Abstract
BACKGROUND & AIMS
There have been conflicting results on a cell of origin in pancreatic regeneration. These discrepancies predominantly stem from lack of specific markers for the pancreatic precursors/stem cells, as well as differences in the targeted cells and severity of tissue injury in the experimental models so far proposed. We attempted to create a model that used diphtheria toxin receptor (DTR) to ablate specific cell populations, control the extent of injury, and avoid induction of the inflammatory response.
METHODS
To target specific types of pancreatic cells, we crossed R26DTR or R26dtR/lacZ mice with transgenic mice that express the Cre recombinase in the pancreas, under control of the Pdx1 (global pancreatic) or elastase (acinar-specific) promoters.
RESULTS
Exposure of PdxCre;R26DTR mice to diphtheria toxin resulted in extensive ablation of acinar and endocrine tissues but not ductal cells. Surviving cells within the ductal compartment contributed to regeneration of endocrine and acinar cells via recapitulation of the embryonic pancreatic developmental program. However, following selective ablation of acinar tissue in ElaCre-ERT2;R26DTR mice, regeneration likely occurred by reprogramming of ductal cells to acinar lineage.
CONCLUSIONS
In the pancreas of adult mice, epithelial cells within the ductal compartment contribute to regeneration of endocrine and acinar cells. The severity of injury determines the regenerative mechanisms and cell types that contribute to this process.
Keywords: Tissue Repair, Mouse Model, Organogenesis, β-Cell
Unraveling the mechanisms involved in the maintenance of the pancreatic cell mass in both physiologic and pathologic conditions has important clinical repercussions. Different models have been used to address the question of whether the pancreas possesses the ability to regenerate after injury, and several “cells of origin” have been proposed to account for this process.1,2 Variation between different studies is likely due to the disparities among the animal species used, as well as the type and extent of injury, which may affect pancreatic cells in different ways. A solution could be cell type-specific ablation, which may be a better method for analyzing the in vivo function of cells during regeneration. This type of injury can be achieved by using streptozotocin (for β-cells) or cerulein (for acinar cells). However, the massive inflammatory response, often accompanying some of the injury models, may act as a confounding factor.
Several studies have suggested that, under physiologic conditions, adult β-cells predominantly arise from already existing β-cells via replication, implying that regeneration might not involve specialized progenitors.3–5 However, during pancreatic organogenesis, progenitors within the pancreatic ductal epithelium give rise to both endocrine and acinar cells.6 Therefore, it seems reasonable to assume that the regeneration process in the adult pancreas following injury would involve ductal cells.1,2,7 In addition, a recent report has argued for the existence of a rare multipotent subpopulation of insulin-expressing cells within the adult human and mouse islets with the capacity to give rise to neuronal and pancreatic cell type lineages.8 These results strongly suggest that while maintenance of the β-cell mass during adult life may primarily involve cell replication, following insult the adult mouse pancreas is capable of robust regeneration by the recruitment of different cell types, including non-β-cells. In particular, the mechanisms involved in β-cell regeneration seem to be determined by the nature and the extent of tissue damage, which apparently dictates whether new β-cells may be generated by replication of existing β-cells,9 differentiation of progenitor cells residing within or in proximity to ducts,7,10,11 or, as recently reported, from transdifferentiation of α-cells.9,10
The regeneration of exocrine pancreas has also been reported following several injury models, including partial pancreatectomy and cerulein treatment. Lineage tracing studies indicated that the recovery of acinar mass in these models occurs not via differentiation of new acini from ductal or other progenitors, but by expansion of those acinar cells that survived injury.12–15
In an effort to determine a potential link between the extent and type of tissue damage and the nature of the regenerative response, we have studied pancreatic regeneration following ablation of different pancreatic cell lineages using the diphtheria toxin receptor (DTR)-mediated conditional targeted cell ablation model. The DTR is a membrane-anchored form of the heparin-binding epidermal growth factor–like precursor (HB-EGF precursor).16 While the human and simian HB-EGF precursors function as receptors for diphtheria toxin (DT), the HB-EGF from mice and rats does not bind the toxin and therefore remains insensitive to DT.17 Thus, transgenic expression of simian or human DDR in mice renders DT sensitive the otherwise naturally DT-resistant mouse cells.18–20 Recently, a mouse strain was generated (R26DTR) in which a loxP-flanked STOP cassette and the open reading frame of simian DTR has been introduced into the ROSA26 locus.21 In the R26DTR strain, the gene encoding DTR is under the control of the Rosa promoter, but its expression is dependent on Cre-recombinase removal of the STOP cassette21; hence, only Cre-expressing cells and their progeny will transcribe DTR. Although viable and normally functioning, the DTR-expressing cells are rapidly killed up on DT administration, allowing control over the extent of injury based on the targeted cells.
Specifically, in our PdxCre;R26DTR transgenic line, all pancreatic epithelial cells are killed via DT administration with the exception of ductal cells, which are serendipitously spared. This represents the most severe (but still survivable) pancreatic injury model of regeneration yet reported. We show that following extensive ablation of both acinar and endocrine tissue, surviving cells within the ductal compartment contribute to regeneration of new endocrine and acinar populations through a process that seems to recapitulate the embryonic pancreatic developmental program. By contrast, following selective ablation of only acinar cells (thus in a less severe type of damage) in the ElacreERT2;R26DTR transgenic line, regeneration occurs through differentiation of ductal cells to acinar lineage.
Materials and Methods
Mice and DT Treatment
Mice used in these studies were maintained according to protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee. The Rosa26DTR21 and ElaCreERT222 strains were generated in the laboratories of Drs Ari Waisman (Johannes-Gutenberg-University, Mainz, Germany) and Craig Logsdon (University of Texas MD Anderson Cancer Center), respectively, whereas the PdxCre23 mice were obtained from the Mouse Models of Human Cancer Consortium. The Rosa26lacZ mice were purchased from The Jackson Laboratory (Bar Harbor, ME).
Description of additional methods and details for reagents are provided in Supplementary Materials and Methods.
