SUMMARY
Biliary tract carcinoma is a rare malignancy. We performed a comprehensive analysis of published prospective clinical trials in advanced biliary tract carcinoma in an attempt to identify active regimens in this setting. We searched PubMed and abstracts presented at the American Society of Clinical Oncology, Gastrointestinal Cancer Symposium, European Society of Medical Oncology and European Cancer Organization conferences for clinical trials in this disease. We found 83 trials. The effect of gemcitabine on overall survival benefit showed a strong trend (p = 0.014) and an improvement in progression-free survival (p = 0.003). Gemcitabine-based regimens containing 5-fluorouracil showed a trend toward an improved overall survival (p = 0.047) relative to platinum agents. Our findings support gemcitabine as the chemotherapy backbone for the treatment of patients with cholangiocarcinoma. Gemcitabine plus 5-fluorouracil combinations warrant further investigations.
KEYWORDS : biliary tract carcinoma, chemotherapy, cholangiocarcinoma, gallbladder carcinoma, treatment
Practice Points.
The combination of gemcitabine with platinum agents compared with other regimens showed a trend toward improved response rate (RR) (p = 0.047) but no significant difference in progression-free survival (PFS) or overall survival (OS) (p = 0.089 and p = 0.43, respectively).
No superior outcomes for the combination of 5-fluorouracil (5-FU) with platinum agents in comparison with other regimens in terms of RR, PFS or OS (p = 0.85, 0.08 and 0.82, respectively).
The regimen of Epirubicin, cisplatin and 5-FU did not demonstrate any statistical significant improvement in RR (p = 0.49), PFS (p = 0.96) or OS (p = 0.14) when compared with the other regimens.
The global effect of gemcitabine on OS showed a statistical trend (p = 0.014) and a significant improvement in PFS (p = 0.003). However, RR was not significant (p = 0.087) for the global gemcitabine effect.
Platinum-containing regimens did not reveal any superior outcomes in OS (p = 0.98), PFS (p = 0.72) or RR (p = 0.15) compared with platinum-free regimens.
There was no statistical differences in OS (p = 0.94) PFS (p = 0.44) or RR (p = 0.79) between regimens containing 5-FU versus combinations without 5-FU.
No significant difference in RR (p = 0.20), PFS (p = 0.31) or median OS (p = 0.25) was found between regimens with irinotecan versus combinations without irinotecan.
Gemcitabine in combination with 5-FU showed a trend toward an improved OS when compared with regimens containing gemcitabine or 5-FU in combination with platinum.
There were no discernable trends in any of the data (RR, PFS or OS) over time.
Background
Biliary tract cancers (BTC) are a heterogeneous group of tumors arising from cholangiocytes lining the biliary tree. Subtypes include gallbladder cancers (GBC) as well as intra-hepatic (ICC) and extra-hepatic cholangiocarcinoma (ECC). BTC comprise a rare cancer type with a high mortality rate. Due to the combined reporting of ICC with hepatocellular carcinoma, it is challenging to determine the exact incidence of BTC. Approximately 10–15% of combined primary liver tumors comprise of ICC. In the USA it is estimated that about 33,000 cases of hepatocellular carcinoma and ICC, and approximately 10,500 cases of gallbladder and other biliary cancers will be diagnosed in 2014 [1–4]. Surgery is the only potentially curative treatment modality but even with surgery recurrence rates are high and the 5-year survival is approximately 30–60%. Moreover, most BTC patients are diagnosed with advanced and inoperable disease [5,6]. Patients with GBC are reported to have a worse prognosis than ICC and ECC [7,8]. Historically, gemcitabine has been widely used in pancreaticobiliary tumors based on its proven activity in pancreatic cancer [9]. Other chemotherapy agents have been incorporated in cholangiocarcinoma treatment including 5-FU, platinum agents, epirubicin, combinations of these agents and others. More recently, the combination of gemcitabine and cisplatin became the standard of care in inoperable BTC based on the ABC-02 trial, a randomized Phase III study that showed an increased median progression free survival (PFS) and median overall survival (OS) of 8.5 and 11.7 months, compared with 5 and 8 months, respectively, for single-agent gemcitabine [10]. The efficacy of this combination was also suggested by a meta-analysis of 104 clinical trials, largely Phase II studies [11].
