by chemiluminescence. Furthermore, SNAP is stable in the E2As polymer, retaining 89% of the initial SNAP after ethylene oxide (EO) sterilization. The SNAP/E2As and E2As control catheters were implanted in sheep veins for 7 d to examine the effect on thrombosis and bacterial adhesion. The SNAP/E2As catheters reduced the thrombus area when compared to the control (1.56 ± 0.76 and 5.06 ± 1.44 cm2, respectively). A 90% reduction in bacterial adhesion was also observed for the SNAP/E2As catheters as compared to the controls. The results suggest that the SNAP/E2As polymer has the potential to improve the hemocompatibility and bactericidal activity of intravascular catheters, as well as other blood-contacting medical devices (e.g., vascular grafts, extracorporeal circuits).
Keywords: bacterial adhesion, blood compatibility, catheter, nitric oxide, S-nitrosothiols, thrombosis
1. Introduction
Blood-material interaction is critical to the success of many medical devices such as intravascular catheters, vascular grafts, stents, and extracorporeal life support circuits.1, 2 Two common factors that can cause complications with blood-contacting devices are thrombosis and infection. As soon as blood comes in contact with foreign surfaces, platelets adhere and become activated, forming thrombus within hours. Clinical use of catheters range from acute catheters placed in operating rooms, emergency rooms, and intensive care units (ICUs) (typically for up to 7 d), to more permanent catheters for long-term nutrition, dialysis access, etc. (months to years). Complications due to thrombosis and infection can result in extended hospital stays, increased healthcare costs, and even patient death. Thrombus formation on catheters decreases their patency and functional lifetime. Venous thrombosis has been detected by Doppler imaging in 33% of intensive care unit patients.3 Infection is another significant problem, with 1.7 million Healthcare Associated Infections that result in 99,000 deaths per year in the United States alone.4 In addition, up to 40% of all indwelling catheters become infected.5 An estimated 80,000 catheter-related bloodstream infections occur in patients within ICUs in the United States, resulting in as many as 28,000 deaths per year.6
Over the last 50 years, much has been learned about surface-induced thrombosis and many different strategies to create hemocompatible materials have been reported.1, 7-9 Some of the surface modifications to improve hemocompatibility include hydrophilic or hydrophobic surfaces, zwitterionic polymers, and immobilized heparin. However, in a clinical setting many devices still require the use of systemic or localized anticoagulation (e.g., heparin lock solutions) to avoid device failure due to thrombosis.10 Long-term use of systemic anticoagulation is not desirable because it can have adverse side effects such as hemorrhage, thrombocytopenia, and thrombosis.11 Catheters with antimicrobial coatings (e.g., containing silver compounds or antibiotics) are available, but are not completely effective at preventing infection and biofilm formation and also do not address complications associated with activation of platelets.5, 12 A recent study reported that catheters coated with a silver alloy had a similar infection rate as control catheters.13 Antibiotic resistant bacterial strains are becoming more common in the hospital setting.14, 15 Further, bacteria have the ability to form biofilms (communities of bacterial encased in a self-synthesized extracellular matrix) that protect the bacteria from antiseptics, antibiotics, and the host's defense system.16-19 Biofilm formation decreases the effectiveness of many antibiotics because they cannot penetrate the biofilm matrix, making the infections difficult to eradicate.20
The use of nitric oxide (NO)-releasing polymers is one approach that has great potential to improve the hemocompatibility and antimicrobial activity of blood-contacting devices such as catheters. Radomski et al. first described NO as a potent vasodilator secreted by the normal endothelium that has the ability to inhibit platelet adhesion and aggregation to the blood vessel wall.21, 22 Nitric oxide is a natural inhibitor of platelet activation that is released from healthy endothelial cells at a flux into the blood stream of 0.5 -4.0 × 10−10 mol cm−2 min−1. 23 In addition, NO that is released within the sinus cavities and by neutrophils/macrophages functions as a potent natural antimicrobial agent.24, 25 Nitric oxide is known to have broad-spectrum antibacterial properties, where both gram-positive and gram-negative bacteria can be killed.26 Therefore, use of NO-releasing polymers has the advantage of enabling the creation of dual-functioning catheters that would possess both antithrombotic and antiseptic properties.
A wide variety of nitric oxide (NO)-releasing polymers have been reported and investigated for their potential biomedical applications.24, 27-29 NO-releasing polymers have been shown to preserve platelet count and reduced thrombus formation when tested in a 4 h rabbit model of extracorporeal circulation (ECC) thrombogenicity.30-34 I-nitrosothiol-modified xerogels were shown to significantly reduce adhesion of platelets and bacteria (Pseudomonas aeruginosa) when tested in vitro.35, 36 Diazeniomdiolate-doped poly(lactic-co-glycolic acid)-based films exhibited antibiofilm properties against both gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) bacteria when tested using a drip-flow bioreactor over a 7 d period.37 Despite these promising literature reports, many of these NO-releasing polymers have not been clinically applied due to instability of the NO donor species within the polymers during storage or sterilization, significant leaching of unbound NO donors, or the requirement of complex chemistry to covalently bind the NO donor to the polymer.30, 38 For example, S-nitrosothiol-modified polyesters were effective at reducing platelet adhesion/activation and had antimicrobial effect against Staphylococcus aureus and the multi-drug resistant Pseudomonas aeruginosa strains, but the NO release capability was significantly influenced by ethylene oxide sterilization.39, 40 In addition, most of the NO-releasing materials reported to date have been tested in vitro or in short-term animal models for their hemocompatibility properties, so testing these materials in longer-term animal models would be beneficial to evaluate more clinically relevant situations.