Results
Cell Ablation and Regeneration of Adult Pancreatic Endocrine and Acinar Cells in PdxCre;R26DTR Mice
Eight-week-old PdxCre;R26DTR mice were injected with DT and killed at different time points (Supplementary Figure 1A). Within 1 week after the last DT injection (early stage), the pancreas had sustained a massive loss of the epithelial cell population, quantifiable in a 97% reduction of acinar tissue and a 96% reduction of insulin- or glucagon-expressing cells. Interestingly, ductal epithelial cells were serendipitously spared (Figure 1A and B). Immunostaining for insulin, glucagon, and amylase (Figure 1C) documented the initial loss and the subsequent recovery of the endocrine and acinar cells. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL) assay analysis showed that the majority of β-cells and the nonduc-tal DBA−/E-cadherin+ epithelial cells surrounding the DBA+ structures were apoptotic 1 day after the injections (Supplementary Figure 2A). The apoptotic rate among the nonductal epithelial cells was almost 50% in the early stage, significantly declined during the following days, and by day 10 it was less than 1% (Figure 1D and Supplementary Figure 2B). The pancreas regained 60% of its original acinar mass and regenerated the endocrine compartment in 3 to 4 weeks (late stage; Figure 1E). Mice exhibited hyperglycemia during the regeneration process for up to 3 weeks (nonfasting blood glucose level >600 mg/dL), after which blood glucose levels eventually declined to approximately basal values (<200 mg/dL) in 63% of mice. Notably, all mice killed at late time points showed recovered acinar tissue, and 80% showed islets characterized by MafA and Glut2 staining, suggesting the presence of functional β-cells. By contrast, 20% of late-stage pancreata exhibited only scattered small hormone+ cell clusters with no β-cells expressing MafA or Glut2 (data not shown).
Figure 1.
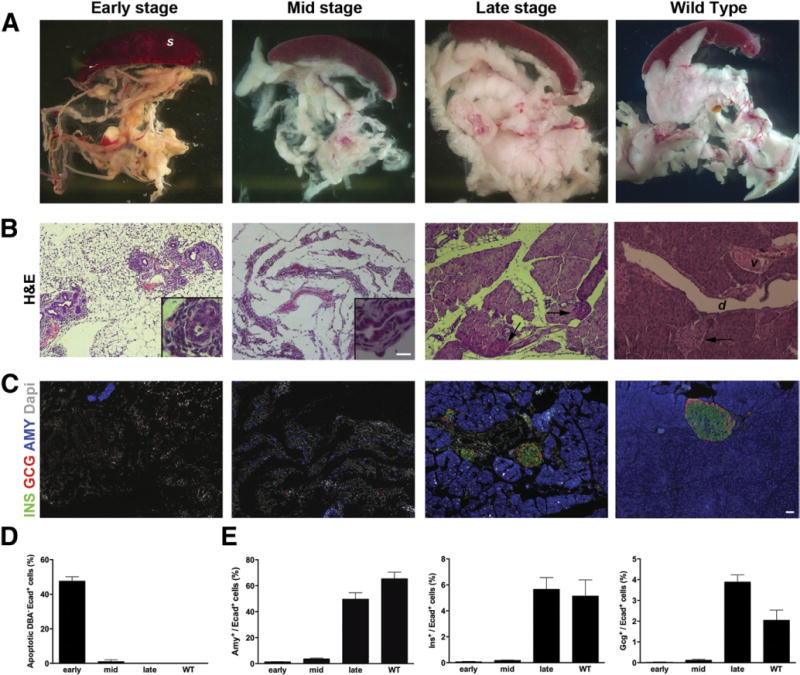
Loss and regeneration of pancreatic mass in DT-treated PdxCre;R26DTR mice. After DT treatment, mice were killed at early, mid, or late stage. (A) Macroscopic appearance shows progressive rescue of pancreatic mass during regeneration. (B) Representative H&E staining of pancreata at the same time points. Notably, upon DT treatment, all epithelial cells were killed but ducts (insets). Arrows show islets. (C) Immunostaining for amylase, insulin, and glucagon confirmed the initial loss and subsequent regeneration of both exocrine and endocrine tissues. Wild type (WT) is an age-matched adult control pancreas. (D) Quantification of apoptotic cells (percent) among the nonductal epithelial (DBA−/E+) population shows the massive cell loss after DT treatment. (E) Quantification (percent) of amylase+, insulin+, and glucagon+ cells normalized by number of E-cadherin+ cells. S, spleen; d, duct; v, vessel. Scale bars = 20 μm.
Survival of the Pancreatic Duct Cells in DT-Treated PdxCre;R26DTR Mice
Based on the widespread positivity (85%–90% of total pancreatic cells) for X-gal staining in the PdxCreR26lacZ pancreas (Supplementary Figure 2C), the apparent resistance of duct cells to DT was a surprising result. To ensure that survival of the duct cells was not due to lack of DTR expression, we stained wild-type and mid stage (post-DT treatment) PdxCre;R26DTR pancreata with antibodies specifically recognizing DTR (simian HB-EGF) (Supplementary Figure 2D–F). As shown in Supplementary Figure 2E, DTR is clearly expressed in surviving DBA+ duct-like structures, but for unknown reasons duct cells remain insensitive to DT.
Lineage Tracing Analysis in the Regenerating Pancreas ofPdxCre;R26DTR/lacZ Mice
To study more rigorously the contribution of cells within the ductal compartment to the regeneration process, 8-week-old PdxCre;R26DTR/lacZ mice were treated with DT and then killed during early, mid, or late stages after DT injection. Shortly after DT treatment, the only X-gal+ structures detectable in the pancreas were the surviving duct cells (Figure 2), whereas during mid and late stages staining revealed extensive contribution of X-gal+ cells to parenchymal restoration, including acinar and endocrine cells (Figure 2A and B). In addition, no cells coexpressing β-galactosidase (β-gal) and insulin, or β-gal and amylase, could be found during early stage (Figure 2C), while β-gal+ cells expressing insulin (Figure 2D) or amylase (Figure 2E) were detected during mid stage. Notably, our analyses also revealed that some segments within the ductal network did not express the Cre recombinase, and as a result ductal branches originating from those areas contained X-gal− cells (inset in Figure 2A).
Figure 2.
