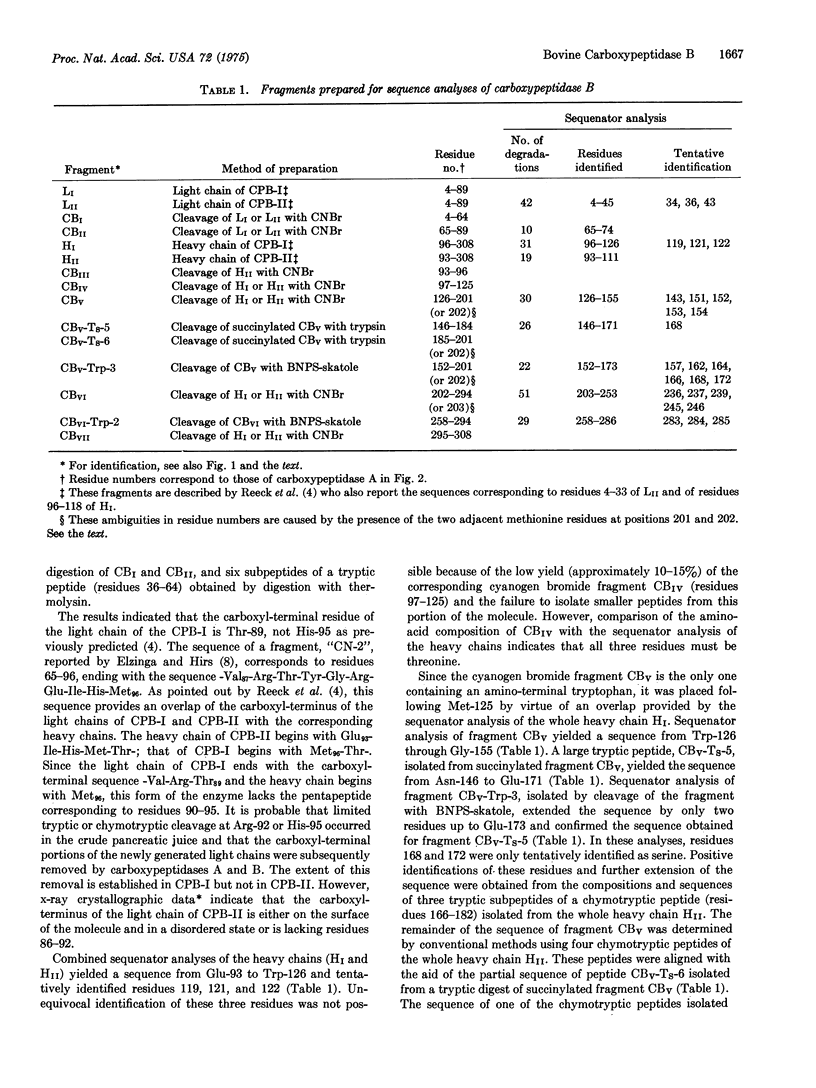
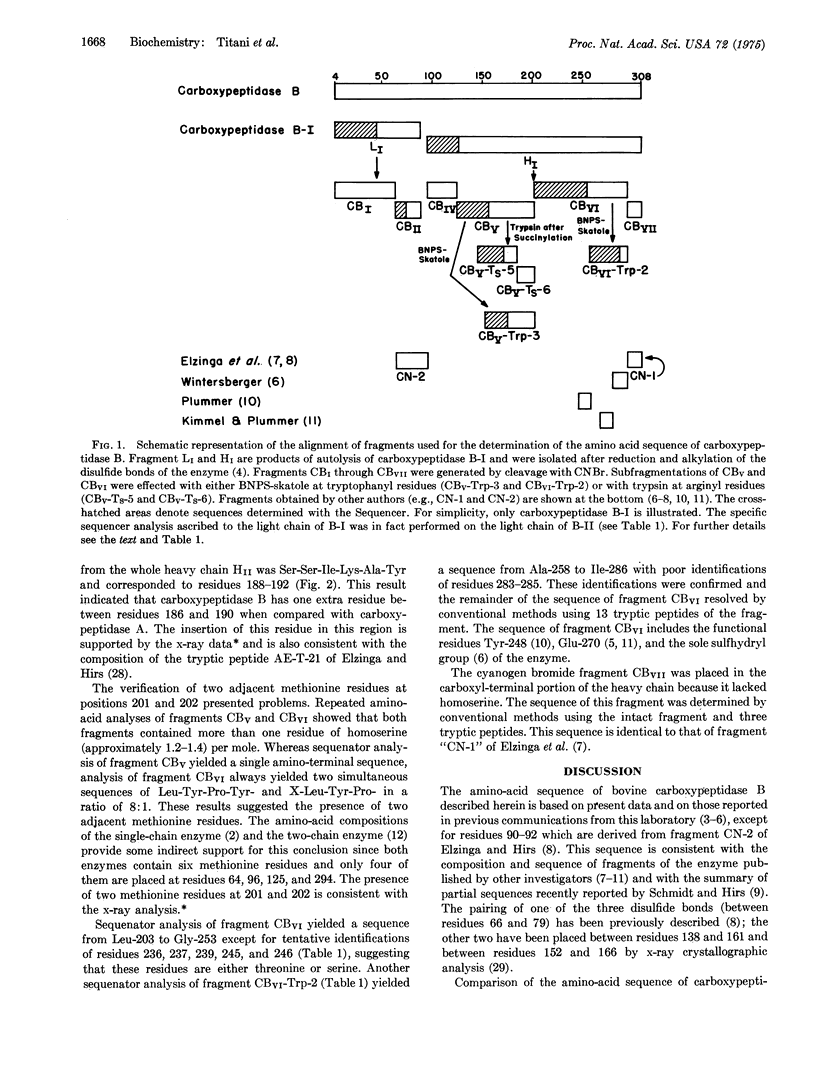
Abstract
The amino-acid sequence of bovine carboxypeptidase B [peptidyl-L-lysine(-L-arginine)hydrolase, EC 3.4.12.3] has been determined using the heavy and light chains of the enzyme isolated from spontaneously activated pancreatic juice. Comparison of the sequence with that of carboxypeptidase A shows that the two enzymes are homologous (49% identity) and that all but one of the functional residues identified in carboxypeptidase A occur in corresponding loci in carboxypeptidase B (peptidyl-L-amino acid hydrolase, EC 3.4.12.2). The exception is the replacement of Ile-255 at the bottom of the substrate binding pocket of carboxypeptidase A, by aspartic acid in carboxypeptidase B. This single change can account for the difference in specificity of the two enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradshaw R. A., Ericsson L. H., Walsh K. A., Neurath H. The amino acid sequence of bovine carboxypeptidase A. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1389–1394. doi: 10.1073/pnas.63.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw R. A., Neurath H., Walsh K. A. Considerations of the concept of structural homology as applied to bovine carboxypeptidases A and B. Proc Natl Acad Sci U S A. 1969 Jun;63(2):406–411. doi: 10.1073/pnas.63.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H., Lai C. Y. Primary structure of bovine carboxypeptidase B. 3. The carboxyl-terminal sequence. Arch Biochem Biophys. 1968 Feb;123(2):353–360. doi: 10.1016/0003-9861(68)90145-8. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H. Primary structure of bovine carboxypeptidase B. II. Tryptic peptides from the reduced, aminoethylated protein. Arch Biochem Biophys. 1968 Feb;123(2):343–352. doi: 10.1016/0003-9861(68)90144-6. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Hirs C. H. Primary structure of bovine carboxypeptidase B. IV. Amino acid sequence of a disulfide-containing loop. Arch Biochem Biophys. 1968 Feb;123(2):361–367. doi: 10.1016/0003-9861(68)90146-x. [DOI] [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- GROSS E., WITKOP B. Nonenzymatic cleavage of peptide bonds: the methionine residues in bovine pancreatic ribonuclease. J Biol Chem. 1962 Jun;237:1856–1860. [PubMed] [Google Scholar]
- Hartley B. S. Homologies in serine proteinases. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):77–87. doi: 10.1098/rstb.1970.0010. [DOI] [PubMed] [Google Scholar]
- Hass G. M., Govier M. A., Grahn D. T., Neurath H. Modification of bovine carboxypeptidase B with N-bromoacetyl-N-methyl-L-phenylalanine. Biochemistry. 1972 Sep 26;11(20):3787–3792. doi: 10.1021/bi00770a018. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- Kimmel M. T., Plummer T. H., Jr Identification of a glutamic acid at the active center of bovine carboxypeptidase B. J Biol Chem. 1972 Dec 25;247(24):7864–7869. [PubMed] [Google Scholar]
- Kycia J. H., Elzinga M., Alonzo N., Hirs C. H. Primary structure of bovine carboxypeptidase B. I. Preparation of enzyme from pancreatic juice. Arch Biochem Biophys. 1968 Feb;123(2):336–342. doi: 10.1016/0003-9861(68)90143-4. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Omenn G. S., Fontana A., Anfinsen C. B. Modification of the single tryptophan residue of staphylococcal nuclease by a new mild oxidizing agent. J Biol Chem. 1970 Apr 25;245(8):1895–1902. [PubMed] [Google Scholar]
- Petra P. H., Hermodson M. A., Walsh K. A., Neurath H. Characterization of bovine carboxypeptidase A (Allan). Biochemistry. 1971 Oct 26;10(22):4023–4025. doi: 10.1021/bi00798a600. [DOI] [PubMed] [Google Scholar]
- Plummer T. H., Jr Isolation and sequence of peptides at the active center of bovine carboxypeptidase B. J Biol Chem. 1969 Oct 10;244(19):5246–5253. [PubMed] [Google Scholar]
- RAFTERY M. A., COLE R. D. Tryptic cleavage at cysteinyl peptide bonds. Biochem Biophys Res Commun. 1963 Mar 25;10:467–472. doi: 10.1016/0006-291x(63)90381-4. [DOI] [PubMed] [Google Scholar]
- Reeck G. R., Walsh K. A., Hermodson M. A., Neurath H. New forms of bovine carboxypeptidase B and their homologous relationships to carboxypeptidase A. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1226–1230. doi: 10.1073/pnas.68.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeck G. R., Walsh K. A., Neurath H. Isolation and characterization of carboxypeptidases A and B from activated pancreatic juice. Biochemistry. 1971 Dec 7;10(25):4690–4698. doi: 10.1021/bi00801a015. [DOI] [PubMed] [Google Scholar]
- Schmid M. F., Herriott J. R. The structure of bovine carboxypeptidase B: results at 5-5 Angstrom resolution. J Mol Biol. 1974 Mar 25;84(1):97–101. doi: 10.1016/0022-2836(74)90214-9. [DOI] [PubMed] [Google Scholar]
- Schmidt J. J., Hirs C. H. Primary structure of bovine carboxypeptidase B. Inferences from the locations of the half-cystines and identification of the active site arginine. J Biol Chem. 1974 Jun 25;249(12):3756–3764. [PubMed] [Google Scholar]
- Spilburg C. A., Bethune J. L., Vallee B. L. The physical state dependence of carboxypeptidase Aalpha and Agamma kinetics. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3922–3926. doi: 10.1073/pnas.71.10.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH K. A., NEURATH H. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:884–889. doi: 10.1073/pnas.52.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERSBERGER E., COX D. J., NEURATH H. Bovine pancreatic procarboxypeptidase B. I. Isolation, properties, and activation. Biochemistry. 1962 Nov;1:1069–1078. doi: 10.1021/bi00912a017. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wikler M., Titani K., Putnam F. W. The amino acid sequence of a lambda type Bence-Jones protein. II. Chymotryptic peptides. J Biol Chem. 1970 Apr 25;245(8):2158–2170. [PubMed] [Google Scholar]
- Wintersberger E. Isolation and structure of an active-center peptide of bovine carboxypeptidase B containing the zinc-binding sulfhydryl group. Biochemistry. 1965 Aug;4(8):1533–1536. doi: 10.1021/bi00884a011. [DOI] [PubMed] [Google Scholar]
- Wintersberger E., Neurath H., Coombs T. L., Wallee B. L. A zinc-binding thiol group in the active center of bovine carboxypeptidase B. Biochemistry. 1965 Aug;4(8):1526–1532. doi: 10.1021/bi00884a010. [DOI] [PubMed] [Google Scholar]
- YAOI Y., TITANI K., NARITA K. N- AND C-TERMINAL RESIDUES IN BAKER'S YEAST CYTOCHROME C. J Biochem. 1964 Sep;56:222–229. doi: 10.1093/oxfordjournals.jbchem.a127984. [DOI] [PubMed] [Google Scholar]


