Abstract
Background
We exploited a large database to investigate the outcome of high-risk neuroblastoma (HR-NB) in the contemporary era.
Methods
We studied all HR-NB patients <12 years old treated during induction at our hospital in 2000–2011, including 118 patients with MYCN-amplified(+) disease, and 127 patients >18 months old with MYCN-non-amplified(−) stage 4.
Results
Complete/very good partial response (CR/VGPR) to induction correlated with significantly superior event-free (EFS) (p<0.001) and overall survival (OS) (p<0.001) compared to partial response or less (≤PR). MYCN(+) and MYCN(−) patients had similar rates of CR/VGPR to induction (p=0.366); MYCN(+) and MYCN(−) patients in CR/VGPR had similar EFS (p=0.346) and OS (p=0.542). In contrast, only MYCN(+) patients had progressive disease (PD) as response to induction (p<0.001), and early death from PD (<366 days post-diagnosis) was significantly more common (p<0.001) with MYCN(+) disease. Overall, among patients with ≤PR, MYCN(+) patients had significantly inferior EFS (p<0.001) and OS (p<0.001) compared to MYCN(−) patients, which accounted for the significantly worse EFS (p=0.008) and OS (p=0.002) of the entire MYCN(+) cohort versus MYCN(−) cohort.
Conclusions
MYCN(−) HR-NB patients display a broad, continuous spectrum as regards response and outcome, whereas MYCN(+) patients have either an excellent response to induction associated with good long-term outcome, or early PD with poor outcome. This extreme dichotomy in the clinical course of MYCN(+) patients points to underlying biological differences with MYCN(+) NB, the elucidation of which may have far-reaching implications, including improved risk classification at diagnosis and identification of targets for treatment.
Keywords: neuroblastoma, contemporary therapy, MYCN, induction
INTRODUCTION
Population-based data1–3 and group studies through the 1990s4–11 showed 5-year event-free survival (EFS) and overall survival (OS) rates of 17–37% for high-risk neuroblastoma (HR-NB), with little difference between EFS and OS (Table 1A). In large studies extending beyond 2000, 5-year EFS and OS rates improved to 35–64% (Table 1B).12–15 The results may represent overestimations of survival given that subsequent analyses revealed a more limited definition of HR-NB. Thus, these studies included: a) patients 12–18 months old with MYCN-non-amplified(−) stage 4 which is now recognized as intermediate-risk disease;16,17 and b) toddlers with MYCN(−) stage 3 with unfavorable histology, which may be cured by limited therapy.18 Indeed, a 1999–2004 group study focusing on a well-recognized high-risk group – i.e., MYCN-amplified(+) NB in infants – yielded 2-year EFS/OS of 29%/30%,19 and an international experience covering 1997–2002 for patients >18 months with MYCN(+) stage 4 revealed EFS 18%.17
Table 1.
Studies of high-risk neuroblastoma with long-term follow-up
| References (years of study)a |
No. of pts |
Stages and age of study population |
Prognostic impact of response/ MYCN(+) |
Staging studies at diagnosis/ follow-up |
Local RT |
CRA | Anti-GD2 immuno- therapy |
Event-free survival |
Overall survival |
|---|---|---|---|---|---|---|---|---|---|
| 1A. Studies in the 1990s | |||||||||
| Zage9 | 142 | 4, >1 yr | …/… | …/post-protocol | yes | no | no | 23% (7 yr) | 28% (7 yr) |
| (1993–95) | 2 or 3, MYCN(+), | every 3–6m, x2 yr | |||||||
| >1 yr | |||||||||
| Matthay10 | 539 | 4, >1 yr | yes/yes | MIBG optional/ | no | ran- | no | 26% (5 yr) | 36% (5 yr) |
| (1991–96) | 4, <1 yr, MYCN(+) | at end of protocol | domised | stage 4: 21% (5 yr) | 32% (5 yr) | ||||
| 3, MYCN(+) | stage 3: 55% (5 yr) | 59%(5 yr)b | |||||||
| 3, UH and/or | |||||||||
| ferritin ≥143 ng/ml | |||||||||
| Berthold7 | 335 | 4, >1 yr | …/… | …/… | no | no | no | 27% (5 yr) | 33% (5 yr) |
| (1990–97) | |||||||||
| DeBernardi6 | 159 | 4, >1 yr | none/yes | …/… | no | no | no | 17% (5 yr) | 28% (5 yr) |
| (1992–97) | |||||||||
| Kaneko5 | 221 | 4, >1 yr | …/none | MIBG optional/ | no | no | no | 34% (5 yr) | 37% (5 yr) |
| (1991–98) | … | ||||||||
| Castel4 | 83 | 4, >1 yr | none/yes | …/… | no | no | no | 33% (4 yr) | 33% (4 yr) |
| (1992–98) | 4, <1 yr, MYCN(+) | … | 20% (5 yr) | ||||||
| 3, MYCN(+) | |||||||||
| Pearson8 | 262 | 4, >1 yr | yes/none | MIBG optional/ | no | no | no | 28% (3 yr) | … |
| (1990–99) | “local preference” | 24% (5 yr) | 26% (5 yr) | ||||||
| 23% (10 yr) | 24%(10yr) | ||||||||
| 1B. Studies completed in the 2000s | |||||||||
| George13 | 97 | 4, >1 yr | …/none | MIBG variable/ | yes | yes | no | 55% (3 yr) | 72% (3 yr) |
| (1994–2002) | 3, 4, 4S, <1 yr, | … | 47% (5 yr) | 60% (5 yr) | |||||
| MYCN(+) | 46% (7 yr) | 53% (7 yr) | |||||||
| 3, >1 yr, UH | |||||||||
| Berthold12 | 295 | 4, >1 yr | yes/yes | …/… | 24 pts | 39 pts | 160 pts | 39% (3 yr) | 58% (3 yr) |
| (1997–2002) | all stages MYCN(+) | 35% (5 yr) | 45% (5 yr) | ||||||
| Canete19 | 35 | 2, 3, 4, 4S, | yes/… | MIBG if available/ | yes | yes | no | 29% (2 yr) | 30% (2 yr) |
| (1999–2004) | <1 yr, MYCN(+) | … | stage 4: 20% (2 yr) | ||||||
| Sung14 | 52 | 4, >1 yr | none/none | MIBG included/ | 35 pts | yes | no | 62% (5 yr) | 64% (5 yr) |
| (1997–2005) | … | ||||||||
| Kreissman15 | 495 | 4, >1 yr | …/… | …/… | yes | yes | 78 pts | 38% (5 yr) | 50% (5 yr) |
| (2001–2006) | 3, 4, 4S, <1 yr, | ||||||||
| MYCN(+) | |||||||||
| 3, >1 yr, UH | |||||||||
Abbreviations: CRA, 13-cis-retinoic acid; MYCN(+), MYCN-amplified; pts, patients; UH, unfavorable histology; RT, radiotherapy
… Indicates not reported or described.