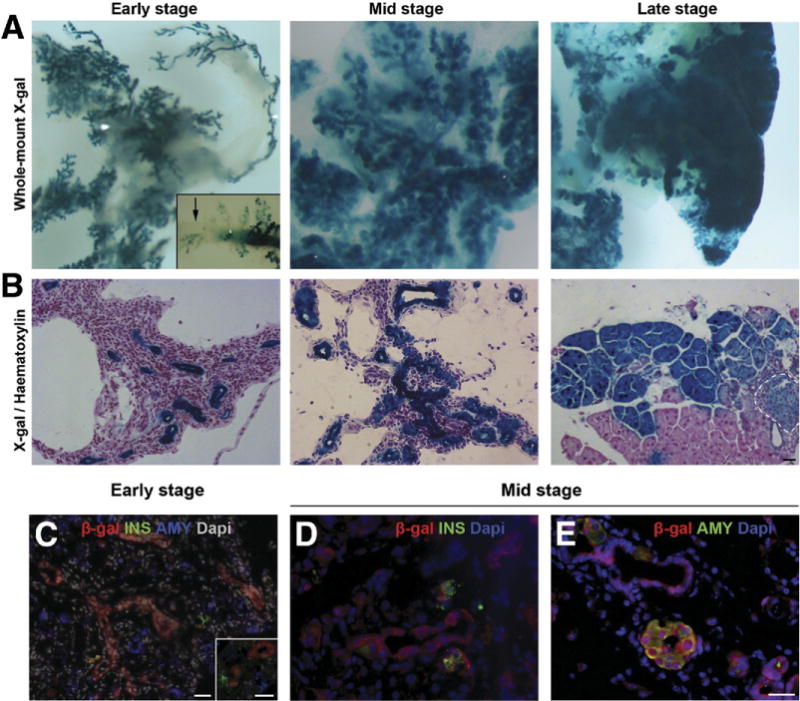
Lineage tracing analyses in the regenerating PdxCre;R26DTR/lacZ model. (A) Whole-mount X-gal staining of early-, mid-, and late-stage regenerating pancreata. Shortly after DT treatment, the only X-gal+ structures detectable were the surviving ducts, whereas mid- and late-stage staining revealed extensive contribution of X-gal+ cells to parenchymal restoration. (Inset) X-gal− segment within the ductal network (arrow). (B) X-gal stained pancreata, counterstained with hematoxylin. Dashed line highlights an islet. (C) β-gal/insulin/amylase immunostaining of PdxCre;R26DTR/lacZ pancreas early after injury showed no β-gal expression in insulin+ or amylase+ cells. Inset shows higher magnification. (D) β-gal/insulin and (E) β-gal/amylase staining of mid stage PdxCre;R26DTR/lacZ pancreata showed double+ cells. Scale bars = 20 μm.
Cells Within the Ductal Compartment Are Highly Proliferative During Regeneration
To identify the proliferating cells within each compartment that may give rise to the regenerated endocrine and acinar cells, representative tissues from different time points were stained for the proliferation marker phospho-histone-H3. Cells within the ductal compartment, as well as surviving acinar cells, were found to be the main proliferating cells during early and mid stages. In particular, the peak of proliferation in acinar tissue was reached during mid stage, whereas ductal cells kept proliferating consistently throughout late stage. Phospho-histone-H3+/endocrine+ cells were detected only from the end of mid stage onward and peaked in late stage (Figure 3A). To better analyze the fate of proliferating cells, we performed a pulse-chase approach. Bromode-oxyuridine (BrdU) was administrated via the drinking water on the final day of DT injections and on the following day; subsequently, pancreata were harvested later on day 1 (Figure 3B), day 10 (Figure 3C and D), or day 30 after last DT injection (Supplementary Figure 3). On day 1 (pulse), the duct-like structures were the main epithelial cells incorporating BrdU (Figure 3B). On day 10 (chase), the presence of insulin+/BrdU+ cells (Figure 3C) and amylase+/BrdU+ cells (Figure 3D) suggested that these cells or their precursors must have been proliferating immediately after injury. Interestingly, at this time point, abundant amylase+/BrdU+ cells were found within both the small and large duct-like structures (inset in Figure 3E), further supporting the hypothesis that the main source of regenerating acinar cells, which peak during mid stage, resides in the ducts rather than in proliferating residual Cre− acinar cells. On day 30, some β-cells, acinar cells, and cells within the larger ducts (but not the smaller ducts) still retained the BrdU labeling (Supplementary Figure 3).
Figure 3.
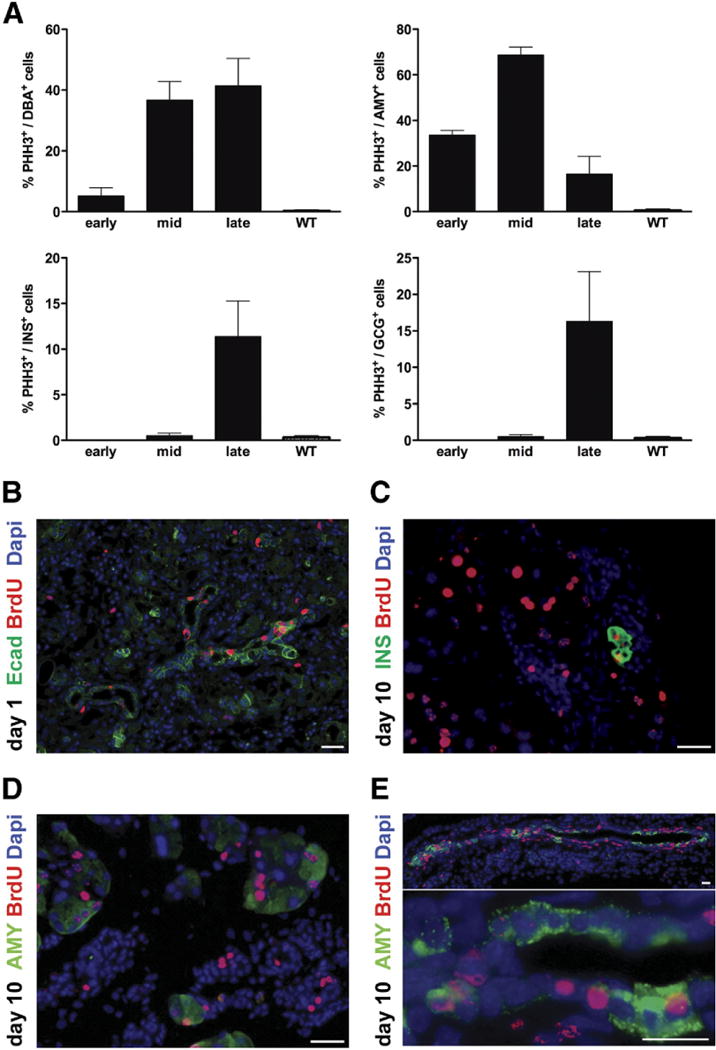
Cells within the ductal compartment are highly proliferative in regenerating PdxCre;R26DTRpancreata. (A) Quantification (percent) of proliferating DBA+, amylase+, or insulin+ cells. PHH3, phospho-histone H3. Wild type (WT) is an age-matched adult control pancreas. (B) Pulse-chase BrdU experiments. On day 1 (pulse), the duct-like structures were the main epithelial cells incorporating BrdU. On day 10 (chase), presence of (C) insulin+/BrdU+ cells and (D) amylase+/BrdU+ cells suggested that these cells or their precursors must have been proliferating mmediately after injury. (E) Several amylase+/BrdU+ cells were found within both the small and large duct-like structures, further supporting the hypothesis that the main source of regenerating acinar cells resides in the ducts rather than in proliferating residual Cre− acinar cells. Scale bars = 20 μm.
