Abstract
Toca 511 (vocimagene amiretrorepvec), a nonlytic, amphotropic retroviral replicating vector (RRV), encodes and delivers a functionally optimized yeast cytosine deaminase (CD) gene to tumors. In orthotopic glioma models treated with Toca 511 and 5-fluorocytosine (5-FC) the CD enzyme within infected cells converts 5-FC to 5-fluorouracil (5-FU), resulting in tumor killing. Toca 511, delivered locally either by intratumoral injection or by injection into the resection bed, in combination with subsequent oral extended-release 5-FC (Toca FC), is under clinical investigation in patients with recurrent high-grade glioma (HGG). If feasible, intravenous administration of vectors is less invasive, can easily be repeated if desired, and may be applicable to other tumor types. Here, we present preclinical data that support the development of an intravenous administration protocol. First we show that intravenous administration of Toca 511 in a preclinical model did not lead to widespread or uncontrolled replication of the RVV. No, or low, viral DNA was found in the blood and most of the tissues examined 180 days after Toca 511 administration. We also show that RRV administered intravenously leads to efficient infection and spread of the vector carrying the green fluorescent protein (GFP)-encoding gene (Toca GFP) through tumors in both immune-competent and immune-compromised animal models. However, initial vector localization within the tumor appeared to depend on the mode of administration. Long-term survival was observed in immune-competent mice when Toca 511 was administered intravenously or intracranially in combination with 5-FC treatment, and this combination was well tolerated in the preclinical models. Enhanced survival could also be achieved in animals with preexisting immune response to vector, supporting the potential for repeated administration. On the basis of these and other supporting data, a clinical trial investigating intravenous administration of Toca 511 in patients with recurrent HGG is currently open and enrolling.
Introduction
Our previous work has demonstrated that Toca 511 (vocimagene amiretrorepvec), a nonlytic, amphotropic retroviral replicating vector (RRV), selectively infects and replicates in the tumor environment. It encodes and delivers a functionally optimized cytosine deaminase gene (CD) selectively to tumors, and the CD enzyme produced converts 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU), resulting in tumor killing, and apparently cures brain cancer in preclinical models.1,2 These mouse models provide support for a dual mechanism of action of Toca 511 combined with 5-FC that includes both direct killing of tumor cells by locally produced 5-FU, and induction of a local and systemic immunotherapeutic response resulting in long-term survival after cessation of 5-FC treatment. Resistance to subcutaneous tumor rechallenge can be demonstrated in these long-term survivors (Huang T, Robbins J, unpublished data). Toca 511, in combination with subsequent oral extended-release 5-FC (Toca FC), is being investigated as a locally administered therapy for recurrent high-grade glioma (HGG) in two clinical studies (www.clinicaltrials.gov: NCT01156584 and NCT01470794). In these studies, Toca 511 is delivered into a tumor transcranially or into the walls of the tumor bed with a blunt-tipped needle after resection. Both of these methods involve a surgical procedure and, given the tumor heterogeneity and unpredictable three-dimensional structure, may present challenges in achieving optimal coverage of the tumor with the virus. An additional method of delivering the vector to an intracranial tumor is by intravenous administration, which is less invasive and can potentially be repeated if necessary. To investigate intravenous administration of Toca 511, a dose escalation clinical study has been launched in patients with recurrent high-grade glioma, using higher doses than in prior studies (NCT01985256). Here we describe the preclinical safety and efficacy studies used to support the clinical investigation of intravenous Toca 511.
We have previously shown that RRV (including Toca 511) have specificity for infecting proliferating cells and replicate better in an immune-suppressed environment, making cancer cells an excellent target for this vector.1,3,4 Initial infection of cancer cells leads to spread to neighboring cancer cells over time.5 Cells infected with Toca 511 express CD, allowing the enzymatic conversion of 5-FC to high levels of the anticancer drug 5-FU directly within the tumor. Anabolites of 5-FU inhibit DNA and RNA synthesis and metabolism in dividing cells, in part by inhibiting thymidylate synthase, leading to cell cycle arrest, necrosis, and apoptosis.6–10 In addition, a “bystander effect” of locally produced 5-FU has been observed11–14 and is presumably generated at least in part by diffusion of 5-FU to neighboring replicating cells to induce more cell death. Initial distribution of Toca 511 throughout the tumor mass, especially to the advancing outer edge of the tumor, is likely to be optimal for maximal spread and tumor killing; however, this may be challenging to achieve reliably with intratumoral or resection bed injection of the vector. Intravenous administration of Toca 511 may allow the vector to be more evenly distributed within the tumor. High-grade gliomas are known to be highly invasive and vascular tumors, and are characterized by “leaky” vasculature as supported by the enhancement observed with gadolinium contrast agents on magnetic resonance imaging (MRI) of high-grade gliomas, especially glioblastomas. In addition to the potential distribution advantage, intravenous administration may allow the vector to reach tumors that are not easily accessible by surgery, and this route of administration is more convenient for patients and their caregivers.
Therefore, we assessed the biodistribution, safety, and efficacy of intravenous administration of Toca 511 in preclinical models. We also compared vector delivery, distribution, and spread after Toca 511 or related marker gene vectors were administered by intravenous or intracranial routes in both immune-competent and immune-compromised animal models. Finally, survival was evaluated in immune-compromised and immune-competent mice when Toca 511 was administered intravenously or intracranially in combination with 5-FC treatment.
Materials and Methods
Drug and reagents
5-FC for in vivo assays was synthesized to order by a contract chemical supplier.
Toca 511 and Toca GFP vectors
Details of Toca 511 design and modification have been previously described.2 Briefly, the plasmid pACE-GFP5 was modified to improve stability during viral replications by reducing unnecessary sequence repeats and increasing the convenience of transgene insertion to yield the vector pAC3-GFP. The plasmid pAC3-yCD2 was generated by substituting a modified CD gene into pAC3-GFP. Genetic enhancements to the wild-type yeast CD gene were as follows: (1) codon usage was optimized for protein synthesis in human cells; and (2) three amino acid changes were introduced (A23L, I140L, and V108I) to increase thermal stability of the yeast CD protein. Toca GFP and Toca 511 are the infectious vector preparations from plasmids pAC3-GFP and pAC3-yCD2, respectively, and were produced and formulated by methods developed for clinical use.
Cell culture
The mouse glioma cell line Tu-244915 was cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, sodium pyruvate, and GlutaMAX (HyClone/GE Healthcare Life Sciences [Logan, UT] or Invitrogen Life Technologies [Carlsbad, CA]). Cells were maintained in a humidified atmosphere with 5% CO2 at 37°C. When ready for in vivo implantation, cells were resuspended in DMEM without any additives.
Mice and intracranial surgeries
Female B6C3F1 or athymic nude-Foxn1nu mice (age, ∼8 weeks) were purchased from Harlan (Indianapolis, IN). Mice were acclimated for 3–7 days after arrival. Depending on the study, mice either underwent surgical placement of an indwelling guide cannula with a 3.0-mm projection implanted into the right striatum, and fitted with a cap containing a 3.5-mm projection; or surgical implantation of the tumor cells by Hamilton syringe (see below). The stereotaxic coordinates for both studies were anteroposterior (AP), 0.5 mm; mediolateral (ML), 1.8 mm; and dorsoventral (DV), 3.5 mm (from bregma).