Although there are retrospective data suggesting that approximately 45% of the patients who progress on first-line treatment are eligible for further treatment, there is a scarcity of prospective trials evaluating optimal treatment regimens in the second-line setting. As a result, no consensus on standard second-line treatment has been established [12].
We performed a comprehensive analysis of published prospective clinical trials in BTC in an attempt to identify active regimens both in the first- and the second-line settings. We also performed subgroup analyzes comparing the outcomes of these regimens in GBC compared with non-GBC cases. Finally, we analyzed the response rate and survival over time.
Patients & methods
We obtained the data for this analysis by searching PubMed, for the years 1992–2013, using a combination of the following terms: 'chemotherapy' plus 'biliary tract cancer', 'cholangiocarcinoma' or 'gallbladder cancer'. In addition, we reviewed relevant abstracts presented in American Society of Clinical Oncology, Gastrointestinal Cancer Symposium, European Society of Medical Oncology and European Cancer Organization conferences. Abstract searches were limited to the time period 2008–2013. We excluded trials that used targeted agents due to the diverse mechanism of action of these agents, and trials that treated ten patients or less. The following data from the studies were recorded: study completion date, number of treated patients, first- or second-line treatment, median RR, PFS, and OS. In trials comprising more than one treatment arm, each arm was analyzed separately as a single-arm study. We also preformed a subgroup analysis for the treatment outcomes in GBC versus ICC and ECC.
• Statistical analysis
In each individual treatment arm, the RR, and the median PFS and OS, were obtained and used as the primary outcome variable of interest. We then compared the distributions of those three outcome variables between the different predefined treatment groups. We restricted the analysis to first-line trials and trials in which <25% of patients were on second-line treatment. For each of the three outcome variables (RR, PFS and OS), we made a variety of two-group comparisons using the Wilcoxon rank sum test. Table 1 indicates the 12 regimens utilized for the analysis and Table 2 postulates which combinations of regimens were used for a given specific comparison. Three general types of comparisons were made: specific combination regimen versus all others, global evaluations, and specific pairwise comparisons. Exact tests were used as appropriate. All reported p values are two tailed. In view of the varying degrees of independence and dependence of the groups compared, and considering the number of tests performed here, the multiplicity of tests performed must be taken account of in a balanced way. Thus, to aid in interpretation of the effects tested, only p < 0.01 can be deemed statistically significant, while 0.05 < p < 0.01 indicates a strong statistical trend. The following abbreviations were used: trials (t), arms (a), and patients (n).
Table 1. . Included chemotherapy regimens, with coupled outcome variables.
| Chemotherapy | Outcome | Arms | Minimum | Lower quartile | Median | Upper quartile | Maximum | Ref. |
|---|---|---|---|---|---|---|---|---|
| 5-FU | OS | 10 | 4.6 | 5.1 | 7.7 | 9.0 | 14.8 | [8,13–21] |