Regeneration Process Recapitulates the Pancreatic Developmental Program in PdxCre;R26DTR/lacZ Mice
To further characterize the cells within the ductal compartment during regeneration, pancreata from DT-treated PdxCre;R26DTR mice were immunostained for markers of committed endocrine progenitors and differentiated acinar, endocrine, or duct cells. Mature duct cells are generally identified as DBA+/SOX9+/PDX1−.24–26 Interestingly, after DT injection, the surviving ductal cells re-expressed PDX1 (Supplementary Figure 4A and B). Notably, once regeneration was completed, Pdx1 expression was once again restricted to the β-cells (Supplementary Figure 4C). These regenerating ductal structures retained Sox9 expression, resembling the DBA+/SOX9+/PDX1+ undifferentiated epithelial cells in the developing pancreas (Supplementary Figure 4D–F).
The presence of NGN3+ (Figure 4A), insulin+ (Figure 4B), amylase+ (Figure 4C), or glucagon+ (Figure 4D) cells within the duct-like structures further supports that in this severe injury model, cells within the ductal network contribute to formation of both endocrine and acinar cells by recapitulation of the pancreatic developmental program. Surprisingly, a subpopulation of PDX1+/glucagon+ cells was also detected in the ducts of regenerating pancreas during mid stage (Figure 4D, arrows), perhaps suggesting α- to β-cell transdifferentiation.
Figure 4.
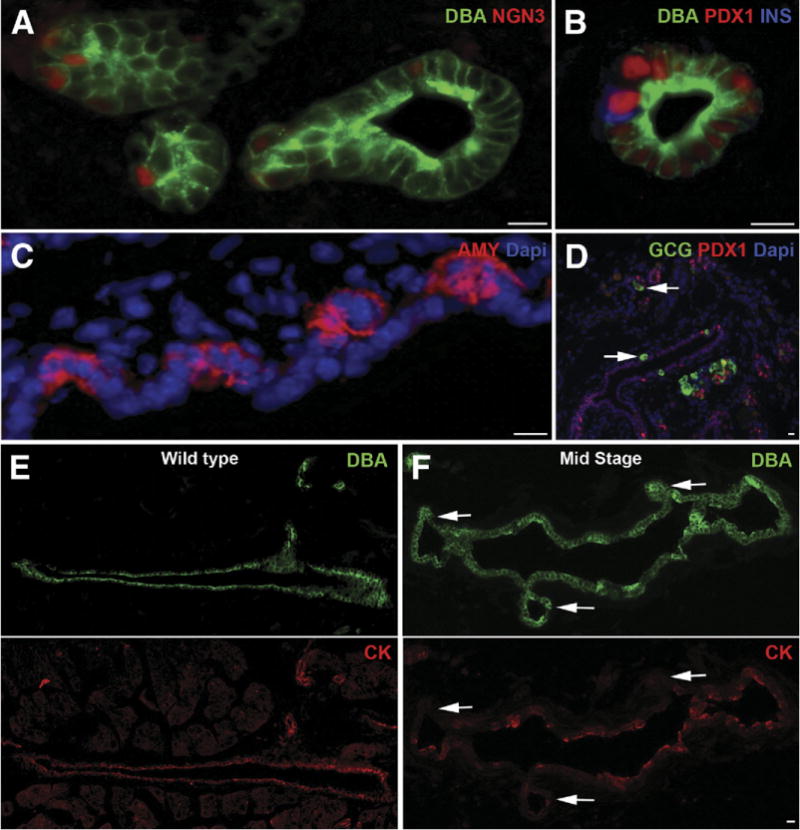
Recapitulation of pancreatic developmental program in regenerating PdxCre;R26DTR pancreata. During mid stage, ductal structures showed presence of endocrine progenitor marker (A) Ngn3+, (B) PDX1+/insulin+, (C) amylase+ (AMY)+, or (D) glucagon+ (GCG) cells. A subpopulation of PDX1+/glucagon+ cells was also detected during mid stage (arrows). (E) Duct cells in wild-type pancreas were all DBA+/cytokeratin+ (CK). (F) In the regenerating pancreas, a significant number of DBA+ cells had lost cytokeratin expression (arrows). Wild type is an age-matched adult control pancreas. Scale bars = 20 μm.
Although duct cells in the control pancreas were all DBA+/cytokeratin+ (Figure 4E), in the regenerating PdxCre;R26DTR pancreas a significant number of DBA+ cells had lost cytokeratin expression (Figure 4F, arrows). This phenomenon was more commonly found in the epithelial buds arising from bigger ducts, thus suggesting these cells were undergoing dedifferentiation and/or transdifferentiation into other cytotypes. During late stage of regeneration, all DBA+ ducts once again expressed cytokeratin (data not shown).
The hypothesis that the regeneration process mirrors the pancreatic developmental program was further supported by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) analysis of tissues from early-, mid-, and late-stage PdxCre;R26DTR pancreata (Figure 5). Throughout early and mid stages, we found expression of genes that are normally transcribed during embryonic pancreatic organogenesis, such as Notch1 and Hes1, and genes that are expressed in endocrine progenitors, such as Ngn3. Parallel to their decrease in the late stage, from mid stage onward we found up-regulation of both genes associated with exocrine acinar tissue (Mist1, Ptf1a) and mature endocrine cells (Ins2, Gcg, Gck, Beta2NeuroD, Glut2). Pdx1, which is normally expressed in both early pancreatic precursors and mature β-cells, was indeed found to be expressed already during early stage and then consistently up-regulated in mid and late stages.
Figure 5.
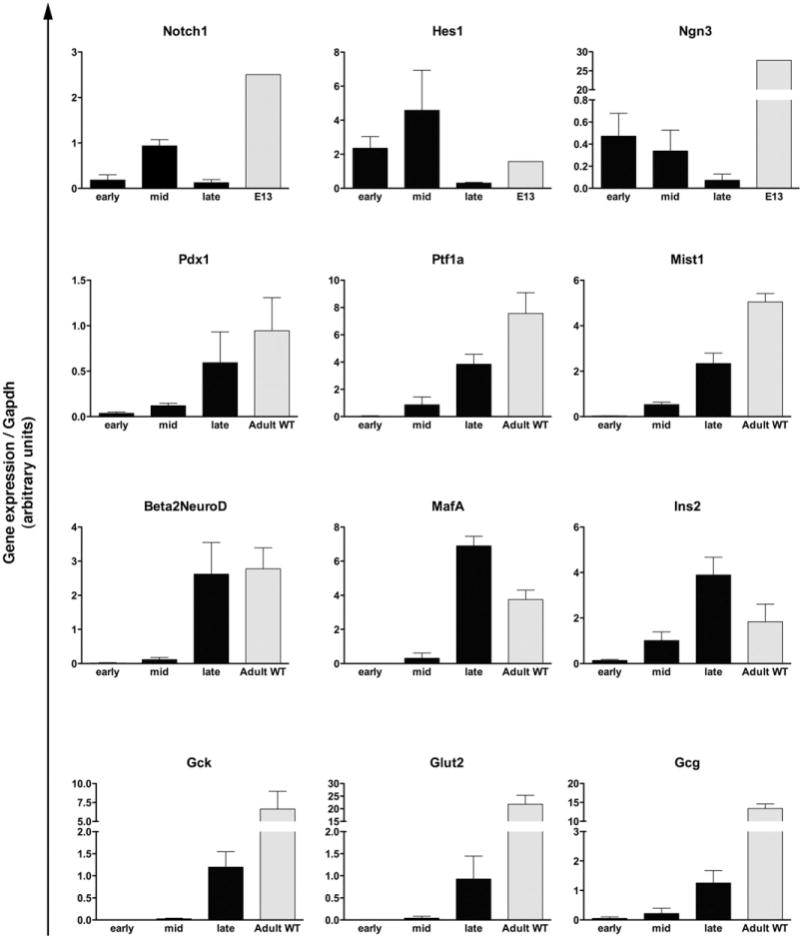
Expression (qRT-PCR) of embryonic and adult pancreatic markers in the regenerating PdxCre;R26DTR pancreata. Bars represent gene expression (mean ± SE) of mice killed at early, mid, or late stage (n = 5 for each time point). Wild type (WT) is age-matched adult control pancreata (n = 5); E13 is pooled pancreata from WT litters (n = 8 embryos).
Acinar-Specific Cell Ablation and Regeneration in the ElaCreERT2;R26DTR Mouse Model
DT treatment in PdxCre;R26DTR mice results in ablation of all pancreatic cells derived from Pdx1+ progenitor cells (cells that had expressed the Cre recombinase and thereby activated the DTR gene). To evaluate whether the mechanisms involved in regeneration are different when the extent of cell ablation is more limited, we selectively targeted acinar cells by using mice expressing a tamoxifen-sensitive Cre under the acinar-specific elastase promoter (ElaCreERT2),22 in combination with the Rosa26DTR construct. In this transgenic line (ElaCreERT2;R26DTR), expression of the DT receptor is restricted to acinar cells, and only after tamoxifen treatment. Mice were then killed 1, 3, 5, 7, and 8 days after DT injection (Supplementary Figure 1B). The peak of injury, identifiable by massive acinar destruction, was reached on day 1 after DT treatment, when sections showed the pancreas consisted mainly of islets, ducts, blood vessels, and adipose tissue (Figure 6A). Remarkably, and presumably due to the apoptotic nature of acinar cell ablation, pancreas did not exhibit significant local inflammation. By day 3, acinar cells were significantly regenerating (Figure 6B), and from days 5 (Figure 6C) to 8 (data not shown) the regeneration process was almost complete. In addition to intact islets (Supplementary Figure 5), the ElaCreERT2;R26DTR mice also displayed normal blood glucose levels throughout the regeneration process (data not shown), further confirming the sparing of endocrine cells. Interestingly, by day 3, we could detect the presence of numerous SOX9+/DBA− duct-like structures (Figure 6C), which were found to be highly proliferating (60%) (Figure 6D). The proliferation rate among these duct-like structures increased to 80% on day 5; however, due to the concomitant decrease in their total number, the main proliferative compartment on day 5 was represented by acinar cells.
Figure 6.
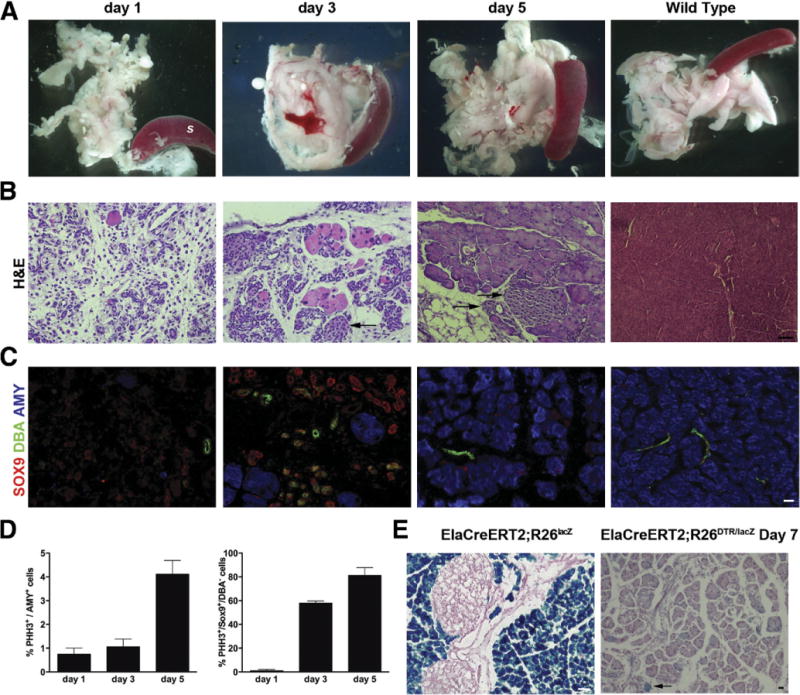
Acinar-specific cell ablation and regeneration in DT-treated ElaCreERT2;R26DTR pancreata. (A) Massive acinar cell loss was observed on day 1 after DT treatment, peaked on day 3, and was nearly complete on day 5. S, spleen. (B) Representative H&E staining of pancreata at the same time points. Arrows highlight islets. (C) Immunostainingfor DBA/SOX9/amylase in the regenerating ElaCreERT2;R26DTR pancreas showed several SOX9+/DBA− duct-like structures during peak of regeneration. By contrast, ducts in wild type are DBA+/SOX9+. (D) X-gal staining showed minimal contribution of preexisting acinar cells to acinar regeneration. (E) Quantification (percent) of proliferating amylase+ and SOX9+/DBA− cells. Arrows highlight X-gal+ acinar cells. Scale bars = 20 μm.
