Abstract
Background
Inotropes are widely used in hospitalized systolic heart failure (HF) patients, especially those with low systolic blood pressure (SBP) or cardiac index. Also inotropes are considered harmful in nonischemic HF.
Methods and Results
We examined the association of in-hospital inotrope use with (1) major events (death, ventricular assist device, or heart transplant) and (2) study days alive and out of hospital during the first 6 months in the ESCAPE trial, which excluded patients with immediate need for inotropic therapy. Predefined subgroups of interest were baseline SBP <100 vs. ≥100 mmHg, cardiac index <1.8 vs. ≥1.8 L/min/m2, and ischemic vs. nonischemic HF etiology. Inotropes were frequently used in both the <100-mmHg (88/165 [53.3%]) and the ≥100-mmHg (106/262 [40.5%]) SBP subgroups and were associated with higher risk for major events in both subgroups (adjusted HR 2.85, 95%CI 1.59–5.12, P<0.001 and HR 1.86, 95%CI 1.02–3.37; P=0.042, respectively). Risk with inotropes was more pronounced among those with cardiac index ≥1.8 L/min/m2 (N=114, HR 4.65, 95%CI 1.98–10.9, P<0.001) vs. <1.8 L/min/m2 (N=82, HR 1.48, 95%CI 0.61–3.58, P=0.39). Event rates were higher with inotropes both in ischemic (N=215, HR 2.64, 95%CI 1.49–4.68, P=0.001) and nonischemic patients (N=216, HR 2.19, 95%CI 1.18–4.07, P=0.012). Across all subgroups, patients who received inotropes spent fewer study days alive and out of hospital.
Conclusion
In the absence of cardiogenic shock or end-organ hypoperfusion, inotrope use during hospitalization for HF is associated with unfavorable 6-month outcomes, regardless of admission SBP, cardiac index, or HF etiology.
Keywords: inotropic agents, heart failure, mortality
Patients hospitalized for heart failure (HF) experience substantial short-term morbidity and mortality.1–3 Although intravenous inotropes are frequently used in the management of patients hospitalized with HF due to systolic dysfunction, data from controlled trials have failed to demonstrate benefit with these agents.4–6 On the contrary, inotropes have been associated with increased risk for adverse outcomes.4–7 Despite these data, however, a substantial proportion of patients hospitalized for HF continue to receive inotropes.8 This discrepant trend is partially rooted in the limited options for management of advanced systolic HF.9 However, it is also rooted in the belief that inotropes may be beneficial when systolic blood pressure (SBP) is relatively low even without clinical hypoperfusion,10 or that benefits may outweigh risks in those patients who are in a low cardiac output state, since previous trials were conducted among hospitalized patients without the specific knowledge of central hemodynamics.5 There are no data however to support these notions. Also, a post-hoc analysis from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) suggested that the adverse impact of milrinone on outcomes occurred primarily in HF of ischemic etiology,7 lessening the concerns about inotrope use in patients with nonischemic HF.
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial evaluated the benefit of pulmonary artery catheterization (PAC) guided HF management.11 In this trial, inotrope use was left to clinical judgment; however, the study enrollment criteria excluded patients with cardiogenic shock or those deemed to need immediate inotropic therapy on admission. Previous findings from the ESCAPE showed that inhospital inotrope use is associated with worse outcomes.6 In the current study, we utilized the ESCAPE database to evaluate the effect of in-hospital inotrope use on outcomes in HF patients across the spectrum of admission SBP and, among those who received PAC, baseline cardiac index. We additionally evaluated for modification effect of ischemic vs. nonischemic etiology.
METHODS
Study Population
Details of the ESCAPE trial have been published previously.11, 12 Briefly, this randomized trial conducted at 26 centers in the United States and Canada was designed to test the long-term safety and efficacy of treatment guided by hemodynamic monitoring with clinical assessment versus clinical assessment alone in patients hospitalized with reduced left ventricular ejection fraction (LVEF). Among the 433 participants, 427 (98.6%) had a supine SBP value recorded on admission and constituted the study population for this analysis. The ESCAPE eligibility criteria included LVEF ≤30%, ≥3 months of therapy with angiotensin-converting enzyme inhibitors and diuretics, supine SBP ≤125 mmHg at randomization; and at least 1 sign and 1 symptom of congestion. The patients either had to be hospitalized or have an emergency department visit for HF within the past year, or receive furosemide >160 mg/day (or equivalent) within 1 month of admission. Initiation of dobutamine, dopamine >3 μg/kg/min, or milrinone prior to randomization, or baseline serum creatinine >3.5 mg/dL were exclusion criteria. Etiology of HF was classified into ischemic vs. nonischemic based on clinical history.
Cardiac Index
Among the 215 patients assigned to PAC-guided therapy, 196 eventually received PAC; in addition, 21 patients in the clinical arm eventually received PAC (i.e. crossed over), for a total of 217 PAC-guided treatment patients. Baseline cardiac index data were available in 196 patients and were included in the cardiac index subset analysis. Hemodynamic measurements, including cardiac index, were assessed twice at baseline and at least twice a day thereafter. For analysis purposes, the first available reading from admission day was taken.
Outcomes
The primary outcome of interest in our study was the time to a major clinical event (death, left ventricular assist device [LVAD] placement, or cardiac transplantation) by day 180 from randomization. The secondary outcome was the number of study days alive and out of hospital by day 180.
Statistical Analysis
Descriptive analyses are presented as mean (standard deviation) for continuous and number (percentage) for categorical variables. Differences between groups were assessed using the nonparametric rank sum test (Mann-Whitney) for continuous variable, Fisher’s exact test for categorical variables, and log-rank Χ2 for time-to event outcomes. The association of inotrope use with primary endpoint was examined with Cox proportional hazard models. Following the analysis scheme of the main ESCAPE study, we initially calculated this endpoint with patients receiving transplant or assist devices coded as dead; then we coded these events as alive in a sensitivity analysis. The association between admission SBP, inotropes use, and outcomes was assessed by categorizing SBP into <100 vs. ≥100 mmHg based on previous literature9, 10 and also by modeling SBP as a continuous variable. We followed a similar approach for cardiac index, using 1.8 L/min/m2 for categorical analysis. In multivariable models, we controlled for age, gender, ischemic vs. nonischemic etiology (except in etiology-specific analyses), body mass index, LVEF, SBP (except in SBP-specific analyses), blood urea nitrogen, sodium, hemoglobin at presentation, and PAC-guided treatment (except in the cardiac index analysis because of collinearity). We did not include serum creatinine in the models because of collinearity with and weaker association than blood urea nitrogen with outcomes. Proportionality of hazards was examined using the Schoenfeld residuals and confirmed by fitting interaction terms with time. For the secondary endpoint (days spent out of hospital; skewed U-shaped distribution), we log-transformed the study days spent in-hospital or dead (lognormal distribution) and treated as a continuous outcome in regression models. Because low-dose dopamine is widely considered to possess renoprotective rather than inotropic properties, we performed a sensitivity analysis in which only >3mcg/kg/min dopamine was considered as an inotrope (using the threshold for inotropic dose set in the ESCAPE design).12 To provide additional insights, we have examined the association between inotropes and outcomes in relation to SBP and CI values obtained on Day 3 (for SBP) and Day 2 (for CI) after randomization. In these analyses we have additionally adjusted for baseline values of SBP and CI, respectively. In secondary analyses, we have assessed the directional association between average in-hospital dose of each inotrope and change (final measurement before removal of PAC minus baseline) in (1) cardiac index; (2) pulmonary capillary wedge pressure; and (3) right atrial pressure. In addition, we compared these changes across groups according to the number of inotropic agents used. A two-sided p<0.05 was accepted as statistically significant. Analyses were performed with STATA 12.1 (StataCorp LP, College Station, TX).
RESULTS
Baseline Characteristics
Mean age of patients was 56±14 years; 74.2% were men; 59.5% were white and 27.6% were black; etiology was ischemic in 49.6%; and mean LVEF was 19.4±6.6%. Mean supine SBP on admission was 106±16 mmHg; SBP was <100 mmHg in 165 patients (38.6%). Overall, 194 patients (45.4%) received in-hospital inotropes including dobutamine in 127 (29.7%), milrinone in 72 (16.9%), and dopamine in 50 (11.7%). The median in-hospital dose was 4.0 μg/kg/min for dobutamine (range: 1.0–15.0), 0.375 μg/kg/min for milrinone (range: 0.100–0.750), and 3.0 μg/kg/min for dopamine (range: 1.5–25.0). One patient received amrinone (0.3 μg/kg/min). Thirty-six patients (8.4%) received 2 inotropic agents (dobutamine and dopamine at median doses 5.0 and 3.1 μg/kg/min respectively, 17 patients; dobutamine and milrinone at median doses 5.0 and 0.375 μg/kg/min respectively, 13 patients; and milrinone and dopamine at median doses 0.300 and 5.0 μg/kg/min respectively, 6 patients) and 10 patients (2.3%) received 3 inotropic agents (dobutamine, milrinone, and dopamine at median doses 5.0, 0.375, and 3.0 μg/kg/min respectively). Table 1 presents the baseline characteristics by admission SBP category. Patients with admission SBP <100 mmHg were more likely to be white, have atrial fibrillation, and be actively evaluated for heart transplantation; had lower body mass index, sodium, and LVEF; and were less likely to have a history of hypertension.
Table 1.
Baseline Patient Characteristics by Systolic Blood Pressure on Admission
| Characteristic | Total (N=427) | SBP <100 mmHg (N=165) | SBP ≥100 mmHg (N=262) | P value* |
|---|---|---|---|---|
| Age, years | 56.1 ± 13.9 | 55.7 ± 14.2 | 56.4 ± 13.7 | 0.66 |
| Men, n (%) | 317 (74.2) | 129 (78.2) | 188 (71.8) | 0.17 |
| Race | 0.004 | |||
| Caucasian, n (%) | 254 (59.5) | 116 (70.3) | 138 (52.7) | |
| African American, n (%) | 118 (27.6) | 34 (20.6) | 84 (32.1) | |
| Hispanic, n (%) | 45 (10.5) | 12 (7.3) | 33 (12.6) | |
| Other, n (%) | 10 (2.3) | 3 (1.8) | 7 (2.7) | |
| Body mass index, kg/m2 | 28 (24, 33) | 27 (23, 32) | 29 (24, 34) | 0.006 |
| Ischemic etiology, n (%) | 212 (49.6) | 90 (54.6) | 122 (46.6) | 0.14 |
| PAC-guided treatment, n (%) | 215 (50.4) | 88 (53.3) | 127 (48.5) | 0.37 |
| In-hospital inotropes, n (%) † | 194 (45.4) | 88 (53.3) | 106 (40.5) | 0.010 |
| Dobutamine, n (%) | 115 (26.9) | 56 (33.9) | 59 (22.5) | 0.018 |
| Milrinone, n (%) | 71 (16.6) | 28 (17.5) | 43 (17.1) | 0.99 |
| Dopamine, n (%) | 42 (9.8) | 24 (14.6) | 18 (6.9) | 0.019 |
| Heart transplant status | 0.001 | |||
| Ineligible, n (%) | 103 (24.1) | 39 (25.0) | 64 (24.9) | |
| Active evaluation, n (%) | 134 (31.4) | 66 (42.3) | 68 (26.5) | |
| No evaluation planned, n (%) | 176 (41.2) | 51 (32.7) | 125 (48.6) | |
| Left ventricular ejection fraction, % | 19.4 ± 6.6 | 18.3 ± 6.8 | 20.0 ± 6.4 | 0.008 |
| Atrial fibrillation, n (%) | 127 (29.7) | 59 (35.8) | 68 (26.0) | 0.039 |
| Smoking | 0.29 | |||
| Never | 117 (27.7) | 49 (29.7) | 68 (26.0) | |
| Current | 51 (12.1) | 17 (10.3) | 34 (13.0) | |
| Quit <6 months | 44 (10.4) | 12 (7.3) | 32 (12.2) | |
| Quit >6 months | 210 (49.8) | 85 (51.5) | 125 (47.7) | |
| Diabetes mellitus, n (%) | 139 (32.6) | 54 (32.7) | 85 (32.4) | 0.92 |
| Hypertension, n (%) | 201 (47.1) | 59 (35.8) | 142 (54.2) | <0.001 |
| Peripheral vascular disease, n (%) | 54 (12.6) | 20 (12.1) | 34 (13.0) | 0.88 |
| Cerebrovascular disease, n (%) | 53 (12.4) | 20 (12.1) | 33 (12.6) | 0.99 |
| Chronic pulmonary disease, n (%) | 72 (16.9) | 22 (13.3) | 50 (19.1) | 0.14 |
| Serum sodium, mEq/L | 137 ± 4 | 135 ± 5 | 137 ± 4 | <0.001 |
| Serum creatinine, mg/dL | 1.5 ± 0.6 | 1.6 ± 0.6 | 1.5 ± 0.6 | 0.29 |
| Serum blood urea nitrogen, mg/dL | 29 (19, 43) | 30 (21, 47) | 27 (19, 41) | 0.020 |
| Baseline ACEI or ARB, n (%) | 386 (90.4) | 147 (89.1) | 239 (91.2) | 0.50 |
| Baseline beta-blocker, n (%) | 264 (61.8) | 107 (64.8) | 157 (59.9) | 0.36 |
| ICD, n (%) | 115 (26.9) | 52 (31.5) | 63 (24.0) | 0.094 |
| Pacemaker, n (%) | 102 (23.9) | 41 (24.8) | 61 (23.3) | 0.73 |
Mann-Whitney test for continuous variables and Fisher’s exact test for categorical variables.
Some patients received >1 inotrope; 1 patient (<100 mmHg) received amrinone.
Continuous variables are expressed as mean ± standard deviation or median (25th, 75th percentile).
ACEI: angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker; ICD: Implantable cardioverter defibrillator; PAC: pulmonary artery catheter; SBP: systolic blood pressure.
Outcomes
There were 117 major events (27.1%) at 180 days (81 deaths, 9 LVAD implantations, and 27 cardiac transplantations). Inotrope use was associated with higher risk for major events (38.1% vs. 18.5%; log-rank Χ2=23.8; P<0.001). The corresponding unadjusted and adjusted hazard ratio (HR) in the entire cohort was 2.47 (95% confidence interval [CI] 1.70, 3.60; P<0.001) and 2.32 (95% CI 1.54, 3.50; P<0.001), respectively. The risk was similar when patients receiving LVAD implantation or cardiac transplantation were censored as alive (unadjusted HR 2.27; 95% CI 1.46, 3.54; P<0.001; adjusted HR 2.09; 95% CI 1.28, 3.42; P=0.003).
Patients spent a median of 159 (IQR 88, 172) study days alive and out of hospital; these days were fewer among patients who received in-hospital inotropes compared with those who did not (144 [35, 167]) vs. 165 [139, 174]; P<0.001). In adjusted models, patients who received inotropes spent 79% more study days in the hospital or dead (95% CI, 41%, 128%).
Systolic Blood Pressure, Inotrope Use, and Outcomes
Inotropes were frequently used in both SBP subgroups (SBP <100 mmHg: 53.3% vs. SBP ≥100 mmHg: 40.5%; P=0.010). Patients who received in-hospital inotropes experienced more clinical events by 180 days compared with those who did not, both in the <100-mmHg (47/88 [53.4%] vs. 18/77 [23.4%]; P<0.001) and the ≥100-mmHg (27/106 [25.5%] vs. 25/156 [16.0%]; P=0.043) subgroups. In adjusted models, inotrope use was associated with increased risk for major clinical events in both SBP subgroups, Table 2. The risk did not substantially change when only death was considered (albeit risk became non-significant in the ≥100-mmHg subgroup) or when 14 patients who received ≤3mcg/kg/min dopamine were classified as not having received inotropic therapy (Table 2). When SBP was examined as a continuous variable, the interaction between SBP and inotrope use was not statistically significant in adjusted models (P=0.23, P=0.32, and P=0.44 for major events, mortality, and with low-dose dopamine not considered as inotrope use, respectively).
Table 2.
In-hospital Inotrope Use and Outcomes According to Systolic Blood Pressure on Admission
| Outcome | SBP <100 mmHg (N=165) | SBP ≥100 mmHg (N=262) | |||
|---|---|---|---|---|---|
|
| |||||
| Major clinical events | HR (95% CI) | P | HR (95% CI) | P | P interaction |
| Death, LVAD implantation, or heart transplantation | 2.85 (1.59–5.12) | <0.001 | 1.86 (1.02–3.37) | 0.042 | 0.32 |
| Death -- LVAD implantation, or heart transplantation censored as alive | 2.87 (1.37–6.04) | 0.005 | 1.64 (0.82–3.27) | 0.16 | 0.28 |
| Death, LVAD implantation, or heart transplantation -- dopamine ≤3mcg/kg/min not considered as inotrope | 2.26 (1.31–3.91) | 0.004 | 1.87 (1.04–3.39) | 0.038 | 0.65 |
|
| |||||
| Study days dead or in hospital | Ratio (95% CI) | P | Ratio (95% CI) | P | P interaction |
|
| |||||
| Any inotrope | 2.04 (1.39–2.99) | <0.001 | 1.69 (1.24–2.32) | 0.001 | 0.46 |
| Dopamine ≤3mcg/kg/min not considered as inotrope | 1.82 (1.24–2.67) | 0.002 | 1.73 (1.26–2.39) | 0.001 | 0.85 |
All ratios refer to effect of in-hospital inotrope use compared with no inotrope use as reference.
All models are adjusted for age, sex, body mass index, left ventricular ejection fraction, etiology of heart failure (ischemic vs. nonischemic), blood urea nitrogen, sodium, hemoglobin, and pulmonary artery catheter-guided treatment.
CI: confidence interval; HR: hazard ratio; LVAD: left ventricular assist device.
Patients who received inotropes spent fewer days (out of 180) alive and out of hospital compared with those who did not, regardless of SBP (SBP <100 mmHg: median [interquartile range] 84 [14, 157] vs. 163 [116, 173, P<0.001; SBP ≥100 mmHg: 158 [86, 172] vs. 167 [144, 175], P=0.002). This difference persisted in adjusted models for both SBP subgroups and did not change when ≤3mcg/kg/min dopamine was not considered as inotrope use (Table 2).
In analyses based on SBP values obtained on Day 3, inotrope use was still associated with increased risk and more in-hospital days in both SBP subgroups. In models adjusting for baseline SBP in addition to other covariates, the direction and magnitude of association was similar to that observed when baseline SBP values were used to classify patients (Supplemental Table 1).
Cardiac Index
Pulmonary artery catheter was placed in 215 (50.4%) patients. Cardiac index was available in 196 patients (median 1.9 L/min/m2; interquartile range 1.6, 2.3). Among these patients, 97/196 (49.5%) received inotropes. Table 3 presents the baseline hemodynamic parameters according to inotrope use. Patients who received in-hospital inotropes had higher right atrial pressure and lower arterial pressure at baseline. Risk with inotropes in the cohort with available cardiac index data was similar to that of the entire cohort in unadjusted (HR 2.82; 95% CI 1.64, 4.85; P<0.001) and adjusted (HR 2.82; 95% CI 1.53, 5.47; P=0.001) models.
Table 3.
Hemodynamic Characteristics According to Inotrope Use Among Patients With Available Pulmonary Artery Catheter Data
| Characteristic | Total (N=196) | Inotrope Use (N=97) | No Inotrope Use (N=99) | P value* |
|---|---|---|---|---|
| Right atrial pressure, mmHg | 12 (7, 18) | 14 (7, 20) | 11 (8, 15) | 0.033 |
| Pulmonary artery systolic pressure, mmHg | 55 ± 14 | 57 ± 15 | 54 ± 15 | 0.10 |
| Pulmonary artery diastolic pressure, mmHg | 27 ± 9 | 28 ± 10 | 27 ± 8 | 0.49 |
| Pulmonary artery mean pressure, mmHg | 37 ± 11 | 37 ± 12 | 37 ± 11 | 0.54 |
| Pulmonary capillary wedge pressure, mmHg | 25 ± 9 | 27 ± 9 | 24 ± 9 | 0.048 |
| Cardiac output, L/min | 3.8 (2.9, 4.6) | 3.7 (2.9, 4.3) | 3.9 (3.1, 4.8) | 0.18 |
| Cardiac index, L/min/m2 | 1.9 (1.6, 2.3) | 1.9 (1.5, 2.2) | 2.0 (1.7, 2.4) | 0.11 |
| Systemic vascular resistance, dyne·s−1·cm−5 | 1319 (994, 1747) | 1289 (834, 1640) | 1384 (1135, 1863) | 0.030 |
| Systolic arterial pressure, mmHg | 110 (97, 119) | 104 (96, 114) | 113 (101, 124) | 0.002 |
| Diastolic arterial pressure, mmHg | 64 (56, 74) | 62 (54, 70) | 68 (60, 77) | 0.006 |
| Mean arterial pressure, mmHg | 79 (71, 89) | 75 (69, 85) | 81 (74, 91) | 0.001 |
| Pulse pressure, mmHg | 43 (35, 53) | 41 (31, 52) | 45 (36, 54) | 0.16 |
Mann-Whitney test for continuous variables
Continuous variables are expressed as mean ± standard deviation or median (25th, 75th percentile).
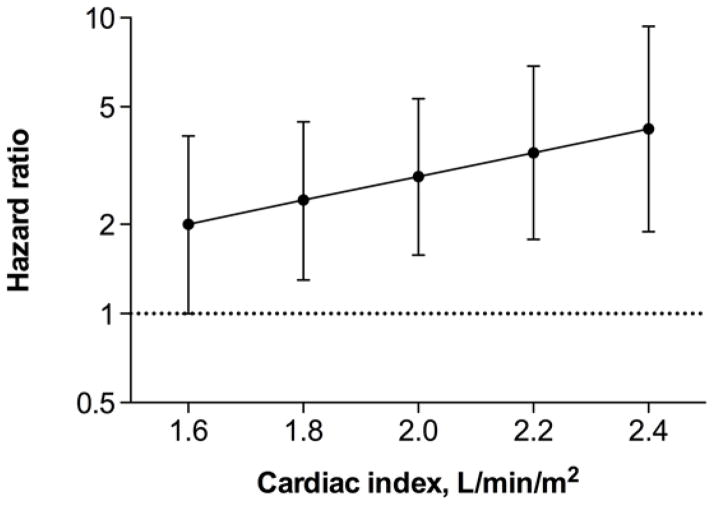
In-hospital inotrope use was associated with elevated risk for major clinical events in the ≥1.8-L/min/m2 cardiac index group, Table 4. This risk was not statistically significant in the <1.8-L/min/m2 group; however, the interaction term did not reach statistical significance (P=0.072 in adjusted models). These results did not materially change when ≤3mcg/kg/min dopamine was not considered as inotrope use; however, the risk differential with inotropes reached statistical significance (P=0.049) in adjusted models. When only mortality was considered, the risk differential between cardiac-index subgroups was diminished, partially because of the small number of events (Table 4). When cardiac index was entered as a continuous variable, there was a trend towards higher risk for major clinical events with use of inotropes with increasing cardiac index, Figure 1. However, the interaction between cardiac index and inotrope use did not reach statistical significance in adjusted models (P=0.092, P=0.47, and P=0.069 for major events, mortality, and with low-dose dopamine not considered as inotrope use, respectively).
Table 4.
In-hospital Inotrope Use and Outcomes According to Cardiac Index at Baseline
| Outcome | Cardiac Index <1.8 L/min/m2 (N=82) | Cardiac Index ≥1.8 L/min/m2 (N=114) | |||
|---|---|---|---|---|---|
|
| |||||
| Major clinical events | HR (95% CI) | P | HR (95% CI) | P | P interaction |
| Death, LVAD implantation, or heart transplantation | 1.48 (0.61–3.58) | 0.39 | 4.65 (1.98–10.9) | <0.001 | 0.072 |
| Death -- LVAD implantation, or heart transplantation censored as alive | 2.59 (0.77–8.71) | 0.12 | 3.16 (1.14–8.77) | 0.027 | 0.80 |
| Death, LVAD implantation, or heart transplantation -- dopamine ≤3mcg/kg/min not considered as inotrope | 1.26 (0.52–2.92) | 0.63 | 4.14 (1.82–9.44) | 0.001 | 0.049 |
|
| |||||
| Study days dead or in hospital | Ratio (95% CI) | P | Ratio (95% CI) | P | P interaction |
|
| |||||
| Any inotrope | 1.95 (1.13–3.38) | 0.017 | 2.11 (1.33–3.35) | 0.002 | 0.83 |
| Dopamine ≤3mcg/kg/min not considered as inotrope | 1.86 (1.08–3.21) | 0.026 | 1.99 (1.25–3.18) | 0.004 | 0.85 |
All ratios refer to effect of in-hospital inotrope use compared with no inotrope use as reference.
All models are adjusted for age, sex, body mass index, left ventricular ejection fraction, etiology of heart failure (ischemic vs. nonischemic), blood urea nitrogen, sodium, and hemoglobin.
CI: confidence interval; HR: hazard ratio; LVAD: left ventricular assist device.
Figure 1.
In-hospital inotrope use and risk for death, ventricular assist device implantation, or heart transplantation by 180 days according to baseline cardiac index (N=196). The hazard ratio represents the effect of in-hospital inotrope use compared with no inotrope use as reference. There was a trend towards higher risk for major clinical events with use of inotropes with increasing cardiac index. The interaction term, however, was not statistically significant (P=0.092) in a model adjusting for age, sex, body mass index, left ventricular ejection fraction, admission systolic blood pressure, blood urea nitrogen, sodium, and hemoglobin.
Patients who received inotropes spent twice the days dead or in hospital (from 180 study days) compared with those who did not, regardless of baseline cardiac index (Table 4).
In analyses based on CI values obtained on Day 2, inotrope use was still associated with increased mortality risk in both the <1.8 and the ≥1.8 L/min/m2 subgroups. In models adjusting for baseline CI in addition to other covariates, the direction of association was consistent with baseline CI analyses, with the exception of a neutral effect of inotropes on hospital-free days among low cardiac index patients (Supplemental Table 2). However, the number of patients with low cardiac index on Day 2 was relatively small (N=43) and therefore these results should be interpreted with caution.
The directional associations of (1) the average in-hospital dose of each inotrope and (2) the number of inotropic agents used with the change (defined as final measurement before removal of PAC minus baseline measurement) in (1) cardiac index; (2) pulmonary capillary wedge pressure; and (3) right atrial pressure are summarized in Supplemental Figures 1–3.
Heart Failure Etiology
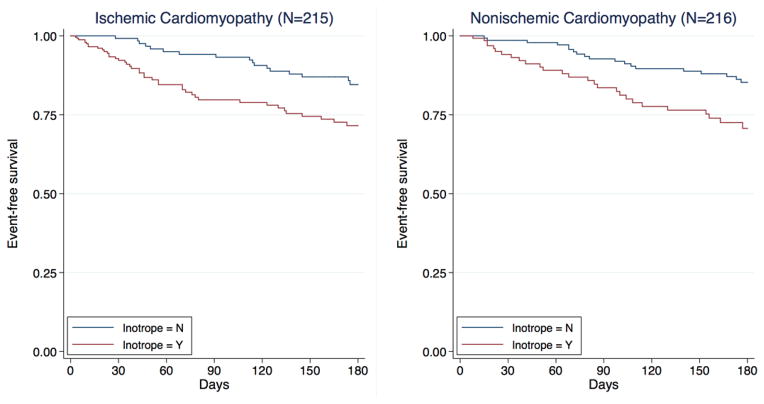
Event rates were higher with inotropes both in ischemic (45/107 [42.1%] vs. 22/108 [20.4%]; P<0.001) and nonischemic patients (29/87 [33.3%] vs. 21/129 [16.3%]; P=0.003), Figure 2. After adjusting for clinical risk factors, the risk for major events remained higher among those who received in-hospital inotropes in both HF etiology groups (Table 5). There was no significant interaction between inotrope use and HF etiology for major events. When LVAD and transplant were censored, patients receiving inotropes were more likely to die, regardless of HF etiology (although risk became non-significant in the nonischemic subgroup). The results did not materially change when ≤3mcg/kg/min dopamine was not considered as inotrope use (Table 5).
Figure 2.
Survival free from left ventricular assist device implantation or heart transplantation according to in-hospital inotrope use in patients with ischemic (left) and nonischemic (right) heart failure. Kaplan-Meier curves are adjusted for age, sex, body mass index, left ventricular ejection fraction, admission systolic blood pressure, blood urea nitrogen, sodium, hemoglobin, and pulmonary artery catheter-guided treatment.
Table 5.
In-hospital Inotrope Use and Outcomes According to Etiology of Heart Failure
| Outcome | Ischemic Etiology (N=215) | Nonischemic Etiology (N=216) | |||
|---|---|---|---|---|---|
|
| |||||
| Major clinical events | HR (95% CI) | P | HR (95% CI) | P | P interaction |
| Death, LVAD implantation, or heart transplantation | 2.64 (1.49–4.68) | 0.001 | 2.19 (1.18–4.07) | 0.012 | 0.44 |
| Death -- LVAD implantation, or heart transplantation censored as alive | 2.37 (1.25–4.48) | 0.008 | 1.88 (0.83–4.22) | 0.13 | 0.42 |
| Death, LVAD implantation, or heart transplantation -- dopamine ≤3mcg/kg/min not considered as inotrope | 2.39 (1.38–4.14) | 0.020 | 2.06 (1.12–3.80) | 0.012 | 0.47 |
|
| |||||
| Study days dead or in hospital | Ratio (95% CI) | P | Ratio (95% CI) | P | P interaction |
|
| |||||
| Any inotrope | 2.15 (1.49–3.09) | <0.001 | 1.65 (1.17–2.32) | 0.004 | 0.19 |
| Dopamine ≤3mcg/kg/min not considered as inotrope | 2.03 (1.40–2.92) | <0.001 | 1.66 (1.17–2.34) | 0.004 | 0.31 |
All ratios refer to effect of in-hospital inotrope use compared with no inotrope use as reference.
All models are adjusted for age, sex, body mass index, left ventricular ejection fraction, etiology of heart failure (ischemic vs. nonischemic), blood urea nitrogen, sodium, hemoglobin, and pulmonary artery catheter-guided treatment.
CI: confidence interval; HR: hazard ratio; LVAD: left ventricular assist device.
Patients who received inotropes spent fewer study days alive and out of hospital (out of 180 study days) compared with those who did not, regardless of HF etiology (Table 5).
DISCUSSION
In this post-hoc analysis of the ESCAPE trial, we observed that in-hospital use of inotropes was associated with unfavorable outcomes regardless of SBP at presentation, baseline cardiac index, or HF etiology. There was a trend towards more pronounced risk among patients with higher cardiac index. Similarly, inotrope use was associated with fewer days alive out of the hospital in all subgroups assessed in this study. The results were consistent when low-dose dopamine was not considered as an inotrope and when inotrope use was assessed in relation to SBP and CI values obtained on subsequent days (Day 3 and Day 2, respectively). These findings raise concerns about routine use of inotropes in patients with systolic HF admitted for decompensation without evidence of cardiogenic shock or end-organ hypoperfusion.
Limited early studies, before the era of neurohormonal therapy, did suggest favorable effects on hemodynamics and symptoms with use of positive inotropes in patients with HF and low cardiac output.13, 14 However, since then, several clinical trials and outcomes studies have demonstrated that the use of inotropes in acute and chronic HF is not beneficial, and in fact, may be associated with increased morbidity and mortality without any gains in cost or length-of-stay.5, 6, 8, 15–18 In the Acute Decompensated Heart Failure National Registry (ADHERE), patients receiving milrinone or dobutamine had considerably higher (45% to 115%) adjusted in-hospital mortality compared with those receiving nitrates or nesiritide. Despite this evidence, inotropes are widely used in the management of HF patients hospitalized for worsening symptoms. This may be related to several reasons.
Data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) and ADHERE registries show that inotrope use among hospitalized HF patients is particularly prevalent among those with low admission SBP despite absence of shock or end organ hypoperfusion.16, 19 In ADHERE, the median SBP of patients on milrinone or dobutamine was 117 and 120 mmHg, respectively, and only 8% of these patients had SBP <90 mmHg.16 The current American College of Cardiology Foundation and American Heart Association Heart Failure management guidelines provide a class I, level of evidence C recommendation for the use of inotropes in HF patients with cardiogenic shock, and a class IIb, level of evidence B recommendation for patients with systolic dysfunction who have low cardiac index and evidence of systemic hypoperfusion and/or congestion.20 However, low SBP alone is not a recommended indication. The relatively low threshold to administer inotropic agents to patients with low SBP in clinical practice reflects thus a belief that SBP represents a surrogate for low cardiac index or that inotropes will somehow mitigate the high risk for adverse events in these patients. Irrespective of the rationale, the use of inotropes in our study was associated with worse outcomes across the spectrum of SBP.
OPTIME-CHF included patients with decompensated HF without the knowledge of central hemodynamics. Thus, one criticism has been that if inotropes were given preferentially to patients in low cardiac output state the benefits of better perfusion might outweigh risks. Our analysis does not provide support for this notion. Among patients who received a PAC and had available cardiac index data, inotrope use was associated with increased risk for adverse events across the spectrum of baseline cardiac index, with a trend towards more pronounced risk with higher cardiac index. Importantly, median cardiac index in these patients was 1.9 L/min/m2, reflecting in general a low-output population for which inotropes are believed to be beneficial.
In a post-hoc analysis of the OPTIME-CHF database, Felker et al. demonstrated that the morbidity and mortality in patients treated with milrinone was largely seen in patients with HF due to ischemic etiology.7 The authors concluded that the use of milrinone might be neutral or beneficial in HF patients with non-ischemic etiology. These findings were not replicated in our analysis; inotrope use was associated with worse outcomes regardless of etiology. Thus, unless prospective studies confirm a differential effect between these two groups of HF patients with depressed EF, our results would caution inotrope use in all HF patients.
It has been theorized that inotropes may provide benefit by extending the window to optimize medications that have proven beneficial in HF, e.g. beta-blockers. Also, implantable cardioverter-defibrillators are thought to be protective against the risk of sudden cardiac death secondary to ventricular arrhythmias,21, 22 presumably related to increased intracellular calcium levels,23 in patients receiving inotropes. However, it is important to recognize that inotropes are not only linked to tachyarrhythmias but also to myocyte apoptosis and ischemia.24, 25 Data also suggest that short-term exposure to inotropic agents could potentially lead to deleterious effects long after the agent is discontinued.26, 27 Thus, even with implantable cardioverter-defibrillators and beta-blockers, inotropes can theoretically still pose a risk for HF patients.
Despite these data, one must differentiate between the potential role of an inotropic agent in HF and the risk associated with currently available inotropes. Much of HF progression is not related to the original insult that led to development of left ventricular dysfunction, but due to the subsequent adverse neurohormonal activation secondary to the low output state. Thus, theoretically, if there were a ‘safe’ inotrope instituted earlier in the course of HF, it would have the potential to mitigate the subsequent adverse cardiac remodeling process. Though arguable, this may be one of the reasons for the morbidity benefit seen with digoxin. Along similar lines, clinical trials with a novel inotropic agent, omecamtiv mecarbil, are ongoing, and other agents, e.g. istaroxime, are under early investigation.
Our study has several limitations. First, the decision about institution of inotropic therapy was left to the investigators. The non-randomized, open-label use of inotropes in ESCAPE renders our findings suggestive rather than definitive. Despite our efforts to minimize indication bias by adjusting for potential confounders, it is still possible that our estimates are confounded by unobserved differences between patients who received inotropes and those who did not. We did not perform a propensity score analysis because of the relatively small number of patients in the two treatment groups. Second, not all patients were optimized on HF therapy with beta-blockers and renin-angiotensin-aldosterone system inhibitors and the rate of cardiac defibrillator use was low. Third, this is a post-hoc analysis with a relatively small number of events, especially for the subset of patients with available cardiac index and for mortality-only analyses. Hence these data should be considered hypothesis generating and suggestive of the fact that, until prospective randomized trials are performed, the use of inotropes in hospitalized systolic HF patients should be limited. Finally, it is also imperative to realize that ESCAPE excluded patients with cardiogenic shock, in whom the current HF guidelines recommend inotrope use and therefore our data should not be extrapolated to these patients. Similarly, we did not assess inotropes as a bridge to transplant or LVAD implantation or as a long-term palliative strategy, which are other recognized indications for inotrope use.20
In summary, there is no substantiated benefit to date with routine use of inotropes in the management of the general population of patients with systolic HF hospitalized for worsening symptoms. Our findings add to the evidence suggesting that inotropes should not be routinely used in these patients in the absence of cardiogenic shock or end-organ hypoperfusion until further, prospective evidence supports the safety of inotropes in this setting.
Supplementary Material
Acknowledgments
Funding Sources:
This study was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454.
Footnotes
ClinicalTrials.gov Identifier: NCT00000619 (http://clinicaltrials.gov/show/NCT00000619)
Disclosures:
There are no conflicts of interest associated with this work.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Kociol RD, Horton JR, Fonarow GC, Reyes EM, Shaw LK, O’Connor CM, Felker GM, Hernandez AF. Admission, discharge, or change in B-type natriuretic peptide and long-term outcomes: data from Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) linked to Medicare claims. Circ Heart Fail. 2011;4:628–636. doi: 10.1161/CIRCHEARTFAILURE.111.962290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand SL, Schreiner G, Spertus JA, Vidan MT, Wang Y, Wang Y, Krumholz HM. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor CM, Gattis WA, Uretsky BF, Adams KF, Jr, McNulty SE, Grossman SH, McKenna WJ, Zannad F, Swedberg K, Gheorghiade M, Califf RM. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1999;138:78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 5.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, Massie BM, O’Connor CM, Pina I, Quigg R, Silver MA, Gheorghiade M. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 6.Elkayam U, Tasissa G, Binanay C, Stevenson LW, Gheorghiade M, Warnica JW, Young JB, Rayburn BK, Rogers JG, DeMarco T, Leier CV. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Felker GM, Benza RL, Chandler AB, Leimberger JD, Cuffe MS, Califf RM, Gheorghiade M, O’Connor CM. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41:997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 8.Partovian C, Gleim SR, Mody PS, Li SX, Wang H, Strait KM, Allen LA, Lagu T, Normand SL, Krumholz HM. Hospital patterns of use of positive inotropic agents in patients with heart failure. J Am Coll Cardiol. 2012;60:1402–1409. doi: 10.1016/j.jacc.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldhaber JI, Hamilton MA. Role of inotropic agents in the treatment of heart failure. Circulation. 2010;121:1655–1660. doi: 10.1161/CIRCULATIONAHA.109.899294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Vaduganathan M, Ambrosy A, Bohm M, Campia U, Cleland JG, Fedele F, Fonarow GC, Maggioni AP, Mebazaa A, Mehra M, Metra M, Nodari S, Pang PS, Ponikowski P, Sabbah HN, Komajda M, Butler J. Current management and future directions for the treatment of patients hospitalized for heart failure with low blood pressure. Heart Fail Rev. 2013;18:107–122. doi: 10.1007/s10741-012-9315-1. [DOI] [PubMed] [Google Scholar]
- 11.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 12.Shah MR, O’Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE): design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 13.Unverferth DV, Magorien RD, Lewis RP, Leier CV. Long-term benefit of dobutamine in patients with congestive cardiomyopathy. Am Heart J. 1980;100:622–630. doi: 10.1016/0002-8703(80)90226-4. [DOI] [PubMed] [Google Scholar]
- 14.Likoff MJ, Weber KT, Andrews V, Janicki JS, Sutton MS, Wilson H, Rocci ML., Jr Amrinone in the treatment of chronic cardiac failure. J Am Coll Cardiol. 1984;3:1282–1290. doi: 10.1016/s0735-1097(84)80189-8. [DOI] [PubMed] [Google Scholar]
- 15.Elis A, Bental T, Kimchi O, Ravid M, Lishner M. Intermittent dobutamine treatment in patients with chronic refractory congestive heart failure: a randomized, double-blind, placebo-controlled study. Clin Pharmacol Ther. 1998;63:682–685. doi: 10.1016/S0009-9236(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 16.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 17.Hauptman PJ, Mikolajczak P, George A, Mohr CJ, Hoover R, Swindle J, Schnitzler MA. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096, e1091–1098. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaney A, Bradford C, McCaffrey J, Bagshaw SM, Lee R. Levosimendan for the treatment of acute severe heart failure: a meta-analysis of randomised controlled trials. Int J Cardiol. 2010;138:281–289. doi: 10.1016/j.ijcard.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation/American Heart Association Task Force on Practice G. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 21.Burger AJ, Horton DP, LeJemtel T, Ghali JK, Torre G, Dennish G, Koren M, Dinerman J, Silver M, Cheng ML, Elkayam U. Effect of nesiritide (B-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the PRECEDENT study. Am Heart J. 2002;144:1102–1108. doi: 10.1067/mhj.2002.125620. [DOI] [PubMed] [Google Scholar]
- 22.Benza RL, Tallaj JA, Felker GM, Zabel KM, Kao W, Bourge RC, Pearce D, Leimberger JD, Borzak S, O’Connor CM, Gheorghiade M. The impact of arrhythmias in acute heart failure. J Card Fail. 2004;10:279–284. doi: 10.1016/j.cardfail.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Stevenson LW. Clinical use of inotropic therapy for heart failure: looking backward or forward? Part II: chronic inotropic therapy. Circulation. 2003;108:492–497. doi: 10.1161/01.CIR.0000078349.43742.8A. [DOI] [PubMed] [Google Scholar]
- 24.Lehtonen LA, Antila S, Pentikainen PJ. Pharmacokinetics and pharmacodynamics of intravenous inotropic agents. Clin Pharmacokinet. 2004;43:187–203. doi: 10.2165/00003088-200443030-00003. [DOI] [PubMed] [Google Scholar]
- 25.Teerlink JR, Metra M, Zaca V, Sabbah HN, Cotter G, Gheorghiade M, Cas LD. Agents with inotropic properties for the management of acute heart failure syndromes. Traditional agents and beyond. Heart Fail Rev. 2009;14:243–253. doi: 10.1007/s10741-009-9153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39:798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 27.Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.