| ADDIN EN.CITE | PFS | 9 | 1.0 | 3.3 | 3.7 | 4.0 | 4.7 | |
| RR | 9 | 0.0 | 7.0 | 14.3 | 32.0 | 35.0 | ||
| 5-FU + platinum | OS | 13 | 3.1 | 8.0 | 9.5 | 10.0 | 12.8 | [13,22–31] |
| ADDIN EN.CITE | PFS | 11 | 1.4 | 3.3 | 3.7 | 4.8 | 6.5 | |
| RR | 13 | 0.0 | 19.0 | 21.4 | 30.0 | 42.9 | ||
| ECF | OS | 6 | 4.9 | 5.8 | 8.5 | 9.1 | 9.9 | [32–37] |
| ADDIN EN.CITE | PFS | 5 | 1.9 | 4.6 | 5.1 | 5.2 | 5.6 | |
| RR | 6 | 10.0 | 10.0 | 19.1 | 22.5 | 40.0 | ||
| Gemcitabine | OS | 10 | 5.8 | 7.5 | 8.4 | 11.5 | 14.0 | [10,38–46] |
| ADDIN EN.CITE | PFS | 9 | 2.5 | 2.6 | 4.3 | 5.6 | 8.1 | |
| RR | 9 | 0.0 | 9.4 | 17.5 | 26.1 | 36.0 | ||
| Gemcitabine+platinum | OS | 24 | 5.0 | 8.7 | 9.5 | 10.8 | 19.9 | [8,10,22,47–66] |
| ADDIN EN.CITE | PFS | 23 | 3.0 | 4.0 | 4.8 | 7.8 | 11.0 | |
| RR | 21 | 14.9 | 21.0 | 29.0 | 32.0 | 50.0 | ||
| Gemcitabine + 5-FU | OS | 17 | 4.7 | 8.9 | 12.5 | 14.0 | 16.0 | [20,38,67–81] |
| ADDIN EN.CITE | PFS | 14 | 2.9 | 4.6 | 6.1 | 7.1 | 9.0 | |
| RR | 15 | 9.5 | 21.4 | 30.0 | 31.4 | 38.0 | ||
| Gemcitabine + 5-FU + platinum | OS | 2 | 9.9 | 9.9 | 10.0 | 10.0 | 10.0 | [82] |
| ADDIN EN.CITE | PFS | 0 | – | – | – | – | – | |
| RR | 2 | 19.0 | 19.0 | 21.0 | 23.0 | 23.0 | ||
| Gemcitabine + pemetrexed | OS | 1 | 6.6 | 6.6 | 6.6 | 6.6 | 6.6 | [83] |
| PFS | 1 | 3.8 | 3.8 | 3.8 | 3.8 | 3.8 | ||
| RR | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| 5-FU+etoposide + leucovorin | OS | 1 | 12.0 | 12.0 | 12.0 | 12.0 | 12.0 | [34] |
| PFS | 1 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | ||
| RR | 1 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | ||
| Gemcitabine + irinotecan | OS | 1 | 7.6 | 7.6 | 7.6 | 7.6 | 7.6 | [84] |
| PFS | 1 | 4.3 | 4.3 | 4.3 | 4.3 | 4.3 | ||
| RR | 1 | 20.5 | 20.5 | 20.5 | 20.5 | 20.5 | ||
| Oxaliplatin + irinotecan | OS | 1 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | [85] |
| PFS | 1 | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | ||
| RR | 1 | 17.9 | 17.9 | 17.9 | 17.9 | 17.9 | ||
| Irinotecan + 5-FU | OS | 1 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | [86] |
| PFS | 1 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | ||
| RR | 1 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | ||
5-FU: 5-fluorouracil; ECF: Epirubicin, cisplatin and 5-FU; OS: Overall survival; PFS: Progression-free survival; RR: Response rate.
Table 2. . Comparisons between specific chemotherapy combinations.
| Chemotherapy regimens | Trial arms | Gemcitibine + platinum vs others | 5-FU + platinum vs others | 5-FU + platinum + epiribucin vs others | Global 5-FU | Global platinum effect | Global gemcitibine effect | Global irinotecan effect |
|---|---|---|---|---|---|---|---|---|
| 5-FU | 10 | - | - | - | + | - | - | - |
| 5-FU + platinum | 13 | - | + | - | + | + | - | - |
| ECF | 6 | - | - | + | + | + | - | - |
| Gemcitabine | 10 | - | - | - | - | - | + | - |
| Gemcitabine + platinum | 24 | + | - | - | - | + | + | - |
| Gemcitabine + 5-FU | 17 | - | - | - | + | - | + | - |
| Gemcitabine + 5-FU + platinum | 2 | - | - | - | + | + | + | - |
| Gemcitabine + Taxol | 1 | - | - | - | - | - | + | - |
| 5-FU + etoposide + leucovorin | 1 | - | - | - | + | - | - | - |
| Gemcitabine + irinotecan | 1 | - | - | - | - | - | + | + |
| Oxaliplatin + irinotecan | 1 | - | - | - | - | - | - | + |
| Irinotecan + 5-FU | 1 | - | - | - | + | - | - | + |
5-FU: 5-fluorouracil ECF: Epirubicin, cisplatin and 5-FU.