It has been shown that following cerulein treatment, preexisting acinar cells in the adult pancreas are the main source for regeneration of new acinar cells.12,13 To determine whether preexisting acinar cells were the origin of newly regenerated acinar cells in DT-treated ElaCreERT2;R26DTR pancreas, we used the ElaCreERT2; R26lacZ/DTR transgenic line. Following tamoxifen and then DT treatment, the pancreata were harvested on day 7 and analyzed by X-gal staining. As shown in Figure 6E, the vast majority of regenerated acinar tissue was X-gal negative.
qRT-PCR on ElaCreERT2;R26DTR pancreata harvested at different time points revealed the expression of embryonic pancreatic markers, such as Notch1 and Hes1, in the first phase of regeneration (peak at day 3) and the up-regulation of acinar-related genes, such as Mist1 and Ptf1a, in the late phase of regeneration (peak at day 8) (Figure 7A). Collectively, these data further support the hypothesis that a mechanism other than replication of preexisting acinar cells is involved in acinar regeneration.
Figure 7.
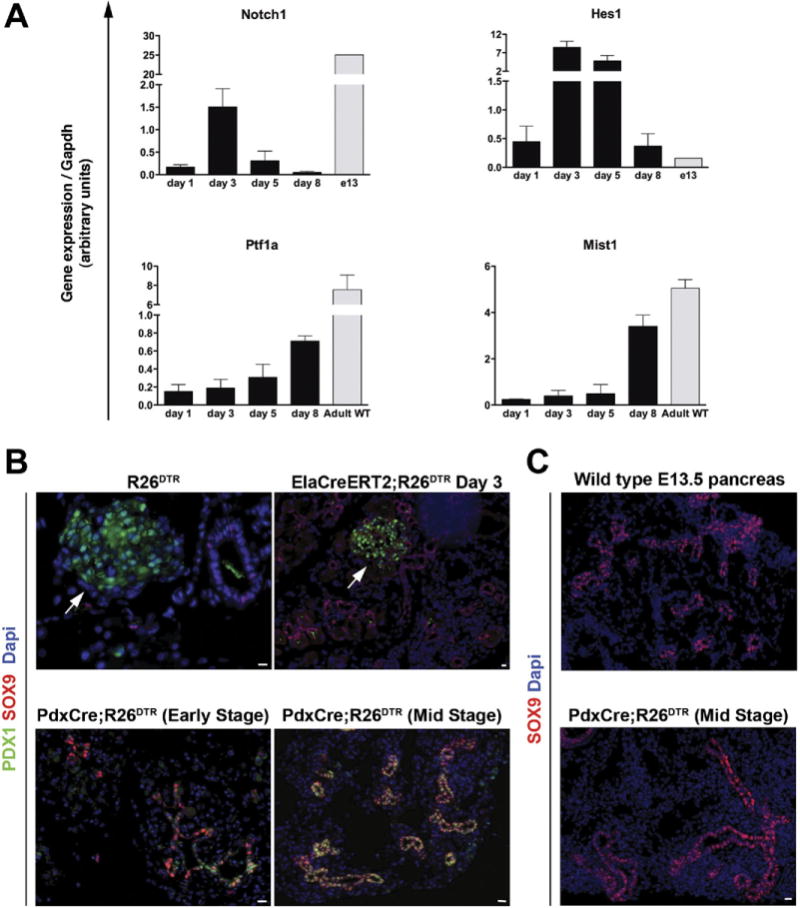
(A) Expression (qRT-PCR) of embryonic and adult exocrine markers in the regenerating ElaCreERT2;R26DTR pancreata. Bars represent gene expression (mean ± SE) of mice killed at different time points (n = 5 for each group). Wild type (WT) is age-matched adult control pancreata (n = 5); E13 is pooled pancreata from WT litters (n = 8 embryos). (B and C) The regeneration mechanism is dictated by the severity of injury. (B) Immunostaining analysis of SOX9 and PDX1 on R26DTR control pancreas, day 3 ElaCreERT2;R26DTR, and early-stage or mid-stage regenerating PdxCre;R26DTR pancreata showed coexpression of these markers only in DT-treated PdxCre;R26DTR pancreas. Arrows highlight islets. (C) SOX9 expression in the regenerating PdxCre;R26DTR pancreas displayed striking similarities to an E13.5 embryonic pancreas. Scale bars = 20 μm.
Regeneration Occurs Through Different Mechanisms in PdxCre;R26DTR and ElaCreERT2;R26DTR Models
Comparison of the regenerative processes in the PdxCre;R26DTR and ElaCreERT2;R26DTR pancreata revealed important differences between the 2 models. Perhaps the most impressive difference was the absence of PDX1 in the ductal structures of the regenerating ElaCre-ERT2;R26DTR pancreas compared with the DBA+/PDX1+/SOX9+ duct-like structures seen in the PdxCre;R26DTR pancreas (Figure 7B). Interestingly, during early stages of regeneration in the PdxCre;R26DTR pancreas, not all SOX9+ cells expressed Pdx1, whereas during mid stage almost all SOX9+ cells also expressed PDX1 (Figure 7A). In addition, the duct-like structures in the regenerating PdxCre;R26DTR pancreata exhibited morphologic resemblance to embryonic pancreatic epithelium (Figure 7C).
Discussion
A number of different animal models have been used to investigate pancreatic plasticity, including duct ligation, partial resection, and chemical ablation. However, there is no firm conclusion as to the origin of the cells destined to become acinar, ductal, or endocrine.
In an attempt to overcome the discrepancies among the different studies, we used a DTR-mediated conditional and targeted cell ablation model, which enabled us to evaluate the extent of injury (ie, the cell type that is damaged) on the regenerative response. In our PdxCre; R26DTR transgenic line, which represents the most severe pancreatic injury model of regeneration yet reported, all pancreatic cells are killed via DT administration, with the exception of ductal cells, which are serendipitously spared. Notably, in this model, cell death is quickly achieved after DT administration via massive apoptosis. Because no relevant local inflammation response is generated during this process, the numerous confounding variables incurred with other models are ruled out.
Lineage tracing studies have shown that all pancreatic epithelial cells originate from a pool of PDX1+ progenitors.27 Thus, our initial assumption was that in PdxCre; R26DTR double-transgenic mice all descendents (endocrine, acinar, and ductal) of Cre+/PDX1+ progenitors would express DTR and therefore should be killed by DT. However, the surprising persistence of numerous DBA+ ductal structures seen after DT injection indicated that cells within the ductal compartment somehow survived the insult. In our rigorous search for the cell(s) of origin, we used PdxCre;R26DTR/lacZ mice in which cells with Cre activity will become DTR+ and lacZ+ double positive. The regenerated PdxCre;R26DTR/lacZ pancreata consisted of mixed populations of X-gal+ and X-gal− cells, indicating a contribution from both preexisting differentiated cells (X-gal−) and ductal cells (X-gal+ or X-gal− as shown in Figure 2D). Our analysis showed a 30% proliferation rate among acinar cells at early stage. However, given the 97% loss of acinar tissue and the actual low number of amy-lase+ cells immediately after injury, these cells most likely did not contribute significantly to overall acinar regeneration. Taken together, these data strongly suggest that the ductal compartment is a potential source of new cells. However, it cannot distinguish whether mature duct cells or putative progenitors residing within ducts were the source of regeneration. Furthermore, it is possible that in some cells, due to low Cre-recombinase activity, only 1 of the 2 floxed genes (the STOP cassette flanking either the DTR or lacZ genes) would be excised. Because it would seem unlikely that any nonductal pancreatic epithelial cell with DTR on its surface would survive the toxin treatment, the main concern would be that some endocrine or acinar cells might express lacZ but not DTR. However, because we could not detect any differentiated X-gal+ cells immediately after injury, it is unlikely that their contribution to regeneration is significant. In a recent report, Solar et al showed that postnatal duct cells do not contribute to new acinar or endocrine cells during normal growth or after injury, based on HNF1b expression as a ductal marker.28 Nevertheless, the injury models used in that report were pancreatic duct ligation or alloxan treatment for β-cell ablation, neither of which is as severe as the injury induced in the PdxCre;RosaDTR pancreas following DT treatment.
Further evidence of ductal involvement during regeneration comes from the BrdU pulse-chase experiments. Because our analysis showed that DBA+ duct-like structures were the main epithelial cells that were proliferating after injury, the presence of several BrdU+/insulin+ or BrdU+/amylase+ cells during chase phase in the mid stage of regenerating pancreas further suggested a progenitor-progeny relationship between the proliferating DBA+ duct-like structures immediately after injury and the regenerated endocrine and acinar cells in subsequent stages. Notably, a few BrdU+ cells could be found within the larger ducts after 30 days from pulse phase. We speculate that this retention of BrdU may reflect their “stemness.” One of the main features of adult stem cells is indeed their ability to divide asymmetrically, ie, to give rise to one daughter stem cell and another daughter cell that undergoes differentiation. Thus, we believe these BrdU+ ductal cells represent the result of perhaps 1 or 2 rounds of asymmetric division, whereas the differentiating cells that underwent extensive proliferation to give rise to the regenerated pancreas eventually became BrdU−.
So far, our data indicate that in this severe ablation model the regeneration process involves proliferation and differentiation of ductal progenitors, mirroring the pancreatic developmental program. This hypothesis is further supported by the finding that, shortly after DT treatment, the surviving ductal cells re-expressed PDX1, resembling the DBA+/SOX9+/PDX1+ undifferentiated epithelial cells in the developing pancreas, and the endocrine progenitor marker Ngn3. In addition, in the epithelial buds arising from bigger ducts during mid stage, a subset of DBA+ cells had lost cytokeratin expression, implying that these cells may be differentiating and/or transdifferentiating into other cytotypes. This would also explain the presence of a subpopulation of PDX1+/glucagon+ cells not only within the ducts, but also in the forming endocrine clusters. Because α-cells typically do not express PDX1, it is tempting to speculate that these PDX1+/glucagon+ cells may represent α-cells converting to β-cells, as recently reported.29,30 Notably, during late stage of regeneration, all DBA+ ducts once again expressed cytokeratin, and PDX1 expression was restricted to β-cells. In addition, qRT-PCR analysis of tissues from early-, mid-, and late-stage PdxCre; R26DTR pancreata confirmed expression of genes that are normally transcribed during embryonic pancreatic organogenesis.
In the PdxCre;R26DTR mice, we saw a consistent level of cell ablation and subsequent regeneration for all pancreatic cell types in the early stage and mid stage pancreata, respectively. The acinar cell recovery then consistently continued through late stage to form normal-appearing acinar tissue. By contrast, only 80% of mice regenerated enough endocrine tissue by the late stage to reverse the diabetic state caused by DT treatment. In light of recent studies reporting that glucose metabolism influences beta cell replication,31 it would be interesting to determine whether in our model maintaining normoglycemia or moderate hyperglycemia affects regeneration.
In response to injury, the exocrine pancreas also activates regenerative processes to maintain tissue homeostasis. We thus investigated whether regeneration would occur through the same mechanisms following selective ablation of acinar tissue. It has been shown that in ceru-lein-induced acinar cell death, surviving acinar cells can serve as the main source for acinar regeneration.12,13 Nevertheless, in our DT-treated ElacreERT2;R26DTR pancreas, where virtually all acinar cells were killed, the acinar compartment still regenerated rapidly. In particular, regeneration coincided with the expansion of DBA−/SOX9+ cells, while proliferation of amylase+ cells was detected only in a subsequent stage. A recent lineage-tracing study has reported that, under normal conditions, SOX9+ cells within the ductal compartment contribute to the maintenance of acinar mass in the adult pancreas.32 Therefore, we speculate that in the absence of a significant number of surviving acinar cells, the regenerative mechanism taps into these SOX9+ cells, as in an accelerated version of a physiological process.
The identity of the cells of origin in our acinar regeneration model is still to be determined as they may be mature duct cells, unidentified progenitors, terminal duct cells, or centroacinar cells (CACs). In the adult pancreas, SOX9 is expressed in duct cells (which are also DBA+) and CACs. To our knowledge, it has not been established whether or not CACs are DBA+. If they do, then the DBA−/SOX9+ cells observed in DT-treated ElaCreERT2; R26DTR pancreas likely do not derive from CACs and may be either dedifferentiated duct cells that lost their ability to bind DBA or another unidentified cell type residing within the ducts. By contrast, if CACs are DBA−, it seems likely that the DBA−/SOX9+ cells derive from CACs. Furthermore, in the adult pancreas, Notch-Hes1 signaling appears particularly high in terminal duct and/or CACs, which can give rise to acinar cells following isolation and culture.33–35 Therefore, reactivation of Notch and Hes1 during acinar regeneration in ElaCreERT2;R26DTR pancreas would argue for terminal duct cells and/or CACs to be the source for this process. Although these data do not provide irrefutable proof of the cell of origin, the lineage tracing studies in ElaCreERT2;R26DTR/lacZ model strongly suggest that preexisting acinar cells are not the major contributor to acinar regeneration in this model.
In conclusion, our findings show that following extensive ablation of acinar and endocrine cells in the PdxCre; R26DTR mice, or acinar-specific ablation in the ElaCreERT2;R26DTR mice, epithelial cells within the ductal network are capable of contributing to both endocrine and acinar regeneration, although through different mechanisms and perhaps through different cell types. However, duct cells might not be the “preferred” regenerative source in physiologic conditions but could be hierarchically recruited based on the severity of injury. During embryonic pancreatic organogenesis, stem cells within the duct pancreatic epithelium give rise to both the endocrine and acinar cells. Therefore, it seems reasonable to assume that the regeneration process in our extremely severe PdxCre; R26DTR injury model would recapitulate the embryonic program. By contrast, in a less severe injury model (exclusive acinar ablation in ElaCreERT2;R26DTR pancreas), reprogramming of ductal cells to acinar lineage is sufficient. This could explain why acinar cell recovery is completed within a week in the ElaCreERT2;R26DTR model, whereas it requires from 3 to 4 weeks to complete in PdxCre; R26DTR model. Identifying signals that may initiate these different responses may provide a key understanding of how new pancreatic cells in general, and β-cells in particular, can be generated.
Supplementary Material
Acknowledgments
The authors thank Dr Ari Waisman for providing the Rosa26DTR strain and Dr Maike Sander for providing the antibody against NGN3.
Funding
Supported by the Concern Foundation (F.E.) and the Children’s Hospital of Pittsburgh.
Abbreviations used in this paper
- β-gal
β-galactosidase
- BrdU
bro-modeoxyuridine
- CAC
centroacinar cell
- DBA
Dolichos biflorus agglutinin
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- HB-EGF precursor
heparin-binding epidermal growth factor-like precursor
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction analysis
- TUNEL
terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling assay
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2011.07.003.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Puri S, Hebrok M. Cellular plasticity within the pancreas—lessons learned from development. Dev Cell. 2010;18:342–356. doi: 10.1016/j.devcel.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonner-Weir S, Li WC, Ouziel-Yahalom L, et al. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59:2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dor Y, Brown J, Martinez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 4.Teta M, Rankin MM, Long SY, et al. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008;105:19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smukler SR, Arntfield ME, Razavi R, et al. The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell. 2011;8:281–293. doi: 10.1016/j.stem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collombat P, Xu X, Ravassard P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, D’Hoker J, Stange G, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Fendrich V, Esni F, Garay MV, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–631. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morris JPt, Cano DA, Sekine S, et al. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–520. doi: 10.1172/JCI40045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai BM, Oliver-Krasinski J, De Leon DD, et al. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen JN, Cameron E, Garay MV, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Naglich JG, Metherall JE, Russell DW, et al. Expression cloning of a diphtheria toxin receptor: identity with a heparin-binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 17.Mitamura T, Higashiyama S, Taniguchi N, et al. Diphtheria toxin binds to the epidermal growth factor (EGF)-like domain of human heparin-binding EGF-like growth factor/diphtheria toxin receptor and inhibits specifically its mitogenic activity. J Biol Chem. 1995;270:1015–1019. doi: 10.1074/jbc.270.3.1015. [DOI] [PubMed] [Google Scholar]
- 18.Saito M, Iwawaki T, Taya C, et al. Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nat Biotechnol. 2001;19:746–750. doi: 10.1038/90795. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha JH, Chang MY, Richardson JA, et al. Transgenic mice expressing the diphtheria toxin receptor are sensitive to the toxin. Mol Microbiol. 2003;49:235–240. doi: 10.1046/j.1365-2958.2003.03550.x. [DOI] [PubMed] [Google Scholar]
- 21.Buch T, Heppner FL, Tertilt C, et al. A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods. 2005;2:419–426. doi: 10.1038/nmeth762. [DOI] [PubMed] [Google Scholar]
- 22.Ji B, Song J, Tsou L, et al. Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis. 2008;46:390–395. doi: 10.1002/dvg.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells JM, Esni F, Boivin GP, et al. Wnt/beta-catenin signaling is required for development of the exocrine pancreas. BMC Dev Biol. 2007;7:4. doi: 10.1186/1471-213X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kobayashi H, Spilde TL, Li Z, et al. Lectin as a marker for staining and purification of embryonic pancreatic epithelium. Biochem Biophys Res Commun. 2002;293:691–697. doi: 10.1016/S0006-291X(02)00278-4. [DOI] [PubMed] [Google Scholar]
- 25.Greenwood AL, Li S, Jones K, et al. Notch signaling reveals developmental plasticity of Pax4(+) pancreatic endocrine progenitors and shunts them to a duct fate. Mech Dev. 2007;124:97–107. doi: 10.1016/j.mod.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Seymour PA, Freude KK, Tran MN, et al. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 28.Solar M, Cardalda C, Houbracken I, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Thorel F, Nepote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464:1149–1154. doi: 10.1038/nature08894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung CH, Hao E, Piran R, et al. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells. 2010;28:1630–1638. doi: 10.1002/stem.482. [DOI] [PubMed] [Google Scholar]
- 31.Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto Y, Maitra A, Ghosh B, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 34.Kopinke D, Brailsford M, Shea JE, et al. Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development. 2011;138:431–441. doi: 10.1242/dev.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rovira M, Scott SG, Liss AS, et al. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A. 2010;107:75–80. doi: 10.1073/pnas.0912589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