Herein we report one of the first longer-term (7 d) in vivo evaluation studies of novel NO-releasing catheters. As we have previously reported, a SNAP-doped Elast-eon E2As polymer formulation has very attractive properties, in terms of its long-term NO release, enhanced shelf-life stability, and its ability to preserve platelets and reduce thrombus during the 4 h extracorporeal circulation, that could make clinical applications feasible.30 In this new study, intravascular catheters were fabricated with the SNAP/E2As polymer using a dip coating method. The NO release from these catheters was monitored over a 20 d period under physiological conditions (37 °C, dark). Another key hurdle for potential clinical applications is the ability of the NO-releasing materials to withstand sterilization. Therefore, the effect of ethylene oxide (EO) sterilization on the SNAP/E2As catheters was also evaluated. Finally, the SNAP/E2As catheters were assessed for their ability to reduce thrombosis and bacterial adhesion after 7 d intravascular implantation in sheep.
2. Materials and Methods
2.1 Materials
N-Acetyl-DL-penicillamine (NAP), sodium chloride, potassium chloride, sodium phosphate dibasic, potassium phosphate monobasic, ethylenediaminetetraacetic acid (EDTA), tetrahydrofuran (THF), sulfuric acid and N,N-dimethylacetamide (DMAc) were purchased from Sigma-Aldrich (St. Louis, MO). Methanol, hydrochloric acid and sulfuric acid were obtained from Fisher Scientific (Pittsburgh, PA). Elast-eon™ E2As was obtained from AorTech International, plc (Scoresby, Victoria, Australia). All aqueous solutions were prepared with 18.2 MΩ deionized water using a Milli-Q filter (Millipore Corp., Billerica, MA). Phosphate buffered saline (PBS), pH 7.4, containing 138 mM NaCl, 2.7 mM KCl, 10 mM sodium phosphate, 100 μM EDTA was used for all in vitro experiments.
2.2 SNAP Synthesis Protocol
SNAP was synthesized using a modified version of a previously reported method.41 Briefly an equimolar ratio of NAP and sodium nitrite was added to a 1:1 mixture of water and methanol containing 2 M HCl and 2 M H2SO4. After 30 min of stirring, the reaction vessel was cooled in an ice bath to precipitate the green SNAP crystals. The crystals were collected by filtration, washed with water, and allowed to air dry. The reaction mixture and resulting crystals were protected from light at all times.
2.3 Preparation of Intravascular Catheters
Catheters were prepared by dip coating polymer solutions on 18 cm long stainless steel mandrels of 2.0 mm diameter (purchased from McMaster Carr). The E2As control catheter solution consisted of E2As dissolved in THF (150 mg/mL). Thirty-five coats of the E2As solution was applied on the mandrel by dip coating at 2 min intervals between each coat. The SNAP-doped E2As catheters had a trilayer configuration, E2As top/base coats and a SNAP-containing middle active layer. The top/base coat solution consisted of E2As dissolved in THF (150 mg/mL). The active solution was formulated to contain 10 wt% SNAP and 90 wt% E2As dissolved in THF with an overall total concentration of 150 mg/mL of solids. The trilayer catheters were prepared by dip coating 5 base coats of E2As solution, 25 coats of active solution, and 5 top-coats of E2As solution. All catheters were allowed to dry overnight under ambient conditions, protected from light. Cured catheters were removed from the mandrels and dried under vacuum for 48 h. Catheters had an i.d. of 2.08 ± 0.06 mm and o.d. of 3.30 ± 0.12 mm, as measured with a Mitutoyo digital micrometer.
2.4 NO Release Measurements
Nitric oxide release from the catheters was measured using a Sievers chemiluminescence Nitric Oxide Analyzer (NOA), model 280 (Boulder, CO). A sample catheter segment (1 cm) was placed in 4 mL PBS buffer at 37 °C. Nitric oxide liberated from the sample was continuously swept from the headspace of the sample cell and purged from the buffer with a nitrogen sweep gas and bubbler into the chemiluminescence detection chamber. The flow rate was set to 200 mL/min with a chamber pressure of 5.4 Torr and an oxygen pressure of 6.0 psi. Catheter segments were incubated in 4 mL of PBS buffer, pH 7.4, at 37 °C and tested for NO release at various time points. The NO release from catheters is normalized by the surface area using the flux unit (×10−10 mol cm−2 min−1). The chemiluminescence nitric oxide analyzer (NOA) measures the NO release from samples in ppb units within the gas phase of nitrogen that purges the solution in which the catheters are placed. The NO flux is calculated based on the surface area of the catheter sample and using this raw data (NO level in ppb) and NOA constant (with units of mol ppb−1 s−1) of the instrument. The buffer was replaced every day. After the 7 d chronic animal studies, 1 cm sections of the implanted NO release catheters were tested for NO release post-blood exposure. For dry vs. humidity experiments, a catheter sample was placed in a dry NOA sample cell incubated at 55 °C. Humidity was introduced by injecting PBS buffer into the cell, with the catheter sample suspended in the air above the buffer. The relative humidity in the sample cell was measured using a Fieldpiece PRH2 psychrometer.
2.5 Effect of Ethylene Oxide (EO) Sterilization
The SNAP-doped E2As catheters were sterilized by ethylene oxide (EO) at the University of Michigan Hospital sterilization facility. Briefly, the EO sterilization procedure consists of the following steps, all at 54 °C: humidity and conditioning (1 h, 40-80% humidity); EO gas exposure (2-3 h, 40-80% humidity); and exhaust/aeration (14 h). Catheters were tested for the wt% SNAP content before and after EO sterilization by UV-Vis analysis. Catheter pieces were weighed and dissolved in N,N-dimethylacetamide (DMAc) for rapid determination of SNAP by UV-Vis (absorbance at 340 nm). UV-Vis spectra were recorded in the wavelength range of 200-700 nm using a UV-Vis spectrophotometer (Lambda 35, Perkin-Elmer, MA) at room temperature. The molar absorption coefficient for SNAP in DMAc at 340 nm was determined to be: εSNAP=1025 M−1 cm−1.
2.6 Catheter Implantation in Sheep Model
Sheep catheter implantation protocol
All animals received care compliant with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guideline for the Care of Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the NIH. This study was approved by the University of Michigan Committee on Use and Care of Animals. Five adult sheep (Valley View Farms, Dexter, MI) were utilized for the large animal model. All experiments were performed under sterile conditions. Implantation of the catheters was performed under general anesthetic. Propofol (APP Pharmaceuticals LLC, Schaumburg, IL) (1 mg/kg) was used for induction followed by isoflurane (Peramal Health Car Limited, Andhra Pradesh, India) (0.1-4%) anesthetic for maintenance. A small 2-3 cm incision was created overlying the jugular vein. The right and left jugular veins were then isolated and either control (E2As) or experimental (SNAP/E2As) cannulas were placed under direct visualization. Small 1-2 cm vertical and transverse incisions were created over the right and left jugular veins. Catheters were then placed using a modified Seldinger technique with one catheter, either control (E2As) or experimental (SNAP/E2As), placed in either the right or left jugular vein, and the other catheter in the other vein. The skin was then re-approximated using skin staples.
Post-operative recovery protocol
Sheep were recovered from anesthesia after the catheter placements and returned to animal housing. The sheep remained in animal housing throughout the remainder of the experiment. Necropsy was performed on day 7. Sheep were anesthetized using the same anesthetic protocol described above. The right and left jugular veins were dissected along their length and isolated. Sheep were heparinized using approximately 100-150 IU/kg bolus dose and activated clotting time of >200 s was confirmed. The jugular veins were then ligated and opened longitudinally. Catheters were removed and placed in sterile saline for further analysis.
Catheter evaluation
After explanting, the catheters were rinsed in sterile saline solution. Pictures were taken of the exterior of the whole catheter using a Nikon L24 digital camera. Catheter sections (1 cm) were cut for NO release testing and bacterial adhesion measurements. To quantitate the viable bacteria on the surfaces of the catheters, a 1 cm piece was cut longitudinally and was homogenized in 1 mL sterile PBS buffer. The resulting homogenate was serially diluted in sterile PBS. Triplicate aliquots of each dilution (10 μL) of each dilution were plated on LB agar plates. The agar plates were incubated at 37 °C for 24 h followed by calculation of colony forming units per catheter surface area (CFU/cm2).
2.7 Statistical Analysis
Data are expressed as mean ± standard deviation (SD). The results between the control and SNAP catheters were analyzed by a comparison of means using Student's t-test. Values of p < 0.05 were considered statistically significant for all tests.
3. Results and Discussion
3.1 In Vitro NO Release from Catheters and Effects of Ethylene Oxide Sterilization
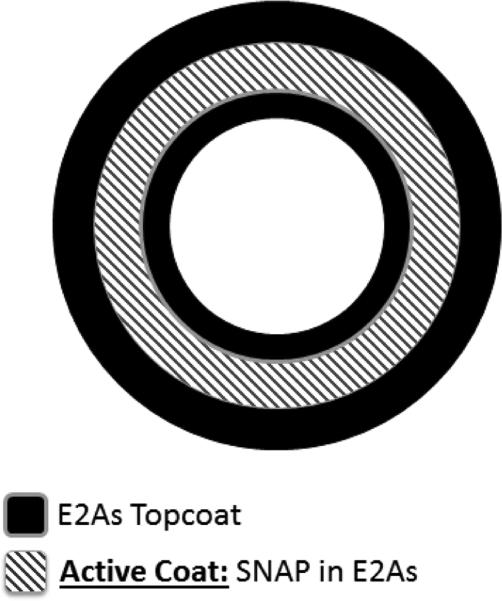
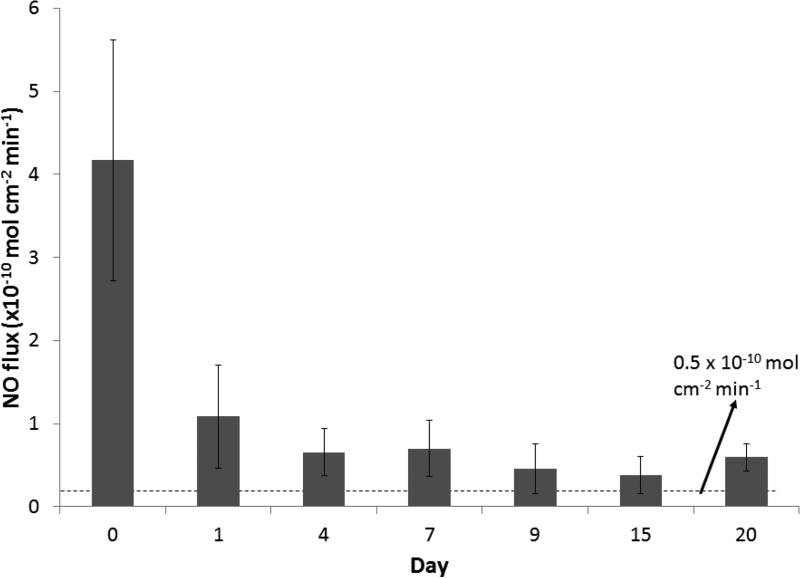
As we have previously reported, the SNAP-doped E2As polymer has many properties that make it desirable for a variety of biomedical applications. The properties include long-term NO release, stability during long-term storage, and ability to preserve platelets during short-term extracorporeal circulation 30. Nitric oxide release from S-nitrosothiols (RSNOs), such as SNAP, can be catalyzed by heat, moisture, metal ions (e.g. Cu+), or light irradiation at the energies that correspond to the S-nitroso absorption bands at 340 and/or 590 nm.42-45 The spontaneous decomposition reaction of RSNOs (no redox reaction) yields a disulfide (RSSR) product and NO. In these catheters the NO release from the SNAP-doped E2As polymer is initiated by the heat and moisture when the devices are placed in the flowing blood. In this study, catheters were prepared with the SNAP-doped E2As polymer using a dip coating method and the trilayer configuration shown in Fig. 1. The top and base coats of E2As were employed to reduce any initial burst of NO from any leaching of SNAP from the surface of the catheters. There is little concern regarding the low levels of SNAP leaching typically observed from the SNAP/E2As polymer, since the parent thiol (NAP) is clinically used to treat heavy metal poisoning.46-49 Hence, the small amounts of SNAP, NAP, or NAP disulfide that could diffuse from the catheter into the blood are not likely to create any toxicity issues. The NO release from SNAP/E2As catheters was monitored using the NOA over a 20 d period (Fig. 2). The catheters exhibited a higher level of NO release upon first exposure to physiological conditions (soaked in PBS at 37 °C) on Day 0. The initial water uptake of the polymer and leaching of SNAP on the outer surface of the catheters contributes to the higher level of NO release observed during this initial test period. In blood, NO has a short half-life due to its rapid scavenging by hemoglobin50; therefore, initial NO release levels that are somewhat higher than physiological levels, such as what is observed on Day 0, are not be a significant concern. This is because the surface areas of the catheters studied here are very small compared to the entire surface areas of the inner walls of all blood vessels within the body that are producing NO at physiological levels continuously. As shown, the SNAP/E2As catheters continue to release physiological levels of NO (> 0.5 × 10−10 mol cm−2 min−1) for up to 20 d. The NO release from the catheters at that time approaches the lower end of the normal endothelium range, which may still prove beneficial to reduce intravascular catheter thrombosis and bacterial adhesion.
Fig. 1.
Schematic of SNAP-doped E2As catheters with a trilayer configuration.
Fig. 2.
NO release from SNAP/E2As catheters under physiological conditions (soaking in PBS buffer at 37 °C in the dark). Data represents the mean ± SD (n=5).
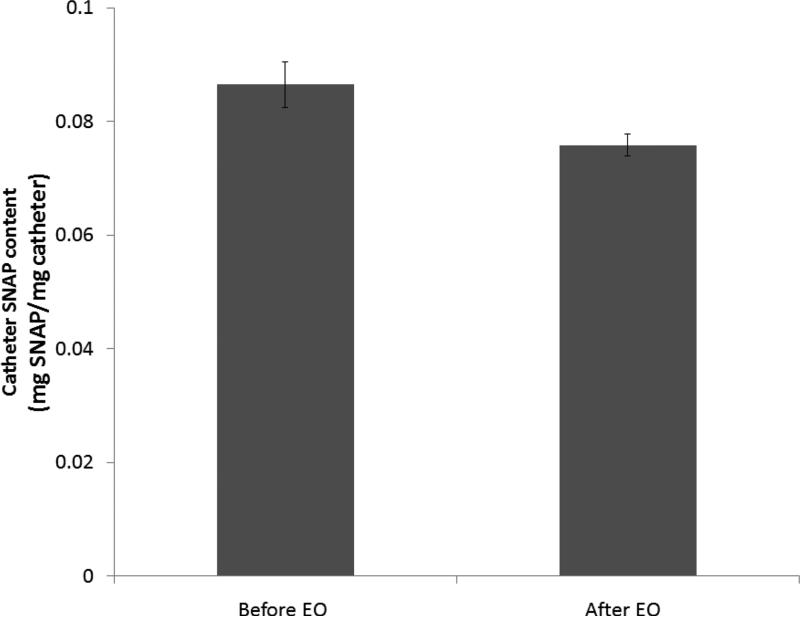
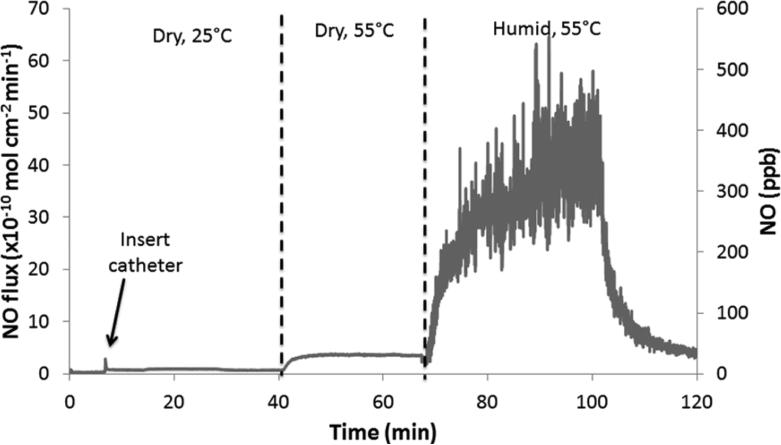
In order for medical devices, such as the SNAP/E2As catheters, to be used clinically they must be able to withstand sterilization and still retain their NO-releasing properties. Ethylene oxide (EO) sterilization is one common method employed to sterilize medical devices. This method is recommended for heat-sensitive devices over other sterilization techniques (e.g., autoclave). Therefore, the SNAP/E2As catheters were also evaluated for their ability to withstand the EO sterilization process. One centimeter sections of the SNAP/E2As catheter were EO sterilized by the University of Michigan Hospital sterilization facility. Precautions were taken to minimize any excessive exposure to light, so that only the effects of the EO sterilization process could be observed. The SNAP/E2As catheters (before and after EO) were dissolved in DMAc to quantitate the wt% SNAP by UV-Vis. The catheters that had been EO sterilized had 88.8 ± 1.7 % of the original SNAP (see Fig. 3), and the NO release profile after sterilization was not significantly affected (data not shown). During the EO sterilization process, the SNAP/E2As catheters were not only exposed to the EO gas, but also were exposed to elevated temperatures (54 °C) and humidity (40-80 %RH). The NO release from catheter sections was observed with the NOA under dry and humid conditions at 55 °C. As shown in Fig. 4, the NO release from the catheters is low under dry conditions, but dramatically increases upon exposure to humidity (~ 50% RH). This demonstrates that the NO loss observed during EO sterilization may, in part, be caused by the high humidity exposure. This loss of SNAP could be significantly reduced if the devices can be EO sterilized under dry conditions.
Fig. 3.
The SNAP content (mg SNAP/mg catheter) of catheters before and after ethylene oxide (EO) sterilization. Data represents the mean ± SD (n=4).
Fig. 4.
Representative NO release from SNAP/E2As catheters under various conditions: dry and room temperature (25 °C); dry and 55 °C; and humid (~50 %RH) and 55 °C.
3.2 Evaluation of Thrombus Formation and Bacterial Adhesion on Catheters in 7 d Sheep Model
Thrombus formation and infection are the two main problems that cause complications with indwelling catheters. The catheters were implanted for 7 d in the jugular veins of sheep (1 SNAP/E2As catheter and 1 E2As control catheter per animal). This 7 d implantation period was selected to mimic potential clinical applications (e.g., Intensive Care Units, etc.) where thrombosis and infection causes complications.51 Following catheter implantation, sheep were monitored closely for changes in behavior, weight, appearance, and activity level. All the sheep recovered rapidly from the surgical procedure and returned to a normal activity level. At the time of catheter explantation, precautions were taken to remove the catheter from the vessel without disrupting the catheter surface. The vessel was cut longitudinally to carefully remove the entire catheter. The catheter was briefly rinsed in sterile saline solution. Any residual thrombus on the catheter was photographed. Explanted catheters were systematically cut into 1 cm sections starting at the distal tip for post-implantation NO release measurements and bacterial adhesion testing.
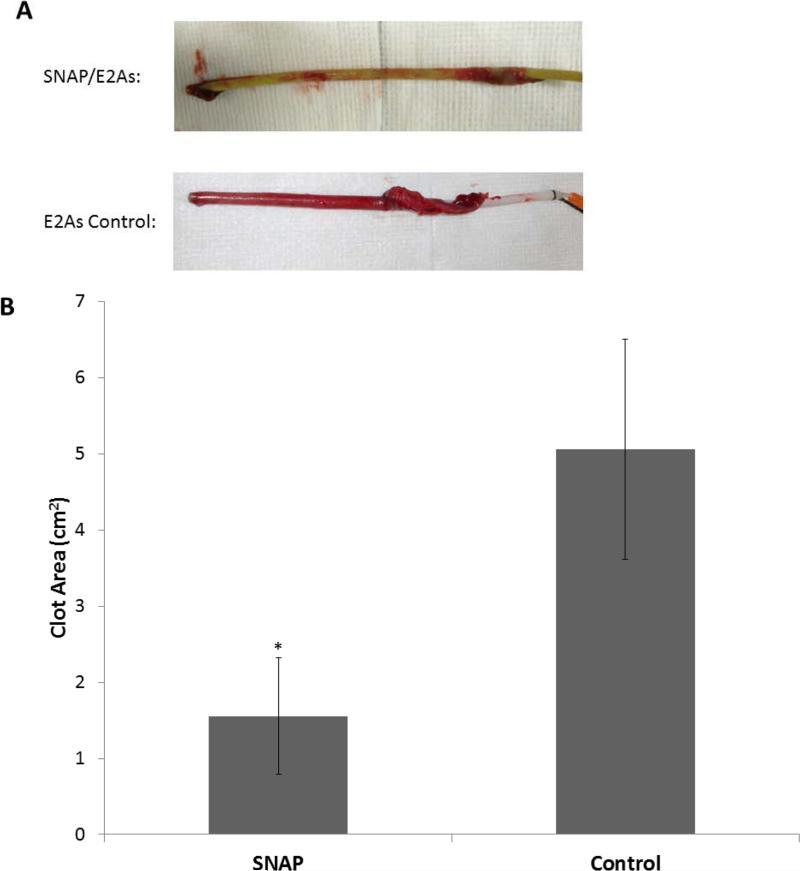
Surface thrombi on the explanted catheters were photographed and the degree of thrombus area was quantitated using Image J imaging software from National Institutes of Health (Bethesda, MD). Fig. 5A, shows representative images of clot formation on the exterior of SNAP/E2As and control catheters. These thrombi area measurements were quantitated and, as shown in Fig. 5B, the thrombus area of the SNAP/E2As catheters was significantly reduced compared to the control catheters, 1.56 ± 0.76 and 5.06 ± 1.44 cm2, respectively (n=5 each).
Fig. 5.
Representative images of SNAP/E2As and E2As control catheters after 7 d implantation in sheep veins (A). The catheters used in this study had an i.d. .of 2.08 ± 0.06 mm, and o.d. of 3.30 ± 0.12 mm, and were 15 cm long. Two-dimensional representation of thrombus formation on the SNAP/E2As and E2As control catheters after 7 d implantation in sheep veins (B). Data represents the mean ± SD (n=5). * = p < 0.05, SNAP/E2As vs. E2As control.
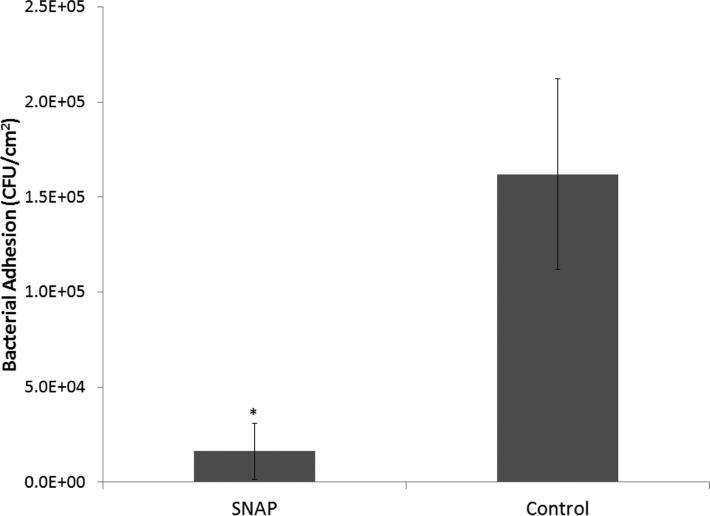
One centimeter catheter sections in 1 mL sterile PBS were homogenized to detach the bacteria from the inner and outer catheter surfaces and cultured as described in the Experimental Section above. The bacterial colonies were counted the following day and are represented as CFU/cm2 in Fig. 6. A 1.0 log reduction (90% reduction) in living bacteria adhered to the surfaces was observed for SNAP/E2As catheters vs. the controls (n=5 each). Post-implanted catheters had a NO flux of 0.6 ± 0.3 × 10−10 mol cm−2 min−1 on the day of explantation, which is at the lower end of the normal range of NO from the endothelium.23 This data is consistent with previously reported animal data, where it was shown that NO release is not compromised due to blood exposure.31, 33 The hemocompatibility of these catheters likely could be further improved by exploring methods to increase the NO flux from the SNAP/E2As polymer, perhaps by adding polymeric additives that increase the degree of water uptake by the E2As polymer.
Fig. 6.
Bacterial adhesion on SNAP/E2As and E2As control catheters after 7 d implantation in sheep veins. Data represents the mean ± SD (n=5). * = p < 0.05, SNAP/E2As vs. E2As control.
4. Conclusions
In this study, the SNAP-doped E2As polymer previously characterized30 was used to fabricate intravascular catheters and these devices were evaluated for their hemocompatibility and antimicrobial properties in a sheep model. The SNAP-doped E2As catheters release physiological levels of NO (> 0.5 × 10−10 mol cm−2 min−1) for up to 20 d. The SNAP/E2As catheters were found to withstand ethylene oxide sterilization, maintaining ca. 89% of the original SNAP after such treatment. It was further determined that the SNAP/E2As catheters significantly reduce the amount of thrombus and bacterial adhesion, in comparison to non-NO release control catheters, after 7 d of intravascular implantation in sheep. This study demonstrates the potential of the SNAP/E2As polymer in clinical applications to improve the hemocompatibility and antimicrobial properties of catheters and other medical devices, even for longer-term implantation periods.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institutes of Health (EB-000783, EB-004527 and K25HL111213). The authors wish to thank AorTech International for the gift of Elast-eon E2As polymer. The authors would also like to thank Dr. Chuanwu Xi and Dr. Jianfeng Wu for their assistance in providing access to the bacteria homogenizer.
References
- 1.Ratner BD. Biomaterials. 2007;28:5144–5147. doi: 10.1016/j.biomaterials.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiener ES, McGuire P, Stolar CJ, Albo VC, Ablin AR, Betcher DL, Sitarz AL, Buckley JD, Krailo MD, Versteeg C. J. Pediatr. Surg. 1992;27:155–164. doi: 10.1016/0022-3468(92)90304-p. [DOI] [PubMed] [Google Scholar]
- 3.Dillon PA, Foglia RP. J. Pediatr. Surg. 2006;41:1582–1587. doi: 10.1016/j.jpedsurg.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Vertes A, Hitchins V, Phillips KS. Anal. Chem. 2012;84:3858–3866. doi: 10.1021/ac2029997. [DOI] [PubMed] [Google Scholar]
- 5.Casey AL, Elliott TS. Br. J. Nurs. 2010;19:78–87. doi: 10.12968/bjon.2010.19.2.46289. [DOI] [PubMed] [Google Scholar]
- 6.O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, Masur H, McCormick RD, Mermel LA, Pearson ML, Randolph A, Weinstein RA. Clin. Infect. Dis. 2002;35:1281–1307. [Google Scholar]
- 7.Werner C, Maitz MF, Sperling C. J. Mater. Chem. 2007;17:3376–3384. [Google Scholar]
- 8.Kidane AG, Salacinski H, Tiwari A, Bruckdorfer KR, Seifalian AM. Biomacromolecules. 2004;5:798–813. doi: 10.1021/bm0344553. [DOI] [PubMed] [Google Scholar]
- 9.Salvagnini C. PhD Dissertation. Universite Catholique de Louvain; Belgium: 2002. [Google Scholar]
- 10.Gaffney AM, Wildhirt SM, Griffin MJ, Annich GM, Radomski MW. BMJ. 2010:341. doi: 10.1136/bmj.c5317. [DOI] [PubMed] [Google Scholar]
- 11.Robinson TM, Kickler TS, Walker LK, Ness P, Bell W. Crit. Care Med. 1993;21:1029–1034. doi: 10.1097/00003246-199307000-00018. [DOI] [PubMed] [Google Scholar]
- 12.Hsu SH, Tseng HJ, Lin YC. Biomaterials. 2010;31:6796–6808. doi: 10.1016/j.biomaterials.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Committee HICPA. Infect. Control Hosp. Epidemiol. 2010;31:319–326. doi: 10.1086/651091. [DOI] [PubMed] [Google Scholar]
- 14.Fischbach MA, Walsh CT. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taubes G. Science. 2008;321:356–361. doi: 10.1126/science.321.5887.356. [DOI] [PubMed] [Google Scholar]
- 16.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Annu. Rev. Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 17.Hall-Stoodley L, Costerton JW, Stoodley P. Nature Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 18.O'Toole G, Kaplan HB, Kolter R. Annu. Rev. Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 19.Parsek MR, Singh PK. Annu. Rev. Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 20.Fux C, Costerton J, Stewart P, Stoodley P. TIM. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Radomski MW, Moncada S. Mechanisms of Platelet Activation and Control. Springer; 1993. pp. 251–264. [Google Scholar]
- 22.Radomski MW, Palmer RM, Moncada S. Biochem. Biophys. Res. Commun. 1987;148:1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 23.Vaughn MW. American Journal of Physiology-Heart and Circulatory Physiology. 1998;274:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 24.Halpenny GM, Mascharak PK. Anti-Infect Agents Med Chem. 2010;9:187–197. [Google Scholar]
- 25.Rouby JJ. Am. J. Respir. Crit. Care Med. 2003;168:265–266. doi: 10.1164/rccm.2305009. [DOI] [PubMed] [Google Scholar]
- 26.Raulli R, McElhaney-Feser G, Hrabie J, Cihlar R. Rec Res Devel Microbiol. 2002;6:177–183. [Google Scholar]
- 27.Carpenter AW, Schoenfisch MH. Chem. Soc. Rev. 2012;41:3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frost MC, Reynolds MM, Meyerhoff ME. Biomaterials. 2005;26:1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Jen MC, Serrano MC, van Lith R, Ameer GA. Adv. Funct. Mater. 2012;22:239–260. doi: 10.1002/adfm.201101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Biomaterials. 2013;34:6957–6966. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Major TC, Brant DO, Reynolds MM, Bartlett RH, Meyerhoff ME, Handa H, Annich GM. Biomaterials. 2010;31:2736–2745. doi: 10.1016/j.biomaterials.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, Meyerhoff ME, Bartlett RH. Crit. Care Med. 2000;28:915–920. doi: 10.1097/00003246-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, Bartlett RH, Meyerhoff ME. J Mater Chem B Mater Biol Med. 2013;1:3578–3587. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. J Mater Chem B Mater Biol Med. 2014;2:1059–1067. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riccio DA, Dobmeier KP, Hetrick EM, Privett BJ, Paul HS, Schoenfisch MH. Biomaterials. 2009;30:4494–4502. doi: 10.1016/j.biomaterials.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riccio DA, Coneski PN, Nichols SP, Broadnax AD, Schoenfisch MH. ACS Applied Materials & Interfaces. 2012;4:796–804. doi: 10.1021/am201443r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai W, Wu J, Xi C, Meyerhoff ME. Biomaterials. 2012;33:7933–7944. doi: 10.1016/j.biomaterials.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joslin JM, Lantvit SM, Reynolds MM. ACS Applied Materials & Interfaces. 2013;5:9285–9294. doi: 10.1021/am402112y. [DOI] [PubMed] [Google Scholar]
- 39.Seabra AB, Da Silva R, De Souza GFP, De Oliveira MG. Artif. Organs. 2008;32:262–267. doi: 10.1111/j.1525-1594.2008.00540.x. [DOI] [PubMed] [Google Scholar]
- 40.Seabra AB, Martins D, Simoes M, da Silva R, Brocchi M, de Oliveira MG. Artif. Organs. 2010;34:E204–E214. doi: 10.1111/j.1525-1594.2010.00998.x. [DOI] [PubMed] [Google Scholar]
- 41.Chipinda I, Simoyi RH. J. Phys. Chem. B. 2006;110:5052–5061. doi: 10.1021/jp0531107. [DOI] [PubMed] [Google Scholar]
- 42.Dicks AP, Swift HR, Williams DLH, Butler AR, Al-Sa'doni HH, Cox BG. J. Chem. Soc., Perkin Trans. 2. 1996:481–487. [Google Scholar]
- 43.Frost MC, Meyerhoff ME. J. Am. Chem. Soc. 2004;126:1348–1349. doi: 10.1021/ja039466i. [DOI] [PubMed] [Google Scholar]
- 44.Wood PD, Mutus B, Redmond RW. Photochem. Photobiol. 1996;64:518–524. [Google Scholar]
- 45.Sexton DJ, Muruganandam A, McKenney DJ, Mutus B. Photochem. Photobiol. 1994;59:463–467. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- 46.Clarckson C. 2012 http://tmedweb.tulane.edu/pharmwiki/doku.php/penicillamine_nap.
- 47.Parameshvara V. Br. J. Ind. Med. 1967;24:73–76. doi: 10.1136/oem.24.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiruppathi C. 2006 http://www.uic.edu/classes/pcol/pcol331/dentalpharmhandouts2006/lecture41.pdf.
- 49.Blanusa M, Varnai VM, Piasek M, Kostial K. Curr. Med. Chem. 2005;12:2771–2794. doi: 10.2174/092986705774462987. [DOI] [PubMed] [Google Scholar]
- 50.Hakim TS, Sugimori K, Camporesi EM, Anderson G. Physiol. Meas. 1996;17:267–277. doi: 10.1088/0967-3334/17/4/004. [DOI] [PubMed] [Google Scholar]
- 51.Didisheim P. ASAIO J. 1994;40:230–237. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.