Results
Our initial search identified 83 published trials and 15 abstracts presented at scientific meetings. These 98 trials comprised of 109 trial arms treating a total of 4572 patients. Seventeen out of 109 arms were excluded from the analysis, 16 arms used targeted therapy and one had less than ten patients treated on the trial. Thus, 83 trials met inclusion criteria and were included in our analysis, comprising 92 treatment arms and treating 3809 patients. Our search results are summarized in Figure 1. The median RR, PFS and OS for all treatment arms (first and second line) was as follows: RR 21.4% (a = 85), PFS 4.3 months (a = 81) and OS 9.2 months (a = 92)
Figure 1. . Study selection.
5-FU: 5-fluororuacil; a: number of arms; ECF: Epirubicin, cisplatin and 5-FU; Etop: Etoposide; Gem: Gemcitabine; Irino: Irinotecan; n: Number of treated patients; Pem:Pemetrexed; t: Number of trials.
In a preliminary analysis, we compared distributions of the three outcome variables (RR, PFS and OS) according to whether the trial enrolled patients with first-line chemotherapy only, first line with <25% second-line treatment, or second-line chemotherapy only. Distributions of first line only versus <25% second line, were not significantly different (p ≥ 0.80). Thus, these two distributions were combined and second line only trials (t = 5) were excluded from further analyses. Accordingly, excluding the second-line only trials, there were 87 treatment arms with 3645 treated patients (Table 1). Chemotherapy regimens were analyzed to determine the most active drug combinations. Subgroups named 5-FU in this analysis comprised fluorouracil, capecitabine or S-1, and platinum agents included were cisplatin, oxaliplatin and carboplatin.
• Specific chemotherapy combinations compared with the other regimens
Gemcitabine in combination with platinum agents
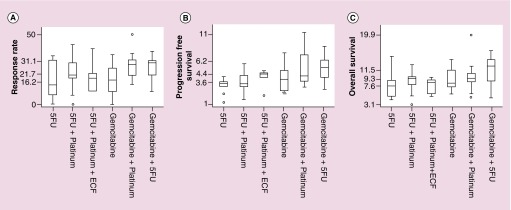
The combination of gemcitabine with cisplatin has become the standard of care treatment for cholangiocarcinoma based on the randomized ABC-02 Phase III trial demonstrating an overall survival benefit for this combination compared with gemcitabine single agent (11.7 vs 8.2 months, p < 0.001) [10]. In our analysis a total of 24 treatment arms, treating 1403 patients, evaluated the efficacy of gemcitabine in combination with platinum agents. In ten out of these 24 trials, including the ABC-02 trial, gemcitabine was combined with cisplatin, treating a total of 655 patients [10,22,47–53]. Out of the remaining 14 trials, 12 trials combined gemcitabine with oxaliplatin [8,54–64], treating a total of 683 patients, while the remaining two trials combined gemcitabine with carboplatin, treating a total of 65 patients [65,66]. The pooled analysis demonstrated a median response rate of 29% (a = 21). The median PFS and OS were 4.8 months (a = 23) and 9.5 months (a = 24), respectively (Figure 2). The combination of gemcitabine with platinum agents compared with other regimens showed a trend toward improved RR (p = 0.047) but no significant difference in PFS or OS (p = 0.089 and p = 0.43, respectively).
Figure 2. . Outcome variables of each of the analyzed studies presented as bubble plots.
The five horizontal lines in each figure represent the minimum, the quartiles and maximum of all the data combined. Circle sizes are proportional to the number of patients on each trial. (A) Response rate, (B) progression free survival and (C) overall survival.
5-FU: 5-fluorouracil; ECF: Epirubicin, cisplatin and 5-FU.