Listed chronologically by year of study’s completion.
5-yr EFS/OS rates for MYCN(+) stage 3 were 25%/27% and for MYCN(−) stage 3 were 77%/79%.11
Better results have been expected with the recent adoption of therapies, many developed in the 1990s, promising more effective induction (dose-dense or dose-intensive chemotherapy),8,20,21 consolidation (13-cis-retinoic acid,10 local radiotherapy,22 anti-GD2 monoclonal antibody [MoAb]23,24), and salvage (131I-metaiodobenzylguanidine [MIBG] therapy,25 topoisomerase 1 inhibitors26–28).
We used the large Memorial Sloan-Kettering Cancer Center (MSKCC) database to investigate the outcome of HR-NB in the contemporary era, i.e., since 2000. We took into account MYCN amplification, since large studies differ whether this finding is4,6,10,12 or is not5,8,13,14 prognostic with HR-NB (Table 1). We also assessed response to induction vis-à-vis EFS and OS, given differences in past studies whether a good response does8,10,12,19 or does not4,6,14 impact survival of HR-NB patients (Table 1). The data revealed a striking dichotomy in the clinical course of patients with MYCN(+) disease, a scenario not seen with MYCN(−) HR-NB.
PATIENTS AND METHODS
Study subjects were identified from the NB patient registry of MSKCC. This source listed 1185 patients in the period 2000–2011. The current study was limited to the 247 patients who were <12 years old when diagnosed with HR-NB and were treated during induction at MSKCC from diagnosis or after starting induction elsewhere. Excluded were patients who came for consultation alone or only surgery. The definition of HR-NB conformed with current criteria: MYCN(+) stage 2, 3, 4S, or 4 disease of any age, or MYCN(−) stage 4 diagnosed at age >18 months.16,17 MYCN amplification was defined by international criteria using fluorescence in situ hybridization.29 In accordance with rules of the MSKCC institutional review board, informed written consents for evaluations and treatments were obtained from guardians, and, for this exploratory study via a retrospective review, a waiver was obtained for examination and analysis of patient records.
Disease status was defined by widely accepted international response criteria,6,8,11,14,15,19,30 including 123I-MIBG findings: complete remission (CR), no evidence of NB in soft tissue, bones, or bone marrow (BM), and urine catecholamines normal; very good partial remission (VGPR), primary mass reduced by ≥90%, no evidence of distant disease in soft tissue, bones, or BM, including negative 123I-MIBG scan, and urine catecholamines normal; partial response (PR), >50% decrease in measurable soft tissue disease and the number of metastatic skeletal lesions in 123I-MIBG scan, and ≤1 positive BM site; mixed response (MR) , >50% decrease of any lesion with <50% decrease in any other, and 123I-MIBG scan improved by <50% decrease in number of (+) sites; no response (NR), <50% decrease but <25% increase in any existing lesion, unchanged MIBG findings; and progressive disease (PD), new, or >25% increase in an existing, lesion.
A complete evaluation of disease status comprised computed tomography or magnetic resonance imaging, 123I-MIBG scan, urine catecholamine levels, and BM histology (aspirates and biopsies from bilateral posterior iliac crests, and aspirates±biopsies from bilateral anterior iliac crests). BM and imaging studies were read by MSKCC specialists outside the Department of Pediatrics unaware of treatment or patient status. A complete evaluation was performed at the end of induction. Patients who achieved CR/VGPR underwent a complete evaluation at least every three months for an additional two years, and then 123I-MIBG scan ± other staging studies every three months for three more years. Patients who achieved only PR or less (≤PR) with induction underwent a complete evaluation every 1–3 months while on therapy; if they achieved CR/VGPR, a complete evaluation was repeated every three months for three more years.
The software SPSS, version 11.0 (SPSS Inc, Chicago, IL), was used for the survival statistical analyses, calculating from the first day of induction chemotherapy. Survival curves were generated according to the Kaplan-Meier method, with point estimates including ±SE, and compared using the two-sided log-rank test. EFS continued through the date of PD, toxic death, or secondary cancer. OS was defined through the date of death from any cause. Two-tailed chi squared test was used for comparisons of response rates to induction therapy.
RESULTS
Patient characteristics
The total series of patients included 118 MYCN(+) patients (1 stage 2, 13 stage 3, 1 stage 4S, 103 stage 4), 127 MYCN(−) patients with stage 4 diagnosed at age >18 months, and two patients with unknown MYCN status. The latter two patients were excluded from further analysis, leaving a total of 245 patients for study (Table 2). Thirty-one patients were infants, i.e., <18 months old, and all had MYCN(+) disease. MYCN(+) and MYCN(−) patients received the same upfront and salvage therapies. Thus, the initial (1st-line) induction regimens were all for HR-NB: 223 (91%) patients received Children’s Oncology Group15,21 (or similar MSKCC20) regimens, and 22 (9%) received other group-wide8,9 or single-institutional programs. Consolidation of CR/VGPR included anti-GD2 MoAb ± autologous stem-cell transplantation (ASCT).24 Initial 2nd-line treatments for refractory or progressive disease with induction included high-dose conventional chemotherapy31–34 or moderate-dose regimens28,35 using agents with known anti-NB activity. Relapse was also treated uniformly, including intra-thecal radioimmunotherapy for central nervous system relapse,36 high-dose conventional chemotherapy31–34 for disseminated or soft-tissue relapse, and moderate-dose chemotherapy28,35 plus radiotherapy for focal skeletal relapse. Patients achieving 2nd CR/VGPR received MoAb.
Table 2.
Patient characteristics
|
MYCN- amplified (n=118) |
MYCN- non-amplified (n=127) |
Entire cohort (n=245) |
|
|---|---|---|---|
| Male:Female (ratio) | 61:57 (1.07) | 47:53 (0.89) | 108:110 (0.98) |
| Age at diagnosis (years) | |||
| Range | 0.12–10.97 | 1.54–11.82 | 0.12–11.82 |
| Median | 2.00 | 3.65 | 3.02 |
| Induction regimens | |||
| A3973/N8 | 89 (75%) | 95 (75%) | 184 (75%) |
| ANBL0532 | 20 (17%) | 19 (15%) | 39 (16%) |
| Other | 9 (8%) | 13 (10%) | 22 (9%) |
| Responses to induction | |||
| CR/VGPR | 68 (58%) | 59 (46%) | 127 (52%) |
| PR | 13 (11%) | 22 (17%) | 35 (14%) |
| MR/NR | 13 (11%) | 46 (36%) | 59 (24%) |
| PD | 24a (20%) | 0 | 24 (10%) |
| Early PD (<366 days)b | 50 (42%) | 17 (13%) | 67 (27%) |
| Early death from PD (<366 days) | 26 (22%) | 6 (5%) | 32 (13%) |
| Non-neuroblastoma events | 4c (3%) | 6d (5%) | 10 (4%) |
| Consolidation of 1st CR/VGPR | |||
| MoAb | 40/68 (59%) | 34/59 (58%) | 74/127 (58%) |
| ASCT + MoAb | 26/68 (38%) | 24/59 (41%) | 50/127 (39%) |
| No ASCT or MoAb | 2/68 (3%) | 1/59 (2%) | 3/127 (2%) |
| Initial treatment for ≤PR | |||
| High-dose chemotherapye | 37/48f (77%) | 52/68 (76%) | 89/116 (77%) |
| ASCT + MoAb | 5/48f (10%) | 5/68 (7%) | 10/116 (9%) |
ASCT, autologous stem-cell transplantation; MoAb, immunotherapy with anti-GD2 monoclonal antibody
Includes one stage 4S patient and three stage 3 patients
Includes patients with PD response to induction and patients who relapsed <366 days from diagnosis.
Two deaths during induction (tumor lysis syndrome, pulmonary failure), one transplant-related toxic death, and one secondary myelodysplastic syndrome (detected at 42 months and successfully treated)
Two transplant-related toxic deaths, two secondary leukemias (diagnosed at 28 and 58 months and successfully treated), one death from hemolytic-uremic syndrome during induction, and one late death in an accident without prior relapse
Not including 2 patients who died of toxicity during 1st-line induction.
Responses to induction (Table 2)
CR/VGPR was achieved with 1st-line induction treatment in 127/245 (52%, 95% confidence interval [CI] 45–58%) patients, including 68/118 (58%, 95% CI 48–67%) MYCN(+) patients and 59/127 (46%, 95% CI 38–56%) MYCN(−) patients (p=0.366). PR rates were also similar between the MYCN(+) cohort (11%, 95% CI 6–18%) and the MYCN(−) cohort (17%, 95% CI 11–25%) (p=0.2). In contrast, a PD response to induction was limited to MYCN(+) disease, occurring in 24/118 (20%, 95% CI 13–29%) MYCN(+) patients, compared to 0/127 MYCN(−) children (p<0.001).
EFS and OS correlated with MYCN
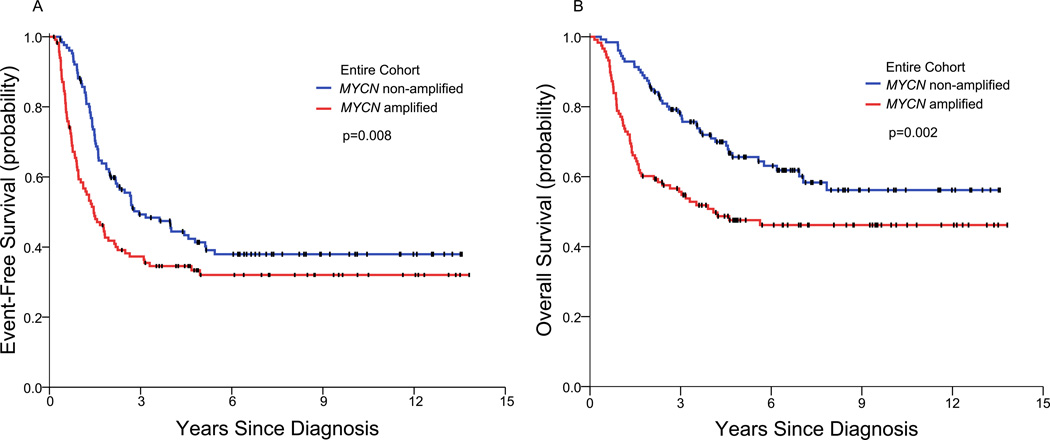
The EFS and OS rates at 3/5/7 years of the entire study cohort of 245 patients were 44/37/35% and 67/57/53%, respectively. The MYCN(+) patients (n=118) had significantly inferior EFS and OS compared to MYCN(−) patients (n=127) (Table 3; Figure 1). Thus, at 3/5/7 years, the EFS rates were 37/32/32% versus 49/41/38% (p=0.008), and the OS rates were 56/48/46% versus 77/66/60% (p=0.002).
Table 3.
Event-free survival (EFS) and overall survival (OS)
| Subsets of patients | EFS rates (%) 3/5/7 years |
p- value |
OS rates (%) 3/5/7 years |
p- value |
|---|---|---|---|---|
| All patients (n=245) | 44/37/35 | 67/57/53 | ||
| MYCN(+) (n=118) vs. MYCN(−) (n=127) | 37/32/32 vs. 49/41/38 | 0.008 | 56/48/46 vs. 77/66/60 | 0.002 |
| CR/VGPR (n=127) vs. ≤PR (n=118) | 58/51/50 vs. 28/21/19 | <0.001 | 80/74/70 vs. 53/38/35 | <0.001 |
| All MYCN(+) patients (n=118) | ||||
| infants (n=31) vs. children (n=87) | 34/34/34 vs. 39/32/32 | 0.824 | 55/55/55 vs. 56/45/43 | 0.671 |
| Patients in CR/VGPR (n=127) | ||||
| MYCN(+) (n=68) vs. MYCN(−) (n=59) | 54/48/48 vs. 63/54/52 | 0.346 | 75/68/68 vs. 86/80/72 | 0.542 |
| Patients in ≤PR (n=118) | ||||
| MYCN(+) (n=50) vs. MYCN(−) (n=68) | 14/9/9 vs. 37/30/26 | <0.001 | 29/18/14 vs. 70/53/50 | <0.001 |
Figure 1.
Event-free survival (A) and overall survival (B) of all MYCN(+) patients and all MYCN(−) patients.
Early treatment failure was significantly more common with MYCN(+) disease: PD at <366 days from diagnosis (including PD response to induction) emerged in 50/118 (42%, 95% CI 33–52%) MYCN(+) patients, compared to 17/127 (13%, 95% CI 8–21%) MYCN(−) patients (p<0.001), and death from PD at <366 days from diagnosis occurred in 26/118 (22%, 95% CI 15–31%) MYCN(+) patients versus 6/127 (5%, 95% CI 2–10%) MYCN(−) patients (p<0.001) (Table 2). Early death of patients who achieved CR/VGPR with 1st-line induction was rare: this occurred in 1/26 MYCN(+) patients consolidated with ASCT+MoAb and 1/40 consolidated with MoAb, and in 0/24 MYCN(−) patients consolidated with ASCT+MoAb and 1/34 consolidated with MoAb.
Among the MYCN(+) patients, infants (n=31) and children (n=87) had overlapping outcomes (Table 3). Thus, at 3/5/7 years, the EFS rates were 34/34/34% compared to 38/31/31% (p=0.828), and the OS rates were 55/55/55% compared to 56/44/42% (p=0.664).
Correlation of EFS and OS with response to induction
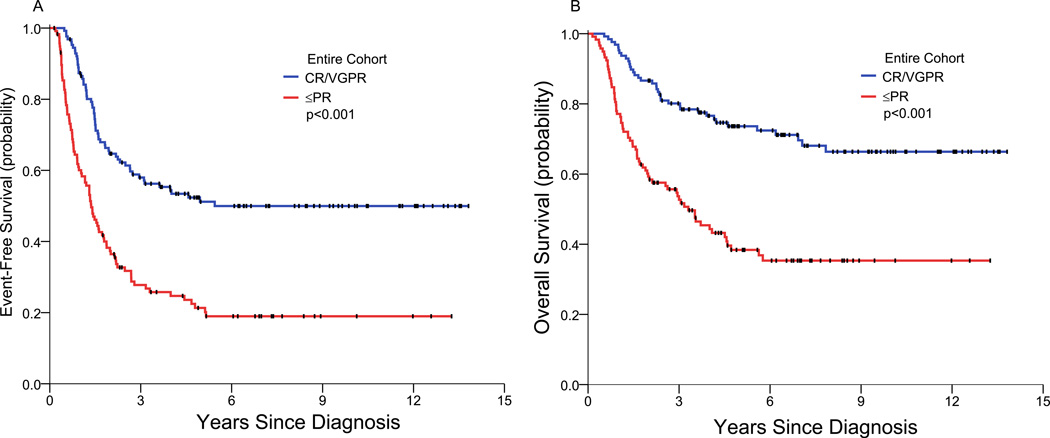
EFS and OS of the patients who achieved CR/VGPR with initial induction therapy (n=127) were significantly better than those of the patients who had ≤PR (n=118) (Table 3; Figure 2). Thus, at 3/5/7 years, the EFS rates were 58/51/50% versus 28/21/19% (p<0.001), and the OS rates were 80/74/70% versus 53/38/35% (p<0.001).
Figure 2.
Event-free survival (A) and overall survival (B) of all patients who achieved complete/very good partial remission compared to all patients who had partial remission or less.
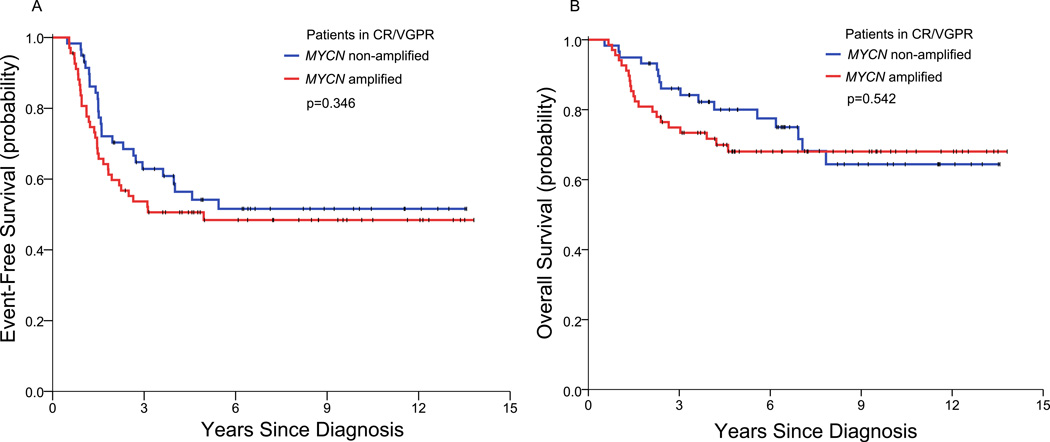
Among the patients who achieved CR/VGPR, EFS and OS of the MYCN(+) patients (n=68) and the MYCN(−) patients (n=59) were similar (Table 2; Figure 3). Thus, at 3/5/7 years, the EFS rates were 54/48/48% versus 63/54/52% (p=0.346), and the OS rates were 75/68/68% versus 86/80/72% (p=0.542). These results show that the long-term OS rates of MYCN(+) patients and MYCN(−) patients in CR/VGPR post-induction were ~20% greater than their respective long-term EFS rates.
Figure 3.
Event-free survival (A) and overall survival (B) of the MYCN(+) patients and the MYCN(−) patients who achieved complete/very good partial remission.
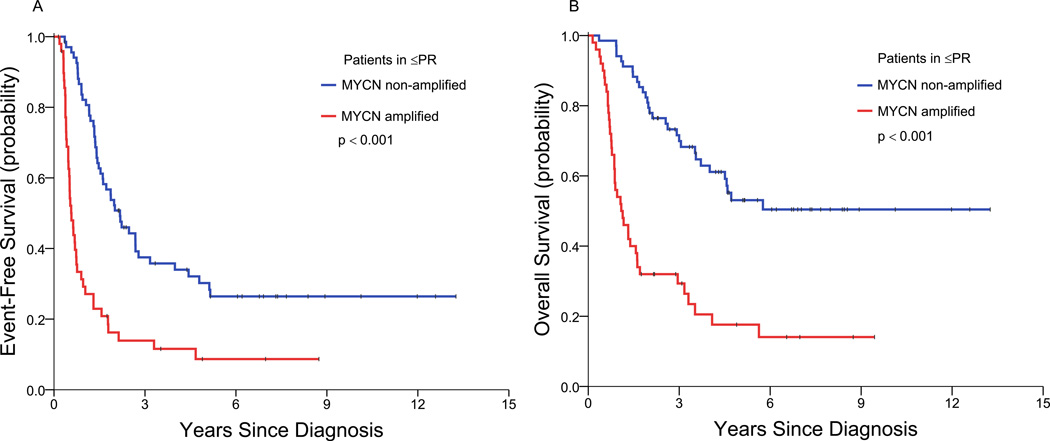
Among the patients with a ≤PR to induction, EFS and OS of the MYCN(+) patients (n=50) were significantly inferior to those of the MYCN(−) patients (n=68) (Table 3; Figure 4). Thus, at 3/5/7 years, the EFS rates were 14/9/9% versus 37/30/26% (p<0.001), and the OS rates were 29/18/14% versus 70/53/50% (p<0.001). These results show that the long-term EFS and OS of these MYCN(+) patients were similar, whereas the long-term EFS and OS of the MYCN(−) patients differed by ~20–30%.
Figure 4.
Event-free survival (A) and overall survival (B) of the MYCN(+) patients and the MYCN(−) patients who achieved partial remission or less.
DISCUSSION
MYCN(−) HR-NB patients display a broad, continuous spectrum as regards response and survival, whereas MYCN(+) patients have either an excellent response to induction associated with good long-term outcome, or early PD with poor outcome. This extreme dichotomy in the clinical scenarios of MYCN(+) patients may result from underlying distinctive genetic features. The elucidation of these divergent biological profiles may have far-reaching implications, including improved risk classification at diagnosis and identification of targets for treatment. Unfortunately, there are currently no known biological findings predictive at diagnosis of outcome for MYCN(+) patients, as MYCN(+) NBs are virtually all associated with certain biomarkers (e.g., chromosome 17q gain and chromosome 1p deletion) and not with others (e.g., chromosome 11q deletion and ATRX gene mutations).
The MYCN(+) and MYCN(−) cohorts received the same induction, consolidative, and salvage treatments (Table 2); this uniformity lent validity to the comparisons of outcome. A key finding was PD response to 1st-line induction with MYCN(+) disease, before consolidation could start. As regards widely used post-induction treatments, among patients who achieved CR/VGPR with 1st-line induction, early death was rare, occurring in 2/66 MYCN(+) patients consolidated with ASCT+MoAb (n=26) or MoAb (n=40), and in 1/58 MYCN(−) patients consolidated with ASCT+MoAb (n=24) or MoAb (n=34). Initial treatment of refractory disease or PD involved chemotherapy with well-recognized anti-NB activity, rather than investigative agents.
MYCN(+) and MYCN(−) patients have equivalent CR/VGPR rates with induction (p=0.366), and MYCN is not prognostic for patients in CR/VGPR at completion of induction. Thus, MYCN(+) and MYCN(−) patients who achieve CR/VGPR with induction have similar long-term EFS and OS (Table 3; Figure 3). In sharp contrast, PD response to induction is limited to MYCN(+) patients (p<0.001), and early PD is significantly associated with MYCN(+) disease (p<0.001). Early treatment failure not only adversely affects EFS but contributes to a dismal OS since PD of HR-NB during, or soon after completion of, induction responds poorly to salvage therapy,31 and short time from diagnosis to PD is a significant adverse factor for OS.37–41Indeed, MYCN(+) patients are significantly more likely than MYCN(−) patients to die early from PD (p<0.001), i.e., <366 days from diagnosis. Overall, among patients with ≤PR to induction, MYCN(+) disease is associated with a significantly inferior EFS (p<0.001) and OS (p<0.001); these results account for the significantly worse long-term EFS (p=0.008) and OS (p=0.002) of the entire MYCN(+) cohort compared to the entire MYCN(−) cohort (Table 3; Figure 1). Age had no prognostic impact with MYCN(+) disease: infants and children with this chromosomal aberration had similar long-term EFS and OS (Table 3).
The PD findings, combined with the significantly worse EFS/OS of MYCN(+) compared to MYCN(−) patients in ≤PR post-induction, suggest that a major response to upfront therapy is crucial for a good outcome of MYCN(+) patients and less so for MYCN(−) HR-NB patients. This point is supported by the absence of continued decline in EFS of MYCN(+) patients over the long term, whereas EFS rates of MYCN(−) patients decrease steadily beyond 3 years (Table 3; Figure 1). The findings reflect a paucity of late relapses among MYCN(+) compared to MYCN(−) HR-NB patients.
The paramount prognostic importance of initial response for MYCN(+) patients, but not for MYCN(−) HR-NB patients, is also illustrated by the relation between EFS and OS rates within subsets of patients. Thus, the MYCN(+) patients with ≤PR to induction have long-term EFS and OS rates that approximate each other (Table 3; Figure 4). This similarity between EFS and OS is consistent with a rapid demise of these patients after PD/relapse and is attributable to persistence of underlying chemo-resistance as well as ineffective second-line therapy (see below). In contrast, the MYCN(+) patients who achieve CR/VGPR with induction have long-term OS ~20% higher than EFS, evidence of prolonged survival post-relapse attributable to continued chemosensitivity (Table 3; Figure 3). This difference between outcomes of MYCN(+) patients in ≤PR versus those in CR/VGPR is not seen with MYCN(−) HR-NB: indeed, all MYCN(−) HR-NB patients - those in CR/VGPR, as well as those in ≤PR following induction - have long-term OS rates that, like the MYCN(+) patients in CR/VGPR post-induction, are ~20% higher than EFS rates (Table 3; Figures 3 and 4).
These large differences between long-term EFS and OS in the 2000–2011 period among all MYCN(−) HR-NB patients and the MYCN(+) patients in CR/VGPR, though not those in ≤PR post-induction, were not seen in the 1990s (Table 1).4–11 In that decade, EFS and OS were closely related, which supported the view that PD/relapse of HR-NB was synonymous with a subsequent rapid demise from PD or toxicity of salvage therapy. Exceptions to this scenario led to the initial reports of long-term survival of children with HR-NB despite persistence or relapse of disease.38,42 This phenomenon of chronic NB was much less common in MYCN(+) than MYCN(−) patients.37–42An underlying biologic factor for chronicity may be mutations of the ATRX gene which were recently found to be significantly associated with MYCN(−) HR-NB in older patients,43 in whom NB is often characterized by an indolent and prolonged, though ultimately lethal, course.44
Consistent with a low likelihood of prolonged survival after MYCN(+) PD/relapse, EFS and OS were poor and virtually identical in the few reports offering details about MYCN(+) patients. Thus, in a 1999–2004 multicenter study of MYCN(+) infants, EFS/OS rates at only 2 years were 29%/30% for all stages (2-year OS was 20% for stage 4);19 reviews of a 1990–2002 international experience found MYCN(+) stage 4 and 4S patients <18 months old to have 5-year EFS/OS rates of 28%/34%45 and MYCN(+) stage 4 patients >18 months old to have 5-year EFS/OS rates of <25%;16 and a 1991–1996 group-wide study of MYCN(+) stage 3 (all ages) yielded 5-year EFS/OS rates of 25%/27%.11
One reason why the above clinical scenario typical in the 1990s - i.e., death soon after PD/relapse - may no longer hold true is the availability of novel, relatively non-toxic (i.e., second-line) salvage therapies that lack cross-resistance with induction. Examples include chemotherapy regimens (e.g., irinotecan-temozolomide28), investigative agents (e.g., fenretinide, ABT-751, crizotinib),46 and targeted radiotherapy (131I-MIBG,25 131I-monoclonal antibodies36). Another factor contributing to prolonged survival may be improved disease surveillance. In the 1990s, MIBG scintigraphy often used 131I and was not regularly performed (Table 2);5,8,10 subsequently, 123I-MIBG became widely adopted and proved to be superior in detecting NB, especially relapse that is asymptomatic (and presumably has lower tumor burden) which may be more amenable to control than bulky metastatic relapse.47 Unfortunately, the welcome prospect that the recent advances in therapy and surveillance might, after relapse, lead to a chronic course with long-term survival plus good quality of life, or even cure, is not relevant to patients with early PD – a subset of patients significantly associated with MYCN(+) NB (p<0.001; Table 3).
In conclusion, results from the 1970s through the contemporary era indicate that better upfront, consolidative, and salvage therapies have improved survival of patients with HR-NB.1–3,12–15,17,23,24 Yet MYCN(+) NB differs significantly from MYCN(−) HR-NB as regards a) PD response to induction, and b) extreme differences in outcome, i.e., early death from disease or excellent PFS and OS. The two sharply divergent clinical scenarios of MYCN(+) patients merit investigation at the molecular/genetic level, analogous to other studies into the underlying biology of NB,43,46 to identify markers predictive at diagnosis of good response/good outcome versus poor response/early demise, and to expand the availability of targets for therapy.
Acknowledgments
Supported in part by grants from the National Institutes of Health (CA10450), Bethesda, MD; the Robert Steel Foundation, NY, NY; and Katie’s Find-A-Cure Fund, NY, NY.
REFERENCES
- 1.Burkhardt-Hammer T, Spix C, Brenner H, et al. Long-term survival of children with neuroblastoma prior to the neuroblastoma screening project in Germany. Med Pediatr Oncol. 2002;39:156–162. doi: 10.1002/mpo.10132. [DOI] [PubMed] [Google Scholar]
- 2.Schroeder H, Wacher J, Larsson H, et al. Unchanged incidence and increased survival in children with neuroblastoma in Denmark 1981–2000: A population-based study. Br J Cancer. 2009;100:853–857. doi: 10.1038/sj.bjc.6604922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haupt R, Garaventa A, Gambini C, et al. Improved survival of children with neuroblastoma between 1979 and 2005: A report of the Italian Neuroblastoma Registry. J Clin Oncol. 2010;28:2331–2338. doi: 10.1200/JCO.2009.24.8351. [DOI] [PubMed] [Google Scholar]
- 4.Castel V, Canete A, Navarro S, et al. Outcome of high-risk neuroblastoma using a dose-intensity approach: Improvement in initial but not in long-term results. Med Pediatr Oncol. 2001;37:537–542. doi: 10.1002/mpo.1248. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko M, Tsuchida Y, Mugishima H, et al. Intensified chemotherapy increases survival rates of patients with stage 4 neuroblastoma with MYCN amplification. J Pediatr Hematol/Oncol. 2002;24:613–621. doi: 10.1097/00043426-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 6.De Bernardi B, Nicolas B, Boni L, et al. Disseminated neuroblastoma in children older than one year at diagnosis: Comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol. 2003;21:1592–1601. doi: 10.1200/JCO.2003.05.191. [DOI] [PubMed] [Google Scholar]
- 7.Berthold F, Hero B, Kremens B, et al. Long-term results and risk profiles of patients in five consecutive trials (1979–1997) with stage 4 neuroblastoma over 1 year of age. Cancer Lett. 2003;197:11–17. doi: 10.1016/s0304-3835(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 8.Pearson A, Pinkerton CR, Lewis IJ, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 9.Zage PE, Kletzel M, Murray K, et al. Outcomes of the POG 9340/9341/9342 trials for children with high-risk neuroblastoma: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2008;51:747–753. doi: 10.1002/pbc.21713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthay KK, Reynolds CP, Seeger RC, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A Children’s Oncology Group study. J Clin Oncol. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JR, Villablanca JG, London WB, et al. Outcome of high-risk stage 3 neuroblastoma with myeloablative therapy and 13-cis-retinoic acid: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52:44–50. doi: 10.1002/pbc.21784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 13.George RE, Li S, Medeiros-Nancarrow C, et al. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: Long-term survival update. J Clin Oncol. 2006;24:2891–2896. doi: 10.1200/JCO.2006.05.6986. [DOI] [PubMed] [Google Scholar]
- 14.Sung KW, Lee SH, Yoo KH, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007;40:37–45. doi: 10.1038/sj.bmt.1705691. [DOI] [PubMed] [Google Scholar]
- 15.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomized phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn SL, Pearson ADJ, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moroz V, Machin D, Faldum A, et al. Changes over three decades in outcome and the prognostic influence of age-at-diagnosis in young patients with neuroblastoma: A report from the International Neuroblastoma Risk Group project. Eur J Cancer. 2011;47:561–571. doi: 10.1016/j.ejca.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Modak S, Kushner BH, LaQuaglia MP, Kramer K, Cheung N-KV. Management and outcome of INSS stage 3 neuroblastoma. Eur J Cancer. 2009;45:90–98. doi: 10.1016/j.ejca.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canete A, Gerrard M, Rubie H, et al. Poor survival for infants with MYCN-amplified metastatic neuroblastoma despite intensified treatment: The International Society of Paediatric Oncology European neuroblastoma experience. J Clin Oncol. 2009;27:1014–1019. doi: 10.1200/JCO.2007.14.5839. [DOI] [PubMed] [Google Scholar]
- 20.Kushner BH, Kramer K, LaQuaglia MP, Modak S, Yataghene K, Cheung N-KV. Reduction from seven to five cycles of intensive induction chemotherapy in children with high-risk neuroblastoma. J Clin Oncol. 2004;22:4888–4892. doi: 10.1200/JCO.2004.02.101. [DOI] [PubMed] [Google Scholar]
- 21.Park JR, Scott JR, Stewart CF, et al. Pilot induction regime incorporating pharmacokinetically guided topotecan for treatment of newly diagnosed high risk neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2011;29:4351–4357. doi: 10.1200/JCO.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose (21 Gy) radiotherapy for high-risk neuroblastoma following intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 23.Yu A, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung N-KV, Cheung IY, Kushner BH, Ostrovnaya I, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony stimulating factor and 13-cis-retinoic acid is effective against chemoresistant marrow MRD among high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthay KK, Yanik G, Messina J, et al. Phase II study on the effect of disease sites, age, and prior therapy on response to iodine-131-metaiodobenzylguanidine therapy in refractory neuroblastoma. J Clin Oncol. 2007;25:1054–1060. doi: 10.1200/JCO.2006.09.3484. [DOI] [PubMed] [Google Scholar]
- 26.Garaventa A, Luksch R, Biasotti S, et al. A phase II study of topotecan with vincristine and doxorubicin in children with recurrent/refractory neuroblastoma. Cancer. 2003;98:2488–2494. doi: 10.1002/cncr.11797. [DOI] [PubMed] [Google Scholar]
- 27.Simon T, Längler A, Harnischmacher U, et al. Topotecan, cyclophosphamide, and etoposide (TCE) in the treatment of high-risk neuroblastoma: Results of a phase-II trial. J Cancer Res Clin Oncol. 2007;133:653–661. doi: 10.1007/s00432-007-0216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushner BH, Kramer K, Modak S, Cheung N-KV. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24:5271–5276. doi: 10.1200/JCO.2006.06.7272. [DOI] [PubMed] [Google Scholar]
- 29.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 31.Kushner BH, Kramer K, Modak S, Qin L-X, Cheung N-KV. Differential impact of high-dose cyclophosphamide, topotecan, and vincristine in clinical subsets of patients with chemoresistant neuroblastoma. Cancer. 2010;116:3054–3060. doi: 10.1002/cncr.25232. [DOI] [PubMed] [Google Scholar]
- 32.Kushner BH, Kramer K, Modak S, Yataghene K, Cheung N-KV. High-dose cyclophosphamide-irinotecan-vincristine for primary refractory neuroblastoma. Eur J Cancer. 2010;47:84–89. doi: 10.1016/j.ejca.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Kushner BH, Kramer K, Modak S, Cheung N-KV. High-dose carboplatin-irinotecan-temozolomide: Treatment option for neuroblastoma resistant to topotecan. Pediatr Blood Cancer. 2011;56:403–408. doi: 10.1002/pbc.22855. [DOI] [PubMed] [Google Scholar]
- 34.Kushner BH, Modak S, Kramer K, Basu EM, Roberts SS, Cheung N-KV. ICE for neuroblastoma: A high-dose salvage regimen and review of the literature. Cancer. 2013;119:665–671. doi: 10.1002/cncr.27783. [DOI] [PubMed] [Google Scholar]
- 35.Saylors RL, Stine KC, Sullivan J, et al. Cyclophosphamide plus topotecan in children with recurrent or refractory solid tumors: A Pediatric Oncology Group phase II study. J Clin Oncol. 2001;19:3463–3469. doi: 10.1200/JCO.2001.19.15.3463. [DOI] [PubMed] [Google Scholar]
- 36.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma. J Neuro-Oncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau L, Tai D, Weitzman S, Grant R, Baruchel S, Malkin D. Factors influencing survival in children with recurrent neuroblastoma. J Pediatr Hematol/Oncol. 2004;26:227–232. doi: 10.1097/00043426-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Santana V, Furman WL, McGregor LM, Billups CA. Disease control intervals in high-risk neuroblastoma. Cancer. 2008;112:2796–2801. doi: 10.1002/cncr.23507. [DOI] [PubMed] [Google Scholar]
- 39.Garaventa A, Parodi S, De Bernardi B, et al. Outcome of children with neuroblastoma after progression or relapse. A retrospective study of the Italian Neuroblastoma Registry. Eur J Cancer. 2009;45:2835–2842. doi: 10.1016/j.ejca.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 40.London WB, Castel V, Monclair T, et al. Clinical and biological features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29:3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon T, Berthold F, Borkhardt A, Kremens B, De Carolis B, Hero B. Treatment and outcomes of patients with relapsed, high-risk neurobastoma: Results of German trials. Pediatr Blood Cancer. 2011;56:578–583. doi: 10.1002/pbc.22693. [DOI] [PubMed] [Google Scholar]
- 42.Kushner BH, Kramer K, Cheung N-KV. Chronic neuroblastoma: Indolent stage 4 disease in children. Cancer. 2002;95:1366–1375. doi: 10.1002/cncr.10800. [DOI] [PubMed] [Google Scholar]
- 43.Cheung N-KV, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA. 2012;307:1062–1071. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conte M, Parodi S, De Bernardi B, et al. Neuroblastoma in adolescents. The Italian experience. Cancer. 2006;106:1409–1407. doi: 10.1002/cncr.21751. [DOI] [PubMed] [Google Scholar]
- 45.Taggart DR, London WB, Schmidt ML, et al. Prognostic value of the stage 4S metastatic pattern and tumor biology in patients with metastatic neuroblastoma diagnosed between birth and 18 months of age. J Clin Oncol. 2011;29:4358–4364. doi: 10.1200/JCO.2011.35.9570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole KA, Maris JM. New strategies in refractory and recurrent neuroblastoma: Translational opportunities to impact patient outcome. Clin Cancer Res. 2012;18:2423–2428. doi: 10.1158/1078-0432.CCR-11-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kushner BH, Kramer K, Modak S, Cheung N-KV. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27:1041–1046. doi: 10.1200/JCO.2008.17.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]