In vivo delivery studies
The syngeneic cell line Tu-2449 was used as an orthotopic brain tumor model in B6C3F1 mice and in nude mice. When only intravenous administration of Toca GFP was required in the study, tumor cells were implanted by Hamilton syringe on day 0 with the following stereotaxic coordinates: AP, 0.5 mm; ML, 1.8 mm; and DV, 3.5 mm (from bregma). On the assigned days, mice were injected with Toca GFP (100 μl/mouse/day) via the tail vein. Tumors were analyzed for green fluorescent protein (GFP) positivity on day 14.
In vivo survival studies
The syngeneic cell line Tu-2449 was used as an orthotopic brain tumor model in B6C3F1 mice and in nude mice. Cell implantation and intracranial vector injections were done through an injection cannula with a 3.5-mm projection inserted through the indwelling guide cannula. B6C3F1 or nude mice underwent intracranial implantation of 1.4×104 tumor cells on day 0. Starting on day 3, mice were injected with Toca 511 (intravenously; 100 μl/mouse/day) for five consecutive days via the tail vein. On day 4, other mice were injected with Toca 511 (intracranially; 5 μl/mouse) by intracranial infusion at 0.33 μl/min (15 min, followed by a hold of 5 min). Starting on day 10, mice were treated with either phosphate-buffered saline (PBS) or 5-FC (500 mg/kg/dose) (intraperitoneal, twice daily) for four consecutive days, followed by 10 days without drug to allow vector spread. Cycles of 4-day on, 10-day off drug treatment were repeated an additional three times.
Two different lots of Toca 511 were used for all in vivo studies. Toca 511 lot T511071-FNL had a starting titer of 6.5×108 transducing units (TU)/ml whereas Toca 511 lot T511082-FNL had a starting titer of 6.3×108 TU/ml. Toca 511 doses are defined as transducing units per gram of brain (TU/g) with the average mouse brain defined as 0.5 g.
In-life observations
Routine general health, in-life observations, and body weights were collected throughout the course of the study. In-life observations were scored on a 0- to 4-point system for severity (1, mild; 2, moderate; 3, severe; and 4, moribund) of each individual symptom such as general clinical signs (including inactivity/lethargy, hunched posture, rough coat, etc.), neurological signs (including leaning, circling, head tilt, etc.). Mice with a cumulative score of 5 were euthanized. Mice with body weight loss of >20% for more than 2 days were euthanized. All animal protocols and experiments were approved by the Institutional Animal Care and Use Committee (A4487-01) of Explora BioLabs (San Diego, CA).
Imaging
Animals were euthanized and brain was harvested. FluorVivo model 100 from INDEC BioSystems (Santa Clara, CA) was used for fluorescence imaging.
Fluorescence-activated cell-sorting analysis
Tumors were harvested from the mice, and minced before being incubated in collagenase for 30 min on a shaker at room temperature. Single tumor cells were obtained by filtering through 40-μm filters. Cells were analyzed for fluorescence percentage by flow cytometry. Spleen was also harvested from the mice, and single splenocytes were obtained after lysing red blood cells. Cells were analyzed for fluorescence percentage by flow cytometry.
In vitro pharmacokinetic study
Tu-2449 cells (5×104) were plated in each well of 6-well plates on day −1. On day 0, Toca GFP (5×104 TU/ml) was incubated with either PBS (control) or serum from naive B6C3F1 mice at 37°C at various time points (0, 5, 10, 30, 60, 120, 240, and 720 min) before addition to the cells for infection. The percentage of GFP-positive cells was analyzed by flow cytometry on day 3.
In vivo pharmacokinetic study
Sera were collected from B6C3F1 mice at 0, 5, 30, 60, 120, and 240 min, and 12 hr after Toca 511 intravenous injection. For qRT-PCR assays, viral RNA was measured by a qRT-PCR two-step procedure consisting of a cDNA synthesis step and an amplification step, with a murine leukemia virus (MLV) reference virus standard for normalization. Forward primer sequences for Env2 were 5′-ACC CTC AAC CTC CCC TAC AAG T-3′ and 5′-GTT AAG CGC CTG ATA GGC TC-3′ for reverse primer, with 5′-FAM-AGC CAC CCC CAG GAA CTG GAG ATA GA-BHQ1-3′ as a probe. qRT-PCR assays were performed on a Bio-Rad (Hercules, CA) CFX 384 instrument. RT-PCR measures viral RNA purified from the selected matrix containing viral particles. This measurement includes all viral RNA released from cells and is not directly equivalent to numbers obtained from the assay for transducing units. The ratio of particle copies to transducing units in these experiments was roughly 200-fold.
Biolocalization
A detailed description of sample processing and PCR analysis has been given previously.1 Briefly, all tissue samples were processed by sterile techniques and using single-use disposable instrumentation per tissue per mouse, and stored at −80°C until processed. For the qPCR assay, genomic DNA was prepared from approximately 3- to 5-mm3 pieces of each tissue. qPCR was used to detect viral sequences in processed samples, using the following MLV-specific primers (Integrated DNA Technologies, San Diego CA): forward primer, 5-MLV-U3-B 5′-AGC CCA CAA CCC CTC ACT C-3′; reverse primer, 3-MLV-Psi 5′-TCT CCC GAT CCC GGA CGA-3′; probe, 5′-FAM-CCC CAA ATG AAA GAC CCC CGC TGA CG-BHQ1-3′, with the qPCR performed in a Bio-Rad C100 thermal cycler with a Bio-Rad CFX real-time system. Four categories of viral levels were used: negative (undetectable); low (below the level of quantitation to 0.1 copy/cell equivalent); medium (0.1–4 copies/cell equivalent); and high (more than 4 copies/cell equivalent). In this calculation we assumed that 1 copy/cell equivalent=150,000 copies/μg genomic DNA.
Hematology
Whole blood (with EDTA) was analyzed with a scil Vet abc analyzer (scil Animal Care, Gurnee, IL). Complete blood counts (CBCs) were determined.
ELISA
Sera from mice were collected on day −11 and were tested for anti-MLV antibody presence.1 Briefly, capture antigen was incubated overnight, and then serum samples were added and incubated before goat anti-mouse horseradish peroxidase (HRP)-conjugated antibody was added for detection. Plates were read at 450 nm with an ELISA reader (SpectraMax 190; Beckman Coulter, Brea, CA).
Immunohistochemistry
To examine tumor angiogenesis, mouse brains were collected and flash frozen in optimal cutting temperature (O.C.T.) compound. Brain sections were stained with anti-CD31 antibody.
To examine vector distribution, mice were perfused with 4% paraformaldehyde (PFA) and brains were collected and fixed in 4% PFA overnight and then immersed in 70% ethanol and embedded into paraffin. Brain sections were stained with anti-CD antibody, clone #9A11, a mouse monoclonal IgG1 antibody raised against recombinant optimized yeast cytosine deaminase protein yCD2 (described previously) for detection of Toca 511 presence, or with anti-GFP antibody (clone #D5.1; Cell Signaling, Danvers, MA) for detection of Toca GFP presence.
Statistical analyses
Survival data were plotted by the Kaplan–Meier method, and were compared by the log-rank test or Student t test as noted. p values less than 0.05 were considered statistically significant in all analyses, which were done with Prism 5 statistical software (GraphPad Software, San Diego, CA).
Results
Pharmacokinetics and biodistribution of Toca 511 after intravenous administration in a non-tumor-bearing mouse model
An in vitro infectivity study and an in vivo viral particle quantification study were performed to assess the pharmacokinetics of Toca 511 in blood after intravenous administration. For the in vitro study, Toca GFP at 1×105 TU to achieve a multiplicity of infection of 1 was incubated with PBS (control) or serum from naive B6C3F1 mice at 0, 5, 10, 30, 60, 120, 240, and 720 min (12 hr) at 37°C before infecting Tu-2449 cells. The percentage of GFP-positive cells was analyzed by flow cytometry 3 days later. As shown in Supplementary Fig. S1a (supplementary data are available online at www.liebertpub.com/hum), Toca GFP vector in PBS (control) was stable at 37°C for at least 2 hr (81% at 0 min and 79% at 120 min). Toca GFP incubated with serum from naive mice had overall a lower percentage of GFP-positive cells compared with the control at every time point examined, indicating that there were some assay-inhibiting factors in the serum (Supplementary Fig. S1a). Toca GFP was reasonably stable in mouse serum for 30 min (68% at 0 min and 61% at 30 min) before the infectivity started decreasing more dramatically. For the in vivo study, 24 B6C3F1 mice were injected with 0.1 ml of Toca 511 intravenously via the tail vein at dose levels of either 106 or 107 TU/g of brain. At 0, 5, 30, 60, 120, 240, and 720 min (12 hr) postinjection, blood was collected for Toca 511 viral particle quantification by qRT-PCR analysis. There was a 100-fold drop from 109 to 107 copies/ml (titer adjusted on the basis of blood volume and vector administration ratio) in detectable Toca 511 in the blood (serum) within 30 min of vector administration (Supplementary Fig. S1b), consistent with the in vitro Toca GFP infectivity experiment shown in Supplementary Fig. S1a (this equates to infectious virus of approximately 5×104 TU/ml at this time point). Toca 511 signal dropped 1000-fold from the starting titer by 120 min and stayed at about 106 viral copies/ml (5×103 TU/ml calculated titer) up to 12 hr. The loss of vector signal appeared to follow second-order rate kinetics.
To understand the biodistribution of Toca 511 when administered intravenously, 20 B6C3F1 mice were injected intravenously with Toca 511 at 1.1×108 TU (5.4×107 TU/g) of brain dose level. All mice were bled on days 30, 90, 120, 150, and 180 postinjection for Toca 511 qPCR analysis. On day 180, these mice were euthanized and tissues were harvested for Toca 511 qPCR analysis. The range of blood and tissue qPCR positivity (expressed as copy number per microgram) and the distribution in the positive mice were assessed. Range allocation was determined as 0, no detectable DNA (Ct>38); low, less than 15,000 copies/μg (<0.1 copy/cell equivalent); medium, 15,000–600,000 copies/μg (0.1–4 copies/cell equivalent); and high, more than 600,000 copies/μg (>4 copies/cell equivalent).
At 30, 90, 120, 150, and 180 days after intravenous administration of Toca 511, blood from almost all treated B6C3F1 mice exhibited measurable Toca 511 DNA signal whereas the negative control showed no signal of Toca 511 (Table 1). Blood measurements for viral sequences have been previously shown to predict the likely level of overall viral replication in an animal. On day 30, viral DNA was highest in the blood, with 11 of 20 mice in the medium category. The viral signal decreased over time, with only two mice in the medium category on day 90. By day 120, all the mice had <15,000 copies/μg (low category) and the viral DNA stayed low until termination on day 180. To check for potential toxicity with the combination of Toca 511 and 5-FC, detailed blood analysis was done using a strain of mice, BALB/c, that is highly permissive to viral infection. Approximately 180 days after intravenous administration of Toca 511, the mice were treated with one 5-day course of 5-FC (500 mg/kg, twice daily), and complete blood counts were compared in viremic versus nonviremic mice. Hematology results indicated there was no significant change or difference between nonviremic and viremic mice (Supplementary Fig. S2).
Table 1.
Toca 511 DNA PCR Signal in Blood from B6C3F1 Mice over Time
| Blood | Distribution | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | Treatment | Cohort | n | No. positive | Range of positivity (copies/μg) | Low | Medium | High |
| 1 | B6C3F1 Control | 30 | 15 | 0 | 0–0 | 0 | 0 | 0 |
| 90 | 15 | 0 | 0–0 | 0 | 0 | 0 | ||
| 120 | 15 | 0 | 0–0 | 0 | 0 | 0 | ||
| 150 | 15 | 0 | 0–0 | 0 | 0 | 0 | ||
| 180 | 5 | 0 | 0–0 | 0 | 0 | 0 | ||
| 2 | B6C3F1 Toca 511 107 IV | 30 | 20 | 20 | 2,030–49,779 | 9 | 11 | 0 |
| 90 | 20 | 20 | 167–23,096 | 18 | 2 | 0 | ||
| 120 | 19 | 18 | 576–9,753 | 18 | 0 | 0 | ||
| 150 | 19 | 18 | 259–11,459 | 18 | 0 | 0 | ||
| 180 | 20 | 20 | 199–14,823 | 20 | 0 | 0 | ||
IV, intravenous.
The tissues were categorized into the following: nervous/special sense system (cerebellum, left brain, right brain, lower spinal cord, upper spinal cord), lymphoid/hematopoietic system (blood, bone marrow, lymph node, spleen, thymus), cardiovascular/respiratory system (heart, lung), digestive system (esophagus, liver, lower esophagus, lower intestine, salivary gland), urogenital system (kidney, urine, ovary), and shedding (skin). Toca 511 viral genomic sequences were detected in some organs in B6C3F1 animals 180 days after they were given intravenous Toca 511 (Table 2). The highest levels of vector DNA were seen mainly in esophagus, and lymphatic tissues including spleen, thymus, and lymph nodes. No organs showed high levels of Toca 511 genomic signal (>600,000 copies/μg) and only a few organs in a few mice showed medium levels of virus (15,000 to 600,000 copies/μg). The genomic DNA signal was below 4000 copies/μg for the majority of organs in the majority of mice analyzed. These data confirm the previous conclusion1 that if any tissue is positive there is also a signal in the blood, and that blood can be used as a sentinel tissue for any viral infection in an individual subject.
Table 2.
Range of Positivity in Organs from B6C3F1 Mice 179 Days After Intravenous Administration of Toca 511
| Organ | No. of positive mice/total no. of mice | Positivity (copies/μg) | Low | Medium | High |
|---|---|---|---|---|---|
| Blood | 20/20 | 199–14,823 | 20 | 0 | 0 |
| Cerebellum | 0/20 | – | 0 | 0 | 0 |
| Left cerebrum | 1/20 | 88 | 1 | 0 | 0 |
| Right cerebrum | 1/20 | 75 | 1 | 0 | 0 |
| Lower spinal cord | 3/20 | 101–160 | 3 | 0 | 0 |
| Upper spinal cord | 1/20 | 76 | 1 | 0 | 0 |
| Bone marrow | 19/20 | 109–3,679 | 19 | 0 | 0 |
| Lymph node | 19/20 | 197–17,916 | 18 | 1 | 0 |
| Spleen | 20/20 | 244–29,413 | 17 | 3 | 0 |
| Thymus | 19/20 | 400–276,846 | 17 | 2 | 0 |
| Heart | 9/20 | 97–4,110 | 9 | 0 | 0 |
| Lung | 17/20 | 111–4,843 | 17 | 0 | 0 |
| Esophagus | 14/20 | 76–6,459 | 14 | 0 | 0 |
| Esophagus (lower) | 16/20 | 221–22,802 | 14 | 2 | 0 |
| Intestine | 14/20 | 78–1,993 | 14 | 0 | 0 |
| Liver | 16/20 | 71–11,534 | 16 | 0 | 0 |
| Salivary gland | 18/20 | 90–5,149 | 18 | 0 | 0 |
| Kidney | 8/16 | 58–2,925 | 8 | 0 | 0 |
| Ovary | 18/20 | 81–18,114 | 17 | 1 | 0 |
| Urine | 0/14 | – | 0 | 0 | 0 |
| Skin | 5/20 | 127–3,661 | 5 | 0 | 0 |
Intravenous administration of Toca GFP results in vector delivery to the intracranial tumor, and overall vector uptake is comparable between intracranial and intravenous delivery routes
High-grade gliomas are known to be highly vascularized.16,17 The vascularity of Tu-2449 tumor at various time points was examined by staining an orthotopic glioma tumor with the endothelial cell marker CD31 to evaluate tumor angiogenesis. Tumor mass after 6 days of implantation showed extensive staining with this endothelial cell marker, indicating high levels of vascularity (Supplementary Fig. S3).
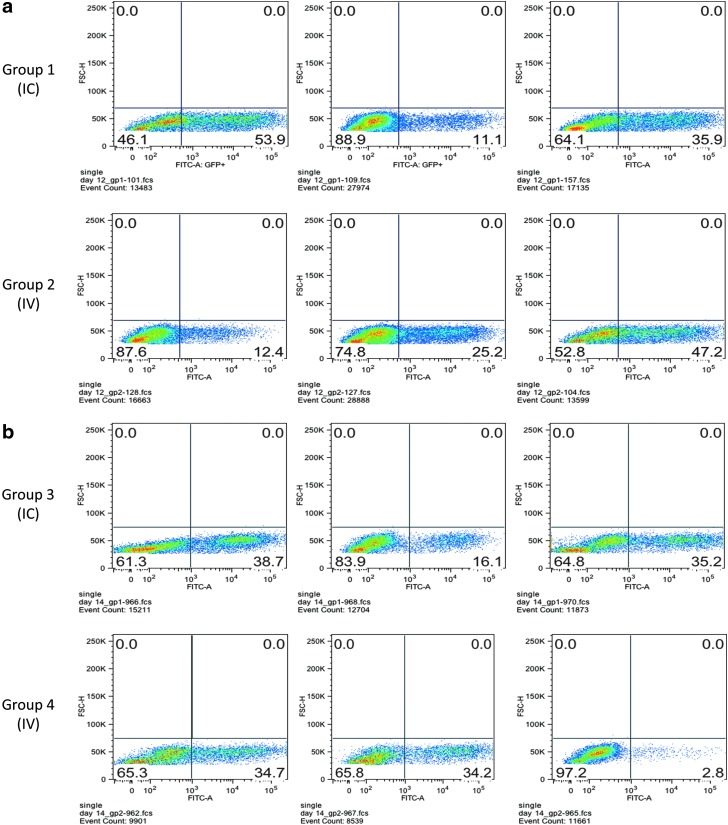
The capability of intravenous administered RRV to infect intracranial tumor was assessed in both immune-competent and immune-compromised mouse models (Fig. 1a and b). In the immune-competent mouse model, Tu-2449 tumor was implanted in B6C3F1 mice intracranially on day 0. Toca GFP was administered either intracranially once (on day 5, group 1) at 2.8×103 TU (1.4×103 TU/g of brain) or intravenously once a day for 3 days (on days 5, 7, and 10, group 2) at 1.7×108 TU (8.4×107 TU/g of brain). Tumor was excised from brain tissue on day 12 to examine GFP spread by fluorescence-activated cell-sorting (FACS) analysis. The average vector spread after intracranial administration was 33.6% of tumor cells (range, 11.1–53.9%), and the average vector spread after intravenous administration was 28.2% (range, 12.4–47.2%) as shown in Fig. 1a. The intravenous and intracranial data in this experiment are statistically indistinguishable.
FIG. 1.
Administration of Toca green fluorescent protein (GFP) intravenously (IV) results in vector delivery to the intracranial tumor, and the percentage of infected cells is comparable to intracranial (IC) delivery. (a) Immune-competent, syngeneic model using the Tu-2449 tumor line on day 12; (b) immune-compromised nude mouse model using the same Tu-2449 tumor line on day 14.
In the immune-compromised mouse model, Tu-2449 tumor was implanted in nude mice intracranially on day 0. Toca GFP was administered either intracranially once (on day 5, group 3) at 5×103 TU (2.5×103 TU/g of brain) or intravenously once a day for five consecutive days (on days 5–9, group 4) at 2.4×108 TU (1.2×108 TU/g of brain). Tumor was excised from brain tissue on day 14 to examine GFP spread by FACS analysis. The average vector spread for the intracranial group was 30% (range, 16.1–38.7%), and the average vector spread for the intravenous group was 23.9% (range, 2.8–34.7%) as shown in Fig. 1b. These results demonstrate that intravenously administered Toca GFP vector successfully crossed the blood–brain barrier (BBB), and infected intracranial tumor in both immune-competent and immune-compromised mouse models. In these experiments vector spread was comparable between intracranial and intravenous administration, with approximately 50,000-fold more vector delivered intravenously.
Percentage of GFP-positive cells in intracranial tumor increases with higher titers of Toca GFP intravenous administration
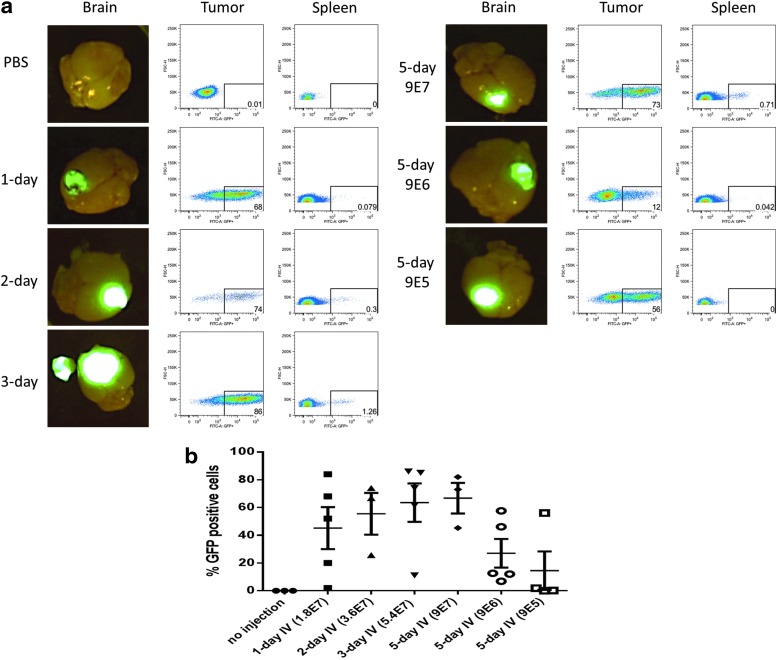
An optimal titer and dosing schedule for intravenous delivery of Toca vector to intracranial glioma was investigated using the immune-competent mouse model. Tu-2449 tumor was implanted in mice intracranially on day 0. Starting on day 3, Toca GFP was administered intravenously to these mice at various titers and schedules. Group 1 received PBS for five consecutive days as a negative control, group 2 received 1 day of Toca GFP (3.6×107 TU or 1.8×107 TU/g), group 3 received two consecutive days of Toca GFP (total dose, 7.2×107 TU or 3.6×107 TU/g), group 4 received Toca GFP every other day for a total of 3 days (total dose, 1.1×108 TU or 5.4×107 TU/g), group 5 received five consecutive days at the highest titer vector (total dose, 9×107 TU/g), group 6 received five consecutive days of a 1:10 dilution of vector (total dose, 1.8×107 TU or 9×106 TU/g), and group 7 received five consecutive days of a 1:100 dilution of vector (total dose, 1.8×106 TU or 9×105 TU/g). On day 14, animals were euthanized, each brain was imaged for GFP fluorescence, and tumor and spleen were collected for FACS analysis.
Representative fluorescence images shown in Fig. 2a demonstrate that PBS control had no GFP positivity whereas the other six groups had a strong GFP signal in the intracranial tumor. Further analyses of the tumor and spleen by FACS revealed that there was medium- to high-level (12–86%) GFP positivity in the tumor but a low level (0–0.7%) in the spleen (Fig. 2a). Comparison of intravenous dosing schedule and titers administered showed that there was a trend of increasing GFP positivity with higher titers and/or more days of intravenous injection (Fig. 2b). Five-day intravenous administration of Toca GFP at 1.8×108 TU (9×107 TU/g of brain) resulted in the highest average GFP-positive cells, and 5-day intravenous administration of 1.8×106 TU (9×105 TU/g of brain) resulted in the lowest average GFP-positive cells.
FIG. 2.
Percentage of GFP-positive cells in intracranial tumor increases with increasing titers of intravenously administered Toca GFP. (a) Representative fluorescence images of mouse brain; FACS data of tumor and spleen on day 14 for all seven groups. (b) Percentage of GFP-positive cells within the tumor from day 11 to day 14.
Blood and tissues were also collected from these mice to examine the biolocalization of Toca GFP 11 days after intravenous vector administration. As shown in Supplementary Tables S1 and S2, group 1 (PBS control) did not have any viral DNA signal in blood or tissues. Most mice from groups 2–7 had no or a low level of viral DNA in blood and tissues. In addition, viral signal, when present, was found most commonly in the lymphatic tissues (spleen, bone marrow, and lymph nodes) in these mice (data not shown), consistent with the results from the biolocalization study done previously in non-tumor-bearing mice (Table 1).
Immunohistochemical staining demonstrates different infection and spread pattern for intracranial and intravenous administration of RRV
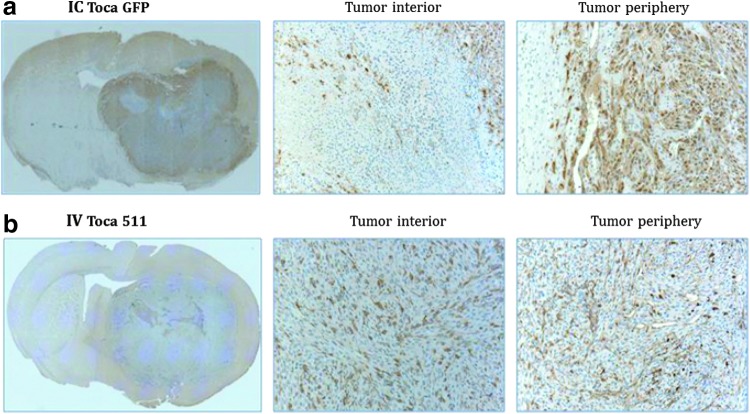
Localization of vector in intracranial tumor was compared between intracranial and intravenous administration of RRV. Mice were implanted with Tu-2449 tumor cells on day 0. Starting on day 3, these mice received Toca 511 intravenously for five consecutive days (days 3–7) for a total of 2.6×108 TU (1.3×108 TU/g of brain). On day 4, these mice also received Toca GFP intracranially at 4.2×106 TU (2.1×106 TU/g of brain). Mice were killed by perfusion when they became moribund, and brains were collected for immunohistochemical (IHC) analysis. The localization of intracranially administered RRV (Toca GFP) was detected with anti-GFP antibody, and the localization of intravenously administered RRV (Toca 511) was detected with anti-CD antibody. IHC stains revealed that GFP protein (intracranially administered) was present mostly in the periphery of the tumor but not the interior of the tumor, and CD protein (intravenously administered) was present in both the periphery and interior of the tumor (Fig. 3a and b). These results demonstrate distinct patterns of localization when vector was delivered via the intracranial or intravenous route.
FIG. 3.
Immunohistochemical staining demonstrates distinct patterns of Toca vector localization after intracranial and intravenous delivery in a Tu-2449 mouse brain tumor model. (a) Toca GFP detected with rabbit anti-GFP antibody; (b) Toca 511 detected with mouse anti-CD antibody. Coronal sections shown; original magnification, ×20. Center: Tumor interior. Right: Tumor periphery.
Intravenous administration of Toca 511 demonstrates survival efficacy in an immune-competent, but not immune-deficient, orthotopic mouse glioma model
Intravenous administration of Toca GFP resulted in vector delivery and spread in intracranial tumor in both immune-compromised and immune-competent mouse models (Fig. 1). Survival efficacy was then assessed in these two mouse models after intravenous administration of Toca 511.
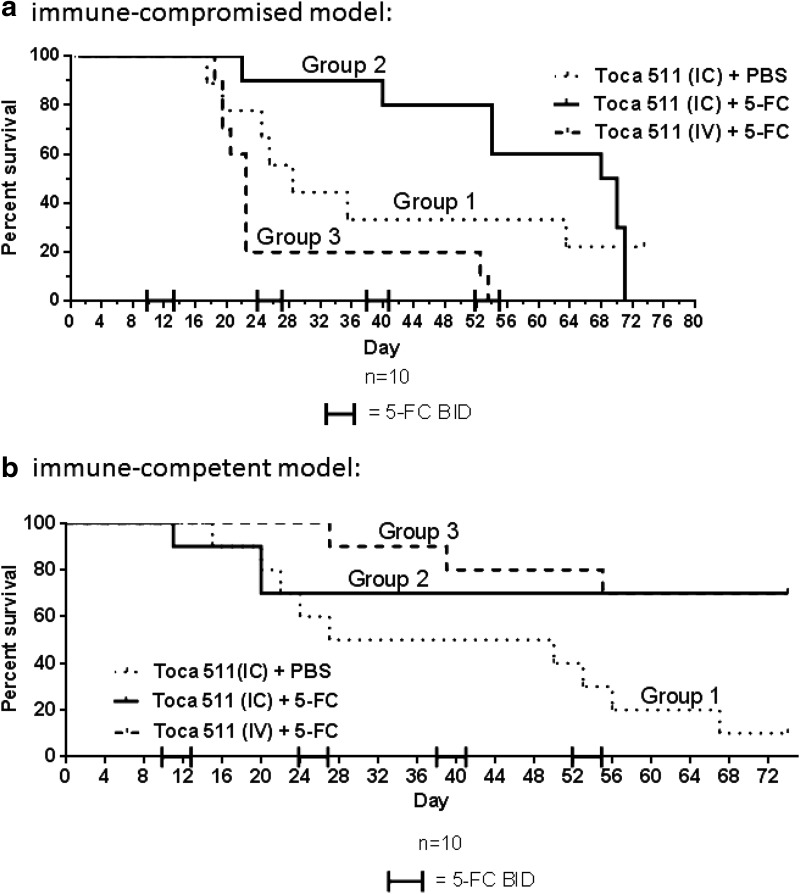
In the immune-compromised model, Tu-2449 cells were implanted intracranially in nude mice on day 0. Starting on day 3, group 3 received intravenous (100 μl) injection of Toca 511 daily for five consecutive days (days 3–7) for a total of 1.3×109 TU (6.3×108 TU/g of brain). On day 4, groups 1 and 2 received intracranial (5 μl) injection of Toca 511 once (1.3×107 TU or 6.3×106 TU/g of brain). Starting on day 10, groups 2 and 3 began four cycles of 5-FC (500 mg/kg in 800 μl, intraperitoneal, twice daily) for four consecutive days, followed by 10 days off drug; group 1 received PBS as control (800 μl, intraperitoneal, twice daily). All groups were analyzed for survival up to day 75. Mice administered Toca 511 intracranially and 5-FC twice daily (group 2) had better median survival compared with the control group treated with PBS (group 1) at 69 and 29 days, respectively, as shown in Fig. 4a. However, mice administered Toca 511 intravenously and 5-FC twice daily (group 3) did not have better median survival (22 days) compared with control group 1. The lack of survival of nude mice after the intravenous administration of Toca 511, in contrast to the benefit in intracranially treated mice, suggests that the peritumoral location of the Toca 511 could be more beneficial, especially in a setting that does not have the contribution of an immune mechanism.
FIG. 4.
Intravenous administration of Toca 511 with 5-FC demonstrates survival efficacy in immune-competent orthotopic mouse glioma model. (a) Tu-2449 tumor in an immune-compromised mouse model; (b) Tu-2449 tumor in an immune-competent mouse model
In the immune-competent mouse model, Tu-2449 tumor cells were implanted intracranially on day 0. Starting on day 3, group 3 received intravenous (100 μl) injection of Toca 511 daily for five consecutive days (days 3–7) for a total of 6.6×108 TU (3.3×108 TU/g of brain). On day 4, groups 1 and 2 received intracranial (5 μl) injection of Toca 511 once for 1.3×107 TU (6.5×106 TU/g of brain). Starting on day 10, groups 2 and 3 began four cycles of 5-FC (500 mg/kg in 800 μl, intraperitoneal, twice daily) for four consecutive days, followed by 10 days off drug; group 1 received PBS as control (800 μl, intraperitoneal, twice daily). Previous in vivo studies have shown that 5-FC treatment alone did not result in any survival efficacy,1,5,14 and thus this control was not included in this study. Survival was also assessed up to day 75. Mice receiving intracranial Toca 511 and 5-FC twice daily (group 2) showed long-term survival at the end of the study compared with the mice treated with PBS (group 1) (p=0.03). Mice treated with intravenous Toca 511 and 5-FC BID (group 3) also showed long-term survival compared with the control group (group 1) (p=0.01) (Fig. 4b). In addition, mice treated with intravenous Toca 511 plus 5-FC twice daily (group 3) survived comparably to mice treated with intracranial Toca 511 plus 5-FC twice daily (group 2) (p=0.8). These results suggest that immune components play a role in maintaining long-term survival in mice treated with Toca 511 plus 5-FC.
Toca 511-preimmunized B6C3F1 mice demonstrate survival efficacy with repeated Toca 511 administration
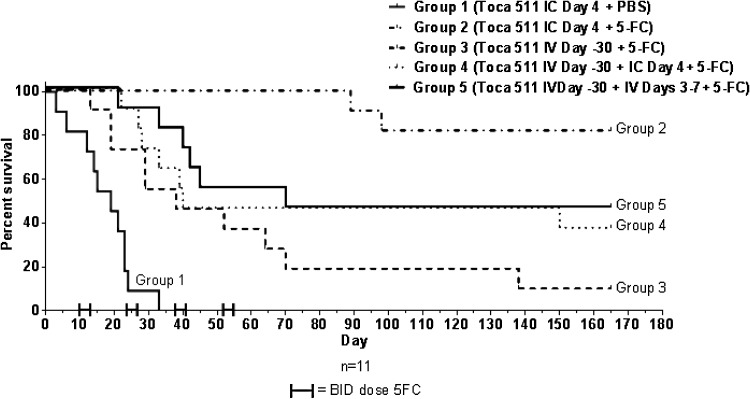
In the previous survival efficacy study, five consecutive days of vector was administered intravenously (before 5-FC cycles) to show survival benefit compared with the untreated group. It is possible that more vector could be administered intravenously during the 5-FC off cycle to obtain additional viral infection. A study to specifically address safety and efficacy in the setting of preexisting antibodies to vector was conducted. Mice were randomized and then either pretreated intravenously with a single, high, Toca 511 dose (2.4×107 TU or 4.8×107 TU/g of brain) to induce antibodies (day −30) or left untreated. On day −11, serum was collected and tested for anti-vector antibodies by ELISA, and all mice that were pretreated with Toca 511 intravenously were positive for anti-vector antibody (Supplementary Table S3). Untreated animals were randomized to groups 1 and 2, and positive animals were randomized to groups 3–5, and then all animals underwent intracranial administration of 1.4×104 Tu-2449 cells. Intracranial groups received 1 day of Toca 511 lot T511082-FNL (3.2×106 TU or 6.3×106 TU/g of brain), and the intravenous groups received either 1 day of Toca 511 (6.7×107 TU or 1.3×108 TU/g of brain) or a total of 3.2×108 TU or 6.3×108 TU/g of brain injected over 5 days. Starting on day 10, groups 2–5 began cycles of 5-FC (500 mg/kg in 800 μl, intraperitoneal, twice daily) for four consecutive days, followed by 10 days off drug; group 1 received PBS. 5-FC treatment was repeated for four cycles. As shown in Fig. 5, a statistically significant increase in survival compared with untreated controls was achieved in the presence of preexisting antibodies to Toca 511 for both transcranial administration and intravenous administration (p<0.0001 for both). There were no obvious safety issues with readministration of vector in these preclinical studies in terms of general health and gross pathology at necropsy. Although survival of immunized animals was somewhat decreased compared with nonimmunized treated animals, this result shows that readministration of vector in the face of preexisting immunity can confer a survival benefit, without signs of toxicity. Thus, repeated intravenous administration should be feasible.
FIG. 5.
Toca 511-preimmunized B6C3F1 mice demonstrate survival efficacy with repeated Toca 511 administration.
Discussion
Adequate delivery of therapeutic agents to the brain for the treatment of glioblastoma is a key first step to potentially eradicating the tumor. However, delivery of all types of drugs may be complicated by the potential exclusion of drugs by the blood–brain barrier, and lack of standardized drug delivery techniques. In addition, the location or shape, structural heterogeneity, and imaging of the tumor and tumor bed may also be factors that influence how therapeutic agents can be delivered. For patients who decline surgical removal of a brain tumor or who are not suitable candidates for brain tumor surgery, transcranial administration of a therapeutic directly into the tumor (intratumoral) is less invasive, but the tumor is not readily accessible in some patients. Furthermore, an unresected tumor may continue to grow aggressively and exert immunosuppressive effects18 resulting in a larger tumor burden that is more difficult to control with drug therapy compared with the treatment of residual tumor after surgical resection. Postresection delivery of a therapeutic agent into the tumor bed may be more practical but the therapeutic agent needs to be distributed throughout the bed to reach residual tumor. Intravenous delivery of drugs may be a solution for these potential limitations if the therapeutic agent can successfully cross the blood–brain barrier and be delivered to the tumor. These factors and considerations play a role in the initial delivery of RRV to brain tumors. Here we showed by FACS that comparable infection and spread can be achieved using intracranial or intravenous delivery of Toca GFP. We also showed by immunohistochemistry that Toca 511 was distributed throughout the intracranial tumor when delivered intravenously (Figs. 1 and 3). This spread pattern may be complementary to intratumoral or postresection delivery because it may enhance the potential for the vector to infect and spread to more tumor cells, and for the bystander effect of 5-FU to be more widespread.
It is not surprising that higher doses of vector will be needed when administered intravenously in order to achieve levels of infection comparable to direct intracranial administration. When vector is delivered intravenously, it is immediately diluted in the bloodstream, and in our experiments is apparently cleared or neutralized, most likely by serum factors, the reticuloendothelial system, or nonproductive binding to nontarget cells and tissues (Supplementary Fig. S1a) or other serum factors. Our experiments use RRV produced in a human cell line and studied in murine blood and prior work reported species-specific complement effects,19 which may be relevant here, and suggests the half-life of Toca 511 in humans may be longer than in mice. In our preclinical models, roughly 5×104-fold more vector for intravenous administration resulted in a similar percentage of GFP-positive cells as intracranial administration (Fig. 1), and this may be due to the need to fill a “sink” as with adenoviral vectors and Kupffer cells.20,21 The dose escalation study (ranging from 1- to 5-day dosing) demonstrated that the percentage of GFP-positive tumor cells increased with higher titers of administered vector (Fig. 2), suggesting a dose-responsive increase in infection. In survival efficacy experiments, consecutive 5-day intravenous administration was used to achieve the highest infection and spread in the tumor before starting 5-FC treatment (Fig. 4). In addition, repeated Toca 511 administration either via the intracranial or intravenous route to animals that previously had antibodies against the vector also showed survival efficacy (Fig. 5), indicating that repeated intravenous administration of Toca 511 may be a possibility to obtain more vector infection and spread even after induction of an immune response against the vector.
One potential concern for delivery of gammaretroviral vectors to patients is the possibility of lymphomagenesis. Lymphomagenesis in susceptible strains of mice is associated with high levels of viremia and recombination of administered MLV with murine-specific endogenous retroviral genomes to generate recombinant MCF virus.22 The occurrence and incidence of lymphomas depends on the murine strain, the age at administration, and several genetic alleles.23–26 In clinical trials, lymphoma was observed in 5 of 25 patients with SCIDS (X-1) treated with autologous hematopoietic stem cells transduced with nonreplicating gammaretroviral vectors carrying a receptor for a growth factor for lymphocytes; however, no lymphomas have been observed in 40 similar subjects with ADA-SCIDS27 treated with a similar gammaretroviral vector encoding a different transgene. Nonreplicating gammaretroviral vectors encoding human factor VIII have been administered intravenously to adult patients with hemophilia at doses up to 8.8×108 TU/kg. This treatment appeared to be safe despite persistence of vector sequences in peripheral blood mononuclear cells for more than 1 year.28 Intracranial administration of Toca 511 in our clinical trials has resulted in quantifiable viral DNA in whole blood DNA or viral RNA in plasma in about 10% of the trial subjects, several weeks after administration; this is rapidly cleared without intervention, so it appears that humans can control this virus at the doses administered to date. In our studies in immune-intact B6C3F1 mice with or without 5-FC1 and in published studies in normal adult monkeys29 neither sustained high-level viremia nor lymphomagenesis has been observed after intravenous treatment with MLV-based gammaretroviruses.
Another possible concern was the targeting efficiency and potential for off-target effects in systemic delivery of Toca 511: either the vector is cleared by the immune system before reaching the intracranial tumor, or the vector might infect off-target proliferating cells within the body. An acute (14-day) biolocalization study using orthotopic glioma-bearing B6C3F1 mice showed no or low viral signals in the blood and tissues examined. When splenocytes and tumor cells were both checked for GFP-positive cells by FACS, few splenocytes were positive for GFP (<2%) whereas tumor cells had medium to high percentages (12–47%) of GFP-positive cells (Fig. 1a and b). A longer term (180 days) biolocalization study in non-tumor-bearing B6C3F1 mice demonstrated that blood and the majority of tissues examined had no or low viral signals, with the exception of a few tissues in the lymphoid/hematopoietic system that had medium levels of Toca 511 DNA signal (Tables 1 and 2). The viral signal in the blood decreased over time (from day 30 to day 180) in this mouse model. No safety issues were observed at any time out to 180 days after Toca 511 was administered intravenously in B6C3F1 mice.
Several phase 1 clinical trials involving intravenous administration of oncolytic viruses for glioblastoma36,37 or metastatic solid tumors38 showed that intravenous administration was well tolerated, and no maximal tolerated dose was reached. In the recently launched clinical study of intravenously administered Toca 511, safety will be examined and the level of viral particles and/or infectious viral units in the blood and tumor will also be monitored in each patient.
Immune components play a role in eradicating intracranial tumor in our preclinical model, as demonstrated in Fig. 4. In the immune-compromised mouse model, mice that received Toca 511 administered intravenously followed by 5-FC showed poor short-term survival compared with mice that received Toca 511 administered intracranially. Immune-compromised mice that received Toca 511 administered intracranially followed by 5-FC showed better short-term survival compared with the PBS control, but all mice eventually succumbed to tumor burden after 5-FC was stopped (Fig 4a). On the other hand, in similar experiments in immune-competent mice, the intracranial and intravenous delivery routes both led to statistically significant efficacy and long-term survivors (Fig. 4b). In Fig. 1, we showed that intracranially and intravenously administered Toca vector had overall comparable infection and spread in both immune-competent and immune-compromised mouse models by examining the percentage of GFP-positive cells, indicating the difference we observed in efficacy was not due to lack of vector presence in the tumor after intravenous administration of Toca 511. In addition, mice from the same immune-competent model that cleared their intracranial tumor with the same dose of Toca 511 plus 5-FC treatment used in Fig. 4b were resistant to tumor rechallenge (subcutaneously), further demonstrating an active role of immune system (Huang T, Robbins J, unpublished data). These results confirm the contribution of immune components in this preclinical model.
Although the immune system is needed to completely eradicate the tumor in this model, there may be an immune response elicited against the vector as well, particularly if the vector is delivered intravenously repeatedly. This has been observed after intravenous administration of other replicating viral vectors.38 On the other hand, Freeman and colleagues showed detectable antibodies against Newcastle disease virus that either plateaued or started to decrease in patients receiving repetitive dosing.36
We previously showed that intracranial administration of Toca 511 followed by 5-FC treatment resulted in long-term survival in intracranial tumor-bearing mice.1 In this current study, we have demonstrated that comparable long-term survival can also be achieved with Toca 511 delivered intravenously in the immune-competent mouse model. These results further support a dual mechanism of action for the combination of Toca 511 and 5-FC that involves both direct tumor chemoablation and the consequent activation of an antitumor immune response. In addition, no safety issues were observed in B6C3F1 mice after intravenous administration of Toca 511. These findings were used to support the clinical investigation of intravenous delivery of Toca 511 followed by Toca FC in patients with recurrent high-grade glioma.
Supplementary Material
Acknowledgments
The authors thank Drs. Nicholas A. Boyle and Alessandro Lobbia for helpful discussions and critical reading of the manuscript. The authors thank the ABC2 Foundation (Washington, DC), the National Brain Tumor Society (Watertown, MA), the American Brain Tumor Association (Chicago, IL), the Musella Foundation (Hewlett, NY), and Voices Against Brain Cancer (New York, NY) for financial support. N.K. was supported by NIH grant U01 NS059821.
Author Disclosure Statement
T.H., S.P., D.O., F.L.E., B.M., C.I., H.G., D.P., D.J., and J.R. are employees and/or shareholders of Tocagen. N.K. is a consultant, has ownership interest in, and is the recipient of a research grant from Tocagen. F.H. is the recipient of a research gift from Tocagen.
References
- 1.Ostertag D, Amundson KK, Lopez Espinoza F, et al. . Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neurooncology 2012;14:145–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez OD, Logg CR, Hiraoka K, et al. . Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther 2012;20:1689–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai CK, Wang WJ, Chen TC, et al. . Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther 2005;12:842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin AH, Burrascano C, Pettersson PL, et al. . Blockade of type I IFN production by retroviral replicating vectors and reduced tumor cell responses to IFN likely contribute to tumor selectivity. J Virol 2014;88:10066–10077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang WJ, Tai CK, Kasahara N, et al. . Highly efficient and tumor-restricted gene transfer to malignant gliomas by replication-competent retroviral vectors. Hum Gene Ther 2003;14:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inada T, Ichikawa A, Kubota T, et al. . 5-FU-induced apoptosis correlates with efficacy against human gastric and colon cancer xenografts in nude mice. Anticancer Res 1997;17:1965–1971 [PubMed] [Google Scholar]
- 7.Pritchard DM, Watson AJ, Potten CS, et al. . Inhibition by uridine but not thymidine of p53-dependent intestinal apoptosis initiated by 5-fluorouracil: evidence for the involvement of RNA perturbation. Proc Natl Acad Sci U S A 1997;94:1795–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Backus HH, Wouters D, Ferreira CG, et al. . Thymidylate synthase inhibition triggers apoptosis via caspases-8 and -9 in both wild-type and mutant p53 colon cancer cell lines. Eur J Cancer 2003;39:1310–1317 [DOI] [PubMed] [Google Scholar]
- 9.Matuo R, Sousa FG, Escargueil AE, et al. . 5-Fluorouracil and its active metabolite FdUMP cause DNA damage in human SW620 colon adenocarcinoma cell line. J Appl Toxicol 2009;29:308–316 [DOI] [PubMed] [Google Scholar]
- 10.Silverstein RA, Gonzalez de Valdivia E, and Visa N. The incorporation of 5-fluorouracil into RNA affects the ribonucleolytic activity of the exosome subunit Rrp6. Mol. Cancer Res 2011;9:332–340 [DOI] [PubMed] [Google Scholar]
- 11.Barresi V, Belluardo N, Sipione S, et al. . Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther 2003;10:396–402 [DOI] [PubMed] [Google Scholar]
- 12.Chen JK, Hu LJ, Wang D, et al. . Cytosine deaminase/5-fluorocytosine exposure induces bystander and radiosensitization effects in hypoxic glioblastoma cells in vitro. Int J Radiat Oncol Biol Phys 2007;67:1538–1547 [DOI] [PubMed] [Google Scholar]
- 13.Chang DY, Yoo SW, Hong Y, et al. . The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer 2010;127:1975–1983 [DOI] [PubMed] [Google Scholar]
- 14.Hlavaty J, Jandl G, Liszt M, et al. . Comparative evaluation of preclinical in vivo models for the assessment of replicating retroviral vectors for the treatment of glioblastoma. J Neurooncol 2011;102:59–69 [DOI] [PubMed] [Google Scholar]
- 15.Pohl U, Wick W, Weissenberger J, et al. . Characterization of Tu-2449, a glioma cell line derived from a spontaneous tumor in GFAP-v-src-transgenic mice: comparison with established murine glioma cell lines. Int J Oncol 1999;15:829–834 [DOI] [PubMed] [Google Scholar]
- 16.Wesseling P, Ruiter DJ, and Burger PC. Angiogenesis in brain tumors; Pathobiological and clinical aspects. J Neurooncol 1997;32:253–265 [DOI] [PubMed] [Google Scholar]
- 17.Linkous AG, and Yazlovitskaya EM. Angiogenesis in glioblastoma multiforme: navigating the maze. Anticancer Agents Med Chem 2011;11:712–718 [DOI] [PubMed] [Google Scholar]
- 18.Bloch O, Crane CA, Kaur R, et al. . Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res 2013;19:3165–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DePolo NJ, Harkleroad CE, Bodner M, et al. . The resistance of retroviral vectors produced from human cells to serum inactivation in vivo and in vitro is primate species dependent. J Virol 1999;73:6708–6714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shashkova EV, Doronin K, Senac JS, et al. . Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res 2008;68:5896–5904 [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, Xu Z, Tian J, et al. . Interaction of systemically delivered adenovirus vectors with Kupffer cells in mouse liver. Hum. Gene Ther 2008;19:547–554 [DOI] [PubMed] [Google Scholar]
- 22.Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol 1997;5:74–82 [DOI] [PubMed] [Google Scholar]
- 23.Dawson PJ, and Fieldsteel AH. The influence of age on chronic remittent Friend disease. Cancer Res 1971;31:974–980 [PubMed] [Google Scholar]
- 24.Yamada Y, Matsushiro H, Ogawa MS, et al. . Genetic predisposition to pre-B lymphomas in SL/Kh strain mice. Cancer Res 1994;54:403–407 [PubMed] [Google Scholar]
- 25.Tang JC, Ho FC, Chan AC, et al. . Clonality of lymphomas at multiple sites in SJL mice. Lab Invest 1998;78:205–212 [PubMed] [Google Scholar]
- 26.Bonzon C, and Fan H. Moloney murine leukemia virus-induced preleukemic thymic atrophy and enhanced thymocyte apoptosis correlate with disease pathogenicity. J Virol 1999;73:2434–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Thrasher AJ, and Gaspar HB. Current progress on gene therapy for primary immunodeficiencies. Gene Ther 2013;20:963–969 [DOI] [PubMed] [Google Scholar]
- 28.Powell JS, Ragni MV, White II GC, et al. . Phase 1 trial of FVIII gene transfer for severe hemophilia A using a retroviral construct administered by peripheral intravenous infusion. Blood 2003;102:2038–2045 [DOI] [PubMed] [Google Scholar]
- 29.Cornetta K, Moen RC, Culver K, et al. . Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum Gene Ther 1990;1:15–30 [DOI] [PubMed] [Google Scholar]
- 30.Roehl HH, Leibbrandt ME, Greengard JS, et al. . Analysis of testes and semen from rabbits treated by intravenous injection with a retroviral vector encoding the human factor VIII gene: no evidence of germ line transduction. Hum Gene Ther 2000;11:2529–2540 [DOI] [PubMed] [Google Scholar]
- 31.McCormack JE, Edwards W, Sensintaffer J, et al. . Factors affecting long-term expression of a secreted transgene product after intravenous administration of a retroviral vector. Mol Ther 2001;3:516–525 [DOI] [PubMed] [Google Scholar]
- 32.Xu L, Haskins ME, Melniczek JR, et al. . Transduction of hepatocytes after neonatal delivery of a Moloney murine leukemia virus based retroviral vector results in long-term expression of β-glucuronidase in mucopolysaccharidosis VII dogs. Mol Ther 2002;5:141–153 [DOI] [PubMed] [Google Scholar]
- 33.Huhtala T, Kaikkonen MU, Lesch HP, et al. . Biodistribution and antitumor effect of Cetuximab-targeted lentivirus. Nucl Med Biol 2014;41:77–83 [DOI] [PubMed] [Google Scholar]
- 34.Tao N, Gao GP, Parr M, et al. . Sequestration of adenoviral vector by Kupffer cells leads to a nonlinear dose response of transduction in liver. Mol Ther 2001;3:28–35 [DOI] [PubMed] [Google Scholar]
- 35.High KA. The gene therapy journey for hemophilia: are we there yet? Blood 2012;120:4482–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman AI, Zakay-Rones Z, Gomori JM, et al. . Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol Ther 2006;13:221–228 [DOI] [PubMed] [Google Scholar]
- 37.Geletneky K, Huesing J, Rommelaere J, et al. . Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 2012;12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nemunaitis J, Cunningham C, Buchanan A, et al. . Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther 2001;8:746–759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.