5-FU in combination with platinum
5-FU in combination with platinum agents has been used with success in several gastrointestinal malignancies including esophageal, gastric and colorectal cancers [87]. In cholangiocarcinoma several trials have evaluated this combination treatment. In our study we identified 13 treatment arms using this combination, treating 421 patients. Four of the 13 trial arms evaluated the efficacy of 5-FU in combination with cisplatin [13,23–24], and two trial arms used 5-FU with oxaliplatin [25,26]. The remaining four trial arms used capecitabine in combination with cisplatin [27,28] (two trial arms) or oxaliplatin [29] (two trial arms). Three of the trials used S-1, two of the trial arms in combination with cisplatin [22,30] and one with oxaliplatin [31]. Pooled analyses showed that the combination of 5-FU compounds with platinum agents had a median RR of 21.4% (a = 13). The median PFS and OS times were 3.7 and 9.5 months, respectively (Figure 2). Our analysis showed no superior outcomes for the combination of 5-FU with platinum agents in comparison with other regimens in terms of RR, PFS or OS (p = 0.85, 0.08 and 0.82, respectively).
The combination of epirubicin, cisplatin &5-FU
The chemotherapy regimen combination of epirubicin, cisplatin and 5-FU (ECF) is currently being used as a standard of care treatment for the management of gastroesophageal cancer [88,89]. In order to study the efficacy of this regimen in cholangiocarcinoma, we analyzed trials that used this combination. Six trials in this analysis were identified, treating 206 patients. Four of the six trials used 5-FU [32–35] and the other two used capecitabine [36] or uracil/tegafur [37]. The median RR was 19%. The median PFS and OS were 5.1 and 8.5 months, respectively (Figure 2). ECF did not demonstrate any statistically significant improvement in RR (p = 0.49), PFS (p = 0.96) or OS (p = 0.14) when compared with the other regimens.
• Specific chemotherapy agent effects
Global effect of gemcitabine
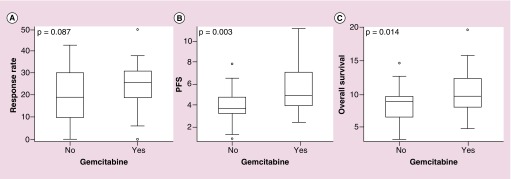
Gemcitabine is a nucleoside analog, which has shown efficacy in pancreatic cancer and hence extrapolation of the data led to great interest in many BTC trials [9]. To further investigate the effect of gemcitabine, all regimens containing gemcitabine were compared with regimens that did not use gemcitabine. We identified 55 treatment arms that used gemcitabine. These regimens had a median OS of 9.7 months, median PFS of 5.0 months (a = 48) and RR 26.1% (a = 49). Thirty-two treatment arms in our analysis did not use gemcitabine. These regimens had a median OS of 8.9 months, median PFS of 3.8 months (a = 28) and RR 19.0% (a = 31). The global effect of gemcitabine on OS showed a statistical trend (p = 0.014) and a significant improvement in PFS (p = 0.003). However, RR was not significant (p = 0.087) with respect to the global gemcitabine effect (Figure 3).
Figure 3. . Global effect of gemcitabine plotted by (A) response rate, (B) progression-free survival and (C) overall survival.
OS showed a statistical trend (p = 0.014) and there was a significant improvement in PFS (p = 0.003). RR was not significant (p = 0.087).
OS: Overall survival: PFS: Progression-free survival; RR: Response rate.
The effect of gemcitabine was also tested in two other pair-wise comparisons. A comparison between regimens that used single agent 5-FU [8,13–21] to regimens that used 5-FU + gemcitabine [20,38,67–81] showed a statistical trend toward improved OS (p = 0.017), and statistically significant improved PFS (p = 0.001) with the combination regimen. However, there was no significant difference in RR (p = 0.17). 5-FU + platinum [13,22–31] versus 5-FU, platinum + gemcitabine [82] was compared, but no difference in RR (p = 0.46), or OS (p = 0.46) was detected (there was insufficient data to test PFS).
Global effect of platinum
In the same manner as above, platinum-containing regimens were compared with platinum-free regimens in an attempt to evaluate the global effect of platinum. This comparison did not reveal any superior outcomes in OS (p = 0.98), PFS (p = 0.72) or RR (p = 0.15).
To further analyze the platinum effect three other pair-wise tests were performed. We found no significant differences between the trials utilizing single agent 5-FU and the 5-FU + platinum trials with respect to any of the three outcome variables (all p > 0.1). The second pair-wise test evaluated gemcitabine single agent [10,38–46] versus gemcitabine in combination with a platinum agent [8,10,22,47–66], to evaluate the effect of platinum given gemcitabine. This comparison indicated a trend toward improved RR when adding platinum to gemcitabine (p = 0.041) but there was no significant difference seen in PFS or OS (p = 0.28 and 0.33, respectively). The third pair-wise comparison was between the combination of gemcitabine and 5-FU with or without platinum agent. No significant difference in RR (p = 0.23), or OS (p = 0.57) was observed (there was insufficient data to test PFS).
5-FU-containing regimens
5-FU-based therapies have been the backbone in many of the randomized Phase II trials evaluating efficacy in BTC. In our analysis 5-FU single agent was used in ten treatment arms, treating 263 patients, resulting in median OS of 7.7 months (a = 10), median PFS 3.7 months (a = 9) and RR 14.3% (a = 9). To evaluate the global 5-FU effect we compared regimens with 5-FU versus combinations without 5-FU, we did not find any statistical differences in OS (p = 0.94), PFS (p = 0.44) or RR (p = 0.79).
The effect of 5-FU was further tested with pair-wise comparisons. Trials treating patients with single agent gemcitabine [10,38–46] were compared with trial arms treating patients with gemcitabine in combination with 5-FU [20,38,67–81]. There was no significant difference in OS (p = 0.13) or PFS (p = 0.08), however, in regards to RR, it was marginally higher in 5-FU + gemcitabine regimens (median 30.0%) compared with gemcitabine alone regimens (median 17.5%) with p = 0.047. In the second pair-wise test we found no statistically significant differences between gemcitabine + platinum regimens compared with gemcitabine + platinum + 5-FU regimens for either OS (p = 0.51) or RR (p = 0.43). PFS was not reported in the two treatment arms utilizing the three-drug regimen gemcitabine + platinum + 5-FU, therefore it was not analyzed.
Global effect of irinotecan
Three trials in our analysis evaluated irinotecan-containing regimens treating 97 patients [84–86]. These trials were compared with all other regimens that did not use irinotecan (Table 1). No significant difference in RR (p = 0.20), PFS (p = 0.31) or median OS (p = 0.25) was found.
• Comparisons between specific chemotherapy combinations
This analysis was performed in an attempt to compare the outcomes of the most frequently used combination regimens in cholangiocarcinoma. These regimens were: 5-FU + platinum agents, gemcitabine + platinum agents, and 5-FU + gemcitabine (Table 1).
5-FU in combination with platinum agents was used in 13 treatment arms, treating 421 patients, and showed a median RR of 21.4%, median PFS of 3.7 (a = 11) months and a median OS of 9.5 months. On the other hand, gemcitabine in combination with platinum compounds was used in 24 treatment arms, treating 1403 patients, and showed a median RR 29%, median PFS of 4.8 months and a median OS of 9.5 months. Comparing regimens containing platinum combined with 5-FU versus gemcitabine demonstrated no significant difference in RR (p = 0.46), or OS (p = 0.59) but a statistical trend (p = 0.041) toward a longer PFS favoring gemcitabine in combination with platinum.
5-FU in combination with platinum [13,22–31] (n = 421 patients, a = 13, median RR 21.4%, median PFS 3.7 months and median OS 9.5 months) was compared with 5-FU in combination with gemcitabine [20,38,67–81] (n = 654, a = 17, median OS 12.5 months, PFS 6.1 months and RR 30%). The outcome of 5-FU with platinum versus gemcitabine showed no difference in RR (p = 0.3), however there was a strong statistical trend (p < 0.05) for both PFS and OS favoring 5-FU in combination with gemcitabine. Comparing regimens containing gemcitabine combined with platinum (a = 24, n = 1403) or 5-FU (a = 17, n = 654) did not show a significant difference in RR or PFS (p = 0.99 and p = 0.49, respectively) but it did show a trend toward an improved OS (median OS 9.5 months vs 12.5 months favoring gemcitabine + 5-FU, p = 0.047). Thus, gemcitabine in combination with 5-FU showed a trend toward an improved OS when compared with regimens containing gemcitabine or 5-FU in combination with platinum.
• Treatment effect over time
In order to study the treatment effect over the past two decades we plotted the RR, PFS and OS for each of the analyzed regimens over the past 18 years (from 1992 to 2010). The median RR was 21.4% (a = 75); the median PFS (a = 70) and OS (a = 81) were 4.4 and 9.4 months, respectively. The number of trial arms differs in this analysis since not all of the trials reported RR or PFS. There were no discernable trends in any of the data over time.
Discussion
Gemcitabine has in recent years been regarded as the main chemotherapeutic agent in the treatment of pancreaticobiliary malignancies [9–10,90–91]. The ABC-02 trial – one of the few Phase III trials in this disease – demonstrated an approximate 30% improvement in median PFS and OS for the combination of gemcitabine and cisplatin compared with gemcitabine alone, thereby establishing a new standard of care in BTC [10]. We performed this analysis in order to identify active regimens in cholangiocarcinoma in both first- and second-line settings. Our analysis evaluating gemcitabine single agent versus gemcitabine in combination with a platinum agent indicated a strong trend toward improved RR when platinum is added to gemcitabine (p = 0.041) but this did not translate into benefit in PFS or OS (p = 0.28 and 0.33, respectively). Our findings are consistent with those reported in a previous meta-analysis of 104 trials, demonstrating superior RR with gemcitabine combined with platinum [11]. To address the question of whether the effect of this combination is due to the gemcitabine component or the platinum agent component we tested the global effect of each of these agents. The treatment effect was evaluated irrespective of dose and schedule of the agent. While the global effect of gemcitabine showed a strong statistical trend in OS (median OS of 8.9 vs 9.7 months, p = 0.014) and a significant improvement in PFS (median PFS of 3.8 vs 5.0 months, p = 0.003) favoring gemcitabine-containing regimens, our analyses indicated no significant difference in RR, PFS, or OS (p = 0.15, 0.72, 0.98, respectively) between regimens that contained platinum versus the ones that did not. Accordingly, we believe that the clinical effect is mainly due to gemcitabine when combined with platinum agents.
5-FU-based therapy has been shown to improve OS and quality of life compared with best supportive care in patients with advanced pancreatic and BTC [92]. Hence, 5-FU-based therapies have been the backbone of chemotherapy regimens in many of the randomized Phase II trials in BTC. In our pooled analyses we compared regimens with 5-FU versus combinations without 5-FU, but we did not find any statistical differences in RR (p = 0.79), PFS (p = 0.44) or OS (p = 0.94). Comparing 5-FU versus gemcitabine, in combination with platinum demonstrated no significant difference in RR (p = 0.46), or OS (p = 0.59) but a statistical trend (p = 0.041) toward a longer PFS favoring gemcitabine in combination with platinum. Therefore, 5-FU-based chemotherapy regimens are not as active as gemcitabine-based regimens in BTC, according to our analysis. Our findings are consistent with those from a retrospective review of 304 patients with BTC that showed lower risk of death in the patients who received gemcitabine-based regimen versus 5-FU-based regimens [93].
The next question was whether combining these two agents together (gemcitabine + fluoropyrimidine) would be an effective strategy in BTC. We addressed this question by comparing gemcitabine in combination with either platinum- or 5-FU-based regimens. This comparison indicated a strong trend toward an improved OS (median OS 9.5 months vs 12.5 months) favoring gemcitabine combined with 5-FU (p = 0.047). