Abstract
A novel selectively targeting gene delivery approach has been developed for advanced hepatocellular carcinoma (HCC), a leading cause of cancer mortality whose prognosis remains poor. We combine the strong liver tropism of serotype-8 capsid-pseudotyped adeno-associated viral vectors (AAV8) with a liver-specific promoter (HLP) and microRNA-122a (miR-122a)-mediated posttranscriptional regulation. Systemic administration of our AAV8 construct resulted in preferential transduction of the liver and encouragingly of HCC at heterotopic sites, a finding that could be exploited to target disseminated disease. Tumor selectivity was enhanced by inclusion of miR-122a-binding sequences (ssAAV8-HLP-TK-122aT4) in the expression cassette, resulting in abrogation of transgene expression in normal murine liver but not in HCC. Systemic administration of our tumor-selective vector encoding herpes simplex virus-thymidine kinase (TK) suicide gene resulted in a sevenfold reduction in HCC growth in a syngeneic murine model without toxicity. In summary, we have developed a systemically deliverable gene transfer approach that enables high-level expression of therapeutic genes in HCC but not normal tissues, thus improving the prospects of safe and effective treatment for advanced HCC.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer worldwide with more than 500,000 new cases annually.1 Surgical resection and liver transplantation can be curative, but only in a small number of patients where the disease is diagnosed early. Other treatment options such as chemo-embolization and radiofrequency ablation are only partially effective in controlling localized tumors, and are associated with a high rate of metastatic recurrence. Advanced HCCs are typically resistant to chemo- and radiotherapy, while targeted therapy with sorafenib has resulted in only modest survival benefits.2 With overall 5-year survival rate for patients with advanced disease remaining disappointingly low at approximately 10%, there is a pressing need to develop novel therapeutic approaches.Gene therapy has emerged as a promising strategy for cancer treatment. Clinical successes have recently been reported in patients with advanced metastatic solid tumors as well as leukemia.3–8 Among vectors available for gene transfer to the liver, there has been considerable interest in adeno-associated viral vectors (AAV) in part because of their excellent safety profile. These vectors when pseudotyped with serotype 8 capsid (AAV8) have a remarkable tropism for the liver, thus making them highly suited for gene therapy of HCC.9,10 Unlike other viral vectors such as adenovirus and poxvirus, the prevalence of neutralizing antibodies to AAV8 in humans is low, enabling effective transduction of the liver following a simple systemic bolus infusion of AAV8, as illustrated in patients with severe hemophilia B.11 Systemic administration of therapeutic vectors offers the possibility of targeting disseminated HCC, which is highly desirable for patients with advanced HCC.
Safe and effective gene therapy mandates selective expression of the therapeutic genes in tumor cells specifically, to maximize efficacy and avoid injury to normal surrounding tissue. However, tumor-selective delivery and/or expression of the therapeutic gene have thus far been elusive. In this study a novel tumor-targeting gene therapy approach was developed by combining the strong liver tropism of AAV8 with transcription control of a small synthetic liver-specific promoter (HLP) and miR-122a posttranscriptional regulation of transgene expression. MiR-122a suppresses gene expression in the liver following binding to partial complementary sequences within target mRNA. MiR-122a is expressed at high levels in the liver but is significantly downregulated or undetectable in advanced HCC.12–18 We, therefore, hypothesized that following systemic administration of AAV8, expression of a transgene containing miR-122a-binding sequences in the 3′-untranslated region will be suppressed in healthy hepatocytes but not advanced HCC (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). To test this hypothesis, the delivery and expression of AAV8 transgenes containing miR-122a-binding sites was systematically evaluated in various models, including a syngeneic murine model of metastatic HCC.
Materials and Methods
Cell lines
HuH-7 and SK-Hep-1 cells were obtained from ATCC. BNL-1h cell line was kindly provided by Dr. Tao MH (Graduate Institute of Life Sciences). MiR-122a expression in the cell lines was assessed by mirVana miRNA Detection Kit (Life Technologies).
Vector design and production
Sequences complementary to miR-122a and miR-223 were cloned downstream of the ORF for luciferase or HSV-tk (TK) genes under the control of the HLP promoter, and flanked by the AAV inverted terminal repeats (ITRs) (Figs. 1a and 2d). rAAV8 vector stocks were produced as described previously.19
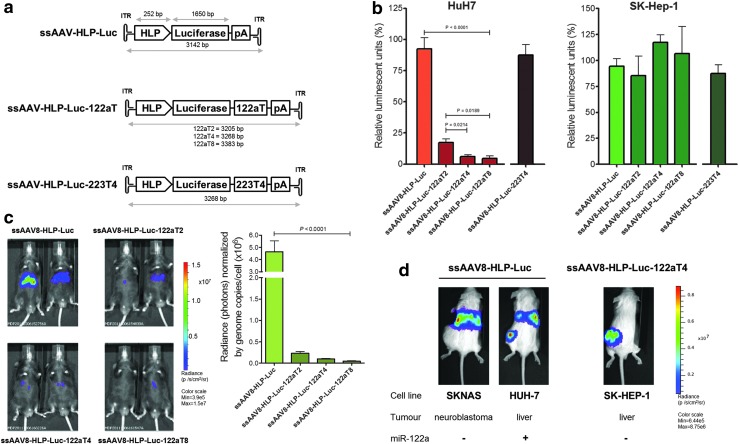
FIG. 1.
Optimization of miR-122a-mediated silencing of rAAV8 transgene expression. (a) AAV8 expression cassette design. Luciferase was cloned downstream the HLP promoter with none, two, four, or eight repeated miR-122a (or four miR-223)-binding sequences in the 3′-UTR region. (b) Luciferase expression in HuH7 and SK-Hep-1 cells was transduced with these vectors (MOI=1×105/cell; n=3). (c) Bioluminescence in C57Bl/6 mice 1 week after intravenous (iv) injection (1×1011 vg/mouse) (left). Genome copy number in liver samples (right). (d) HUH-7, SK-Hep-1, or SKNAS cell lines were injected in the flank of three cohorts (n=6/cohort) of NOD/SCID immunodeficient mice, followed 4 weeks later by tail vein injection of ssAAV8-HLP-Luc or ssAAV8-HLP-Luc-122aT4 vectors (1×1011 vg/mouse). Representative bioluminescence imaging of HUH-7 (left) and SKNAS (middle) mice injected with ssAAV8-HLP-Luc. Luciferase expression in mice bearing SK-Hep-1 xenograft injected with ssAAV8-HLP-Luc-122aT4 (right).
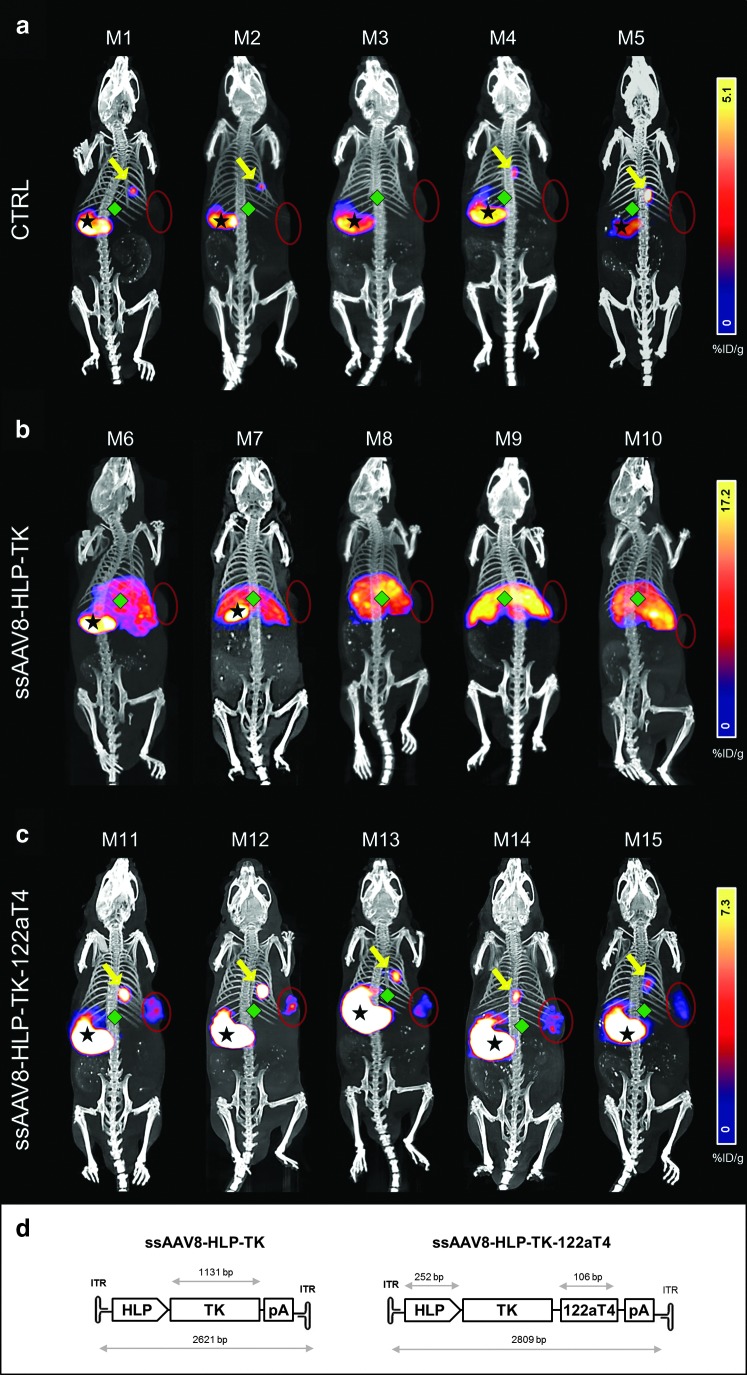
FIG. 2.
SPECT/CT imaging showing cancer-specific expression of TK following iv administration of ssAAV8-HLP-TK-122aT4. NOD/SCID mice (n=5/cohort) bearing human SK-Hep-1 xenografts (red circles) transduced with (a) no vector, (b) ssAAV8-HLP-TK vector, or (c) ssAAV8-HLP-TK-122aT4 vector. Four days after vector injection, SPECT-CT images were acquired (a and b) 30 min and (c) 3 hr after injection of [125I]FIAU. Bars represent radiotracer uptake (%ID/g). Stars indicate stomach, diamonds indicate liver, and arrows indicate gallbladder. (d) TK gene cloned into our expression cassette to generate AAV-HLP-TK and AAV-HLP-TK-122aT4.
In vitro AAV functional studies
An amount of 1×105 vector particles of rAAV8 vectors were used to transduce various cell lines. Luciferase activity was measured by the Luciferase Assay System (Promega). TK activity was assessed by adding 5 μg/ml of ganciclovir (GCV) to the media the day after cell transfection. Cells were counted by flow cytometry on day 5 using counting beads.
Western blot and IHC
Whole cell lysates were prepared in RIPA lysis buffer and subjected to immunoblotting using goat anti-HSVtk (vN-20, 1:200) or mouse anti-GAPDH (6C5, 1:1000) (Santa Cruz Biotechnology). Secondary antibodies were coupled to HRP and detected by ECL (Thermo Scientific). The same TK antibody was used also for IHC.
In vivo AAV studies
Animal work was performed under the authority of UK Home Office Project and Personal Licences regulations and was compliant with University College London ethical review committee guidelines. Mice were obtained from Charles River Laboratories Inc. AAV8 vectors encoding luciferase gene were injected by tail vein (1×1011 vg/mouse) suspended in 200 μl of X-vivo10. Luciferase expression was detected using D-Luciferin (Melford Laboratories), which was injected intraperitoneally (IP) at a dose of 200 μg/mouse, as substrate, using the IVIS Imaging System 100 Series (Perkin Elmer).
In vivo HCC animal models
Six- to eight-week-old male BALB/c mice received 1×106 BNL-1h cells by intrasplenic injection under anesthesia. An amount of 1×1011 vg/mouse of ssAAV8-HLP-TK-122aT4 resuspended in 200 μl of X-vivo10 were injected into the tail vein on day 21 after tumor implantation, followed 5 days later by daily injection of GCV (25 mg/kg/mouse). An amount of 1×107 SK-Hep-1 cells were injected in the flank of 6–8-week-old NOD/SCID immunocompromised mice to establish subcutaneous xenografts. An amount of 1×1011 vg/mouse of ssAAV8-HLP-TK-122aT4 vector (phosphate buffered saline [PBS] as control) were injected by tail vein followed by daily IP injection of GCV (25 mg/kg/day) for 12 days. Tumor volume was calculated using the ellipsoidal formula: (length×width2) 1/2. ALT and AST activity was measured using a Beckman Coulter AU680 analyzer (Beckman Coulter).
SPECT-CT imaging
FIAU was radiolabeled with [125I] as previously described.20 Six- to eight-week-old NOD/SCID mice received 1×107 SK-Hep-1 cells by subcutaneous injection in the flank. Twenty-one days later, 1×1011 vg/mouse of vectors or PBS as control were administered via tail vein. SPECT-CT images were obtained 4 days later using a dedicated small animal scanner (Mediso). Anesthetized mice were then injected intravenously with 100–200 μl 20–30 MBq [125I]FIAU. SPECT-CT images were collected 5, 30, and 180 min postinjection. Whole-body CT images were acquired and reconstructed using the Nucline software (Mediso). Images were fused using VivoQuant software (inviCRO), and then normalized to the administered activity to parameterize images in terms of %ID/g. Manually drawn three-dimensional volumes of interest were used to determine radiotracer accumulation in units of %ID/g.
Vector distribution
A qPCR assay SYBR Green (QuantiFast SYBR Green PCR Kit; Quiagen) was used to evaluate the biodistribution of rAAV8 vectors in genomic DNA using the following primers: HLP forward 5′-CAGGACGCTGTGGTTTCTG-3′ and reverse 5′-TGCCTGAAGCTGAGGAGAC-3′; GAPDH forward 5′-GGAGTCCACTGGCGTCTTCAC-3′ and reverse 5′-GAGGCATTGCTGATGATCTTGAGG-3′.
Statistical analysis
Data analysis was performed using unpaired and one-sample t-test (GraphPad Software Inc.). p<0.05 was considered statistically significant.
Results
Optimizing miR-122a-mediated silencing of AAV transgene expression
Three serotype 8-pseudotyped, single-stranded AAV vectors were developed containing our previously described liver-specific promoter (HLP) in preference to constitutively active promoters.21 This is because our preliminary studies with AAV8 vectors showed significant transgene expression in nonhepatic tissues in addition to high level of expression in the liver with constructs containing constitutively active promoters, which increases the risk of off-target toxicity (Supplementary Fig. S2). Included in these HLP-driven vectors was the firefly luciferase reporter gene containing either two, four, or eight tandem repeats of miR-122a-binding sequences in the 3′-UTR (AAV-HLP-Luc-122aT2, AAV-HLP-Luc-122aT4, and AAV-HLP-Luc-122aT8, respectively) (Fig. 1a). The control vector (AAV-HLP-Luc) did not contain any miR-122a-binding sites. Since AAV transgene expression peaks at 3–5 days and primary human hepatocytes can only be maintained in culture for ∼3 days, the initial assessment of these vectors was performed in two cell lines with different levels of miR-122a expression.22 The SK-Hep-1 is an immortalized, human endothelial cell line derived from the ascitic fluid of a patient with adenocarcinoma of the liver, which has been used in numerous studies of liver cancer.23–25 These cells have undetectable levels of miR-122a and therefore serve as an miR-122a-negative cell line for the assessment of our AAV vectors. HuH7, a well-differentiated human HCC cell line, has some residual miR-122a, although approximately 10-fold lower than normal hepatocytes26 and was therefore used as an miR 122a-positive cell line (Supplementary Fig. S3).
An amount of 1×105 HuH7 or SK-Hep-1 cells were transduced with AAV-HLP-Luc-miR-122aT vectors and the AAV-HLP-Luc control vector at a multiplicity of infection (MOI) of 1×105. Incorporation of miR-122a-binding sites downstream of a luciferase gene had no effect on bioluminescence in the SK-Hep-1 (Fig. 1b). However, luminescence in HuH7 cells declined from 91±9% RLU (percentage of relative luminescence units, normalized to nontransduced [NT] cells) in cells transduced with the ssAAV8-HLP-Luc vector to 17±3%RLU in ssAAV8-HLP-Luc-122aT2 (p=0.0012) and 6±1%RLU in ssAAV8-HLP-Luc-122aT4 (p=0.0006). The difference in luminescence levels between HUH7 cells transduced with ssAAV8-HLP-Luc-122aT4 and ssAAV8-HLP-Luc-122aT8 was not significant (p=0.60). Importantly, overall cell numbers at 72 hr after gene transfer were similar, suggesting that overexpression of mir-122a-binding site did not affect the cell proliferation or survival rate (Supplementary Fig. S4).
AAV-mediated luciferase expression was assessed in vivo 1 week after tail vein administration of 1×1011 vg/mouse into male C57Bl/6 mice. As described before, the liver was preferentially transduced after tail vein administration of ssAAV8-HLP-Luc vector because of the natural liver tropism of AAV8.9,10,27 Two miR-122a-binding sites in the ssAAV8-HLP-Luc-122aT2 vector decreased luciferase expression by 35-fold. Luminescence was almost undetectable in the liver of mice transduced with ssAAV8-HLP-Luc-122aT4 and ssAAV8-HLP-Luc-122aT8 vectors (Fig. 1c). Analysis of the transgene copy number in liver by qPCR assay showed that the average copy number/cell in each cohort of mice was similar (0.70±0.2) and did not account for the difference in transgene expression observed between different vectors.
These data indicated that inclusion of a minimum of four tandem repeats of miR-122a-binding sites in the 3′-UTR of the AAV expression cassette allows repression of transgene expression in miR-122a-positive HuH7 cells or murine hepatocytes but not in cells lacking miR-122a. That this was an miR-122a-specific effect was suggested by the fact that similar moderation of expression was not seen with an identical vector containing four miR-223-binding sites in place of miR-122a target sequences (Fig. 2b).
Preferential transduction of human HCC xenografts in ectopic sites following systemic administration of AAV8 vectors
Although AAV8 has a strong liver tropism, it was unclear if the serotype 8 would be equally efficient at transducing HCC cells in ectopic sites—an important issue in patients with metastases. Therefore, 1×107 HuH-7 or SKNAS (miR-122a-negative human neuroblastoma cell line) were each injected in the subcutaneous space of 12 male NOD/SCID mice. Four weeks later, when the tumors in these animals were of equal size, mice bearing each of the tumors types were divided into equal groups that received a single tail vein injection of either ssAAV8-HLP-Luc or ssAAV8-HLP-Luc-122aT4 (1×1011 vg/mouse).
A week later, bioluminescence was observed in the liver of both cohorts of tumor-bearing mice following transduction with ssAAV8-HLP-Luc. Additionally, bioluminescence was detected in the HCC xenografts within the subcutaneous space but not the neuroblastoma xenografts (Fig. 1d, left). Quantitative PCR analysis of tissues isolated 6 weeks after gene transfer showed that the liver had the highest proviral copy number, with 6±2 and 10±3 copies/cell in HuH7 and SKNAS xenograft cohorts, respectively. The transgene copy number in HuH7 xenografts was 0.9±0.2 copies/cell, as compared with 0.01±0.003 copies/cell in SKNAS xenografts. Transgene copy number in kidneys of both cohorts was comparable at approximately 0.05±0.004 copies/cells (Supplementary Fig. S5). These results suggest preferential transduction of HuH7 cells at ectopic sites by AAV8 vectors. In separate studies using miR-122a-negative SK-Hep-1 cells, luciferase expression was detected only in SK-Hep-1 xenografts following tail vein administration of ssAAV8-HLP-Luc-122aT4 vector with no signal from the normal liver (Fig. 1d, right). This is consistent with downregulation of AAV transgene expression in the miR-122a-positive normal murine liver cells but not in the miR-122a-negative SK-Hep-1 tumor cell line.
Efficacy of AAV8 vectors encoding the TK suicide gene against human HCC cells in vitro
The luciferase sequence was replaced with the TK suicide gene to generate AAV-HLP-TK and AAV-HLP-TK-122aT4 (Fig. 2d). An amount of 1×105 HuH7 cells were transduced with ssAAV8-HLP-TK or ssAAV-HLP8-TK-122aT4 at an MOI of 105. Three days later, cells were exposed to varying concentrations of GCV (0.1–5 μg/ml). Cell survival was assessed after 72 hr of GCV treatment and expressed as a percentage of NT cells. GCV at a concentration of 5 μg/ml was not toxic to NT HuH7 cells. Dose-dependent killing of ssAAV8-HLP-TK-transduced HuH7 cells was observed with only 14±3% cell survival following exposure to 5 μg/ml of GCV (Supplementary Fig. S6B). By comparison, the ssAAV8-HLP-TK-122aT4 vector-transduced HuH7 cells appeared to be less sensitive to killing by GCV, as illustrated by the survival of approximately 53±4% of cells following 3 days of treatment with 5 μg/ml of the GCV. Western blot analysis demonstrated significantly lower TK expression in the ssAAV8-HLP-TK-122aT4-transduced HuH7 cells when compared with cells transduced with an identical vector lacking the miR-122a-binding sequences (Supplementary Fig. S6B). This is most likely because of a reduction in translation of the proviral mRNA following binding of miR-122a to its target sequences within the AAV expression cassette. In contrast, in miR-122a-negative cell lines, SK-Hep-1 and BNL-1h (a murine embryonic liver cell; Supplementary Fig. S3) showed no difference in GCV-mediated killing following transduction with either ssAAV8-HLP-TK or ssAAV-HLP8-TK-122aT4 (Supplementary Fig. S6C and D).
Radionucleotide imaging confirms selective gene transfer to HCC xenografts resulting in potent antitumor activity following systemic administration of ssAAV8-HLP-TK-122aT4
SK-Hep-1 cells were injected subcutaneously in the flank of 6–8-week-old male NOD/SCID mice, and 3 weeks later the animals were divided into 3 groups (n=5/cohort), each receiving 1×1011 vg/mouse of ssAAV8-HLP-TK, ssAAV8-HLP-TK-122aT4, or an equivalent volume of PBS (control group) via the tail vein. The radiolabeled TK substrate [125I]FIAU was injected 4 days after vector administration followed by SPECT-CT imaging at regular intervals. As expected, there was nonspecific radiochemical uptake in the stomach and gallbladder in all groups of animals, including PBS controls. A high level of 125I radioactivity was observed within 5 min of administration of substrate in the liver of animals transduced with ssAAV8-HLP-TK, consistent with high TK levels within hepatocytes (Fig. 2b and Supplementary Figs S7 and S8).
This radioactivity signal gradually declined over a period of 1 hr. In contrast, the 125I radioactivity signal in the liver of mice transduced with ssAAV8-HLP-TK-122aT4 vector was low or undetectable at all time points and comparable to levels observed in control animals that received PBS. This suggests that expression of TK is suppressed in normal murine hepatocytes by the interaction of high levels of endogenous miR-122a with the complementary binding sequences within the HLP-TK-122aT4 transgene (Fig. 2a and c, and Supplementary Figs S7 and S8). A relatively strong radioactive signal was observed in the SK-Hep-1 xenografts 3 hr after [125I]FIAU injection in the cohort transduced with ssAAV8-HLP-TK-122aT4, suggesting that despite the lack of signal in the liver of these animals the TK transgene was expressed at significant levels in the miR-122a-negative tumor cells (Fig. 2c and Supplementary Figs S7 and S8). Signal in the SK-Hep-1 xenografts in the ssAAV8-HLP-TK mice remained negative at all time points presumably because of the rapid breakdown of [125I]FIAU by the high levels of TK in the liver of these animals (Fig. 2b).
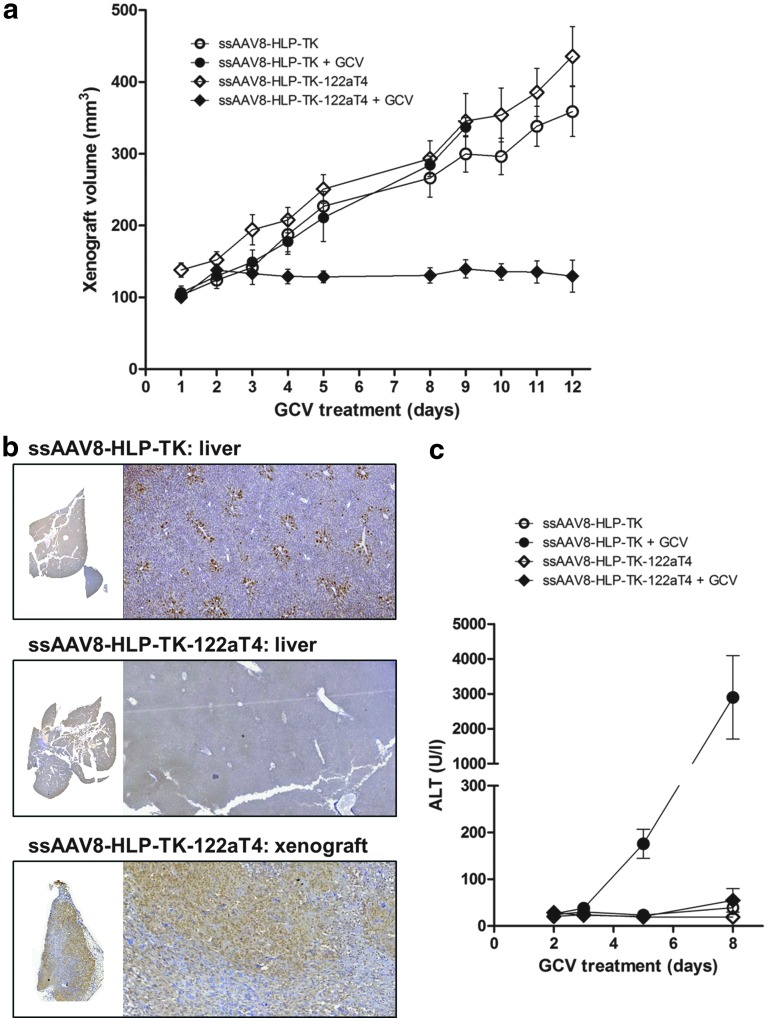
Consistent with the imaging data, tail vein administration of ssAAV8-HLP-TK-122aT4 followed by 12 days of GCV (25 mg/kg) treatment resulted in reduction of growth of SK-Hep1 tumors in the subcutaneous space (from 100±10 pre-GCV to 129±49 mm3 post-GCV) (Fig. 3a). TK expression in the xenografts was confirmed by immunohistochemistry (Fig. 3b), which showed diffuse staining, consistent with efficient transduction of >70% of tumor cells in the subcutaneous implant following systemic administration of ssAAV8-HLP-TK-122aT4. In mice transduced with ssAAV8-HLP-TK-122aT4 or ssAAV8-HLP-TK but treated with PBS as opposed to GCV, the tumor size increased approximately threefold from 138±22 to 436±92 mm3 and 103±19 to 359±78 mm3 over a 12-day period, respectively (Fig. 3a). GCV treatment in mice transduced with ssAAV8-HLP-TK resulted in death of all animals within 8 days associated with a >100-fold (2900±1197 IU/I) increase in ALT levels over that observed in naïve untransduced mice treated with GCV alone (23±3 IU/I). This is consistent with killing of hepatocytes expressing TK (Fig. 3b) as a result of conversion of GCV to phosphorylated GCV, which inhibits replication of DNA. ALT values in mice transduced with ssAAV8-HLP-TK-122aT4 vector were marginally higher at day 8 of GCV when compared with naïve untransduced controls at 55±25 IU/I, suggesting that liver toxicity was minimized by the inclusion of miR-122a-binding sequences within the transgene cassette (Fig. 3c and Supplementary Table S1).
FIG. 3.
Efficacy of ssAAV8-HLP-TK-122aT4/GCV in a xenografts model of human liver cancer. (a) SK-Hep-1 xenograft volumes in NOD/SCID mice after transduced with ssAAV8-HLP-TK and ssAAV8-HLP-TK-122aT4 vectors followed GCV (or PBS) treatment (n=6/cohort). (b) Representative IHC for TK detection in the liver and xenograft. (c) ALT values. GCV, ganciclovir; PBS, phosphate buffered saline.
Systemic administration of ssAAV8-HLP-TK-122aT4 is effective in a syngeneic metastatic murine HCC model
Tumor selectivity of the ssAAV8-HLP-TK-122aT4 vector was further assessed in a challenging syngeneic model of HCC in which BNL-1h miR-122a-negative murine cells, subclones of the BALB/c chemically transformed BNL-1ME A.7R.1 hepatic cell line, were injected into spleens of BALB/c mice to establish tumor in disseminated sites. Three weeks later the mice received a bolus tail vein injection of 1×1011 vg/mouse of ssAAV8-HLP-TK-122aT4. Five days after gene transfer, half of the animals received (n=6) GCV IP at a dose of 25 mg/kg/day for 5 days. The remaining mice (control) received PBS. Five days after GCV treatment the mice were sacrificed and the number of hepatic and extra-hepatic lesions was counted in a blinded manner.
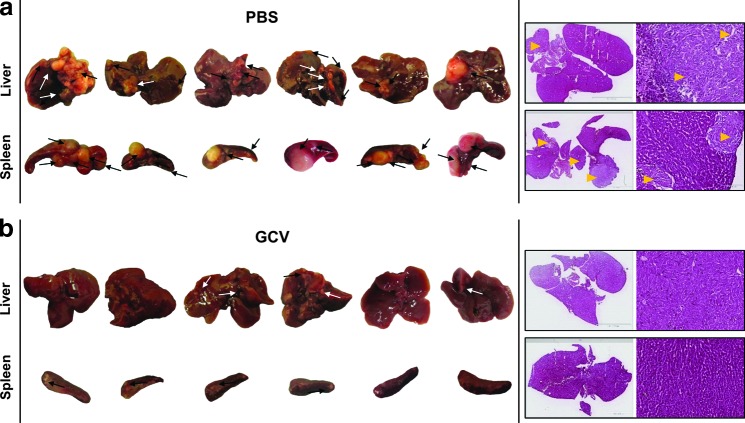
The combination of ssAAV8-HLP-TK-122aT4/GCV resulted in a significant reduction in the number of grossly visible lesions in the liver (Fig. 4) (mean=0.8; range 1–4) compared with control mice (mean=6; range 4–9). This difference was highly significant (p=0.004). Similarly, there was a reduction in the number and size of tumor nodules in the spleen, as well as tumors outside of the hepato-splenic tissues, in ssAAV8-HLP-TK-122aT4/GCV-treated mice (Table 1). Consistent with these macroscopic findings, there was little or no evidence of tumor on histological evaluation of sections of liver derived from mice treated with GCV (Fig. 4).
FIG. 4.
Efficacy of ssAAV8-HLP-TK-122aT4/GCV in a metastatic syngeneic model of hepatocellular carcinoma (HCC). Balb/C mice were injected intrasplenically with BNL-1h cells followed 3 weeks later by a single iv injection of ssAAV8-HLP-TK-122aT4 and treated 5 days after GCV (PBS as control) (n=6/cohort). Liver and spleen from (a) control and (b) GCV mice. Arrows indicate sites of tumors. Representative H&E staining of two liver showing healthy tissue and tumors (yellow arrows).
Table 1.
Total Number of Tumor Nodules in the Two Cohorts of ssAAV8-HLP-TK-122aT4 Mice After GCV Treatment (PBS as Control)
| Organs | |||||
|---|---|---|---|---|---|
| Treatment | Liver | Spleen | Mesentery | Gut | Kidney |
| PBS | 36 | 21 | 9 | 1 | 1 |
| GCV | 5 | 4 | 1 | 0 | 1 |
GCV, ganciclovir; PBS, phosphate buffered saline.
BALB/c mice were implanted with the syngeneic BNL-1h cells. Three weeks later, animals received a tail vein injection of ssAAV8-HLP-TK-122aT4 (1×1011 vg/mouse). Five days later, animals received GCV (PBS as control) intraperitoneally (25 mg/kg/day) for 5 days. Mice were killed at day 31 of the study and the number metastases in the major organs was counted.
Discussion
In this study we aimed to extend our clinical success with AAV8 vectors in hemophilia B patients to develop a new selectively targeting gene therapy approach for advanced HCC, a leading cause of cancer deaths worldwide. Recent insights into the pathogenesis of HCC were exploited to develop a systemically deliverable gene transfer strategy that facilitated HCC-specific expression of transgenes, not only within primary tumor, but also in associated metastases. Specifically, our approach combined three aspects of vector design: a vector serotype with preferential liver tropism, a liver-specific promoter, and posttranscriptional regulatory mechanism inhibiting gene expression in healthy liver tissue. Bioluminescence and clinically translatable noninvasive molecular-genetic imaging using TK and radiolabeled TK substrate analog28 showed that AAV8 is highly efficient at transducing HCC in the liver and heterotopic sites following systemic administration.
Our comparative studies demonstrated that HCC xenografts in the subcutaneous space had a higher transgene copy number than neighboring tissues, or other tumors such as neuroblastoma, following systemic administration of AAV8 vector. This ability to “home” to tumors in heterotopic sites after systemic administration of AAV8 raised the possibility of targeting disseminated tumor, thus offering an advantage over other cancer gene therapy approaches.29 This aspect was exploited in murine models to show a reduction in tumor growth in miR-122a-negative human SK-Hep1 xenografts implanted into the subcutaneous space. Additionally, efficacy of our tumor-targeting approach was established in a syngeneic mouse model of metastatic HCC following tail vein administration of ssAAV8-HLP-TK-122aT4 without toxicity. Thus, the hitherto unknown ability of AAV8 vectors to preferential transduce tissue of liver origin provides a unique opportunity to target disseminated disease in patients with advanced stage HCC. While the mechanism behind this remains unclear, we speculate that the upregulation of laminin receptor on HCC cell may play a role.30
A critical aspect of our strategy was the ability to minimize “off-target toxicity” of the therapeutic gene, which was achieved by exploiting the differential expression of miR-122a, which is abundantly expressed in normal hepatocytes but almost undetectable in HCCs. Specifically, incorporating a minimum of four tandem repeats of the miR-122a-binding sequence in the 3′ end of an AAV8 expression cassette significantly downregulated TK suicide gene expression in normal liver tissue, presumably secondary to proviral mRNA degradation following the binding of miR-122a with its complementary target sequence in the AAV expression cassette. This reduced hepatocellular toxicity without compromising antitumor potency of ssAAV8-HLP-TK-122aT4 in miR-122a-negative tumor cell lines. Several studies demonstrate absence or very low levels of miR-122a in biopsy samples from over 70% of patients with advanced HCC. These individuals generally have aggressive poorly differentiated HCC and have a particularly poor prognosis even with radical therapy involving sorafenib in combination with chemo- and/or radiotherapy.12,31–33
These patients need new treatment options and would, therefore, be highly suited to our HCC targeted gene therapy approach. Serum levels of circulating miR-122a have been proposed as a new marker for prediction of survival of patients with liver cirrhosis.34 However, miR-122a is not downregulated in cirrhosis to the same extent as in HCC, thus enabling differential targeting of HCC tumor cells with our AAV expression cassette.35 The potential benefits of AAV-based gene therapy for HCC will need to be balanced with the potential risk of AAV-mediated oncogenesis. This risk is relatively low as AAV proviral DNA integrates in the host genome at a much lower frequency than is the case with integrating vectors such as those based on onco-retroviruses.36–41 This is consistent with the fact that wild-type AAV infection in humans, though common, is not associated with oncogenesis. However, an increased incidence of HCC has been reported in the mucopolysaccharidoses type VII mouse model following perinatal gene transfer of AAV.42 Studies on other murine models have failed to recapitulate this finding; in addition, tumors have not been observed in larger animal models or over 500 patients who have received AAV vectors.10,43 Therefore, the available data do not preclude the use of AAV vectors for gene therapy of HCC.
Differential expression of miR-122a in normal liver tissue and advanced HCC has been exploited previously for the purpose of constructing oncolytic viruses that replicate in HCC but not in normal hepatocytes, thus reducing off target toxicity.44,45 Similarly, Xiao and colleagues demonstrated that the insertion of miR-122a target sequences into the 3′-UTR of AAV vectors encoding marker genes or secretable proteins resulted in reduction in transgene mRNA and protein levels by over 50-fold in the liver.46 However, ours is the first study to systematically examine the use of miRNA-based posttranscriptional regulatory mechanisms in the context of AAV vectors to deliver therapeutic gene to HCC in animal models.
Collectively, the ability to administer vector systemically to target disseminated malignancy, together with HCC-restricted expression of the transgene, provides significant advantages over other gene therapy approaches involving adenovirus or oncolytic poxvirus that have been tested.29,47,48 In some of these studies, the vector had to be administered intratumorally to minimize “off target” toxicity as well as overcome antiviral immunity arising from previous exposure to wild-type virus or immunization.48 Consequently, the long-term efficacy was impaired, as only tumors easily accessed by intervention radiological techniques could be treated.
In summary, the synergistic effect of the three elements described, natural tropism of a viral vector, promoter specificity, and posttranslational regulation, provides a tumor-selective strategy for targeting advanced HCC. This approach provides a new opportunity for delivering a variety of therapeutic genes to HCC without concerns of significant toxicity to normal hepatocytes.
Supplementary Material
Acknowledgments
Experimental support from Dr. Jenny McIntosh and Doyoung Lee is gratefully acknowledged. This work was supported by the Foundation for Liver Research, UK, NIHR University College London Hospitals Biomedical Research Centre, NIHR Programme Grant (RP-PG-0310-1001), King's College London, UCL Comprehensive Cancer Imaging Centre CR-UK, as well as The ASSISI Foundation of Memphis, the American Lebanese Syrian Associated Charities. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health in the UK.
Author Disclosure Statement
A.M.D. and A.C.N. are listed as inventors in a patent filing for HLP promoter. All other coauthors have no conflicts of interest to disclose.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90 [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008;7:3129–3140 [DOI] [PubMed] [Google Scholar]
- 3.Bonini C, Ferrari G, Verzeletti S, et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 1997;276:1719–1724 [DOI] [PubMed] [Google Scholar]
- 4.Dranoff G, Soiffer R, Lynch T, et al. A phase I study of vaccination with autologous, irradiated melanoma cells engineered to secrete human granulocyte-macrophage colony stimulating factor. Hum Gene Ther 1997;8:111–123 [DOI] [PubMed] [Google Scholar]
- 5.Lupo-Stanghellini MT, Provasi E, Bondanza A, et al. Clinical impact of suicide gene therapy in allogeneic hematopoietic stem cell transplantation. Hum Gene Ther 2010;21:241–250 [DOI] [PubMed] [Google Scholar]
- 6.Okada T, Shah M, Higginbotham JN, et al. TK-mediated killing of subcutaneous tumors in situ results in effective immunization against established secondary intracranial tumor deposits. Gene Ther 2001;8:1315–1322 [DOI] [PubMed] [Google Scholar]
- 7.Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathwani AC, Gray JT, McIntosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood 2007;109:1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathwani AC, Rosales C, McIntosh J, et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther 2011;19:876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulouarn C, Factor VM, Andersen JB, et al. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 2009;28:3526–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu X, Rivera A, Tao L, et al. Construction of an oncolytic herpes simplex virus that precisely targets hepatocellular carcinoma cells. Mol Ther 2012;20:339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutay H, Bai S, Datta J, et al. Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J Cell Biochem 2006;99:671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol 2002;12:735–739 [DOI] [PubMed] [Google Scholar]
- 16.Murakami Y, Yasuda T, Saigo K, et al. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 2006;25:2537–2545 [DOI] [PubMed] [Google Scholar]
- 17.Tsai WC, Hsu PW, Lai TC, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 2009;49:1571–1582 [DOI] [PubMed] [Google Scholar]
- 18.Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 2012;122:2884–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allay JA, Sleep S, Long S, et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther 2011;22:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan R, Sander K, Galante E, et al. A one-pot three-component radiochemical reaction for rapid assembly of 125I-labeled molecular probes. J Am Chem Soc 2013;135:703–709 [DOI] [PubMed] [Google Scholar]
- 21.McIntosh J, Lenting PJ, Rosales C, et al. Therapeutic levels of FVIII following a single peripheral vein administration of rAAV vector encoding a novel human factor VIII variant. Blood 2013;121:3335–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki T, Sakurai F, Nakamura S, et al. miR-122a-regulated expression of a suicide gene prevents hepatotoxicity without altering antitumor effects in suicide gene therapy. Mol Ther 2008;16:1719–1726 [DOI] [PubMed] [Google Scholar]
- 23.Heffelfinger SC, Hawkins HH, Barrish J, et al. SK HEP-1: a human cell line of endothelial origin. In Vitro Cell Dev Biol 1992;28A:136–142 [DOI] [PubMed] [Google Scholar]
- 24.Herraez E, Lozano E, Macias RI, et al. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology 2013;58:1065–1073 [DOI] [PubMed] [Google Scholar]
- 25.Lin LI, Ke YF, Ko YC, et al. Curcumin inhibits SK-Hep-1 hepatocellular carcinoma cell invasion in vitro and suppresses matrix metalloproteinase-9 secretion. Oncology 1998;55:349–353 [DOI] [PubMed] [Google Scholar]
- 26.Chang J, Nicolas E, Marks D, et al. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol 2004;1:106–113 [DOI] [PubMed] [Google Scholar]
- 27.Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 2006;107:2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paneda A, Collantes M, Beattie SG, et al. Adeno-associated virus liver transduction efficiency measured by in vivo [18F]FHBG positron emission tomography imaging in rodents and nonhuman primates. Hum Gene Ther 2011;22:999–1009 [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Alcoceba R, Sangro B, Prieto J. Gene therapy of liver cancer. World J Gastroenterol 2006;12:6085–6097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozaki I, Yamamoto K, Mizuta T, et al. Differential expression of laminin receptors in human hepatocellular carcinoma. Gut 1998;43:837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–943 [DOI] [PubMed] [Google Scholar]
- 32.Han DH, Choi GH, Kim KS, et al. Prognostic significance of the worst grade in hepatocellular carcinoma with heterogeneous histologic grades of differentiation. J Gastroenterol Hepatol 2013;28:1384–1390 [DOI] [PubMed] [Google Scholar]
- 33.Oishi K, Itamoto T, Amano H, et al. Clinicopathologic features of poorly differentiated hepatocellular carcinoma. J Surg Oncol 2007;95:311–316 [DOI] [PubMed] [Google Scholar]
- 34.Waidmann O, Koberle V, Brunner F, et al. Serum microRNA-122 predicts survival in patients with liver cirrhosis. PLoS One 2012;7:e45652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koberle V, Kronenberger B, Pleli T, et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer 2013;49:3442–3449 [DOI] [PubMed] [Google Scholar]
- 36.Deyle DR, Russell DW. Adeno-associated virus vector integration. Curr Opin Mol Ther 2009;11:442–447 [PMC free article] [PubMed] [Google Scholar]
- 37.Donsante A, Vogler C, Muzyczka N, et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther 2001;8:1343–1346 [DOI] [PubMed] [Google Scholar]
- 38.Kay MA. AAV vectors and tumorigenicity. Nat Biotechnol 2007;25:1111–1113 [DOI] [PubMed] [Google Scholar]
- 39.Miller DG, Trobridge GD, Petek LM, et al. Large-scale analysis of adeno-associated virus vector integration sites in normal human cells. J Virol 2005;79:11434–11442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakai H, Wu X, Fuess S, et al. Large-scale molecular characterization of adeno-associated virus vector integration in mouse liver. J Virol 2005;79:3606–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakai H, Yant SR, Storm TA, et al. Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol 2001;75:6969–6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science 2007;317:477. [DOI] [PubMed] [Google Scholar]
- 43.Bell P, Moscioni AD, McCarter RJ, et al. Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther 2006;14:34–44 [DOI] [PubMed] [Google Scholar]
- 44.Leber MF, Bossow S, Leonard VH, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther 2011;19:1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CY, Rennie PS, Jia WW. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin Cancer Res 2009;15:5126–5135 [DOI] [PubMed] [Google Scholar]
- 46.Qiao C, Yuan Z, Li J, et al. Liver-specific microRNA-122 target sequences incorporated in AAV vectors efficiently inhibits transgene expression in the liver. Gene Ther 2011;18:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013;19:329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian C, Idoate M, Bilbao R, et al. Gene transfer and therapy with adenoviral vector in rats with diethylnitrosamine-induced hepatocellular carcinoma. Hum Gene Ther 1997;8:349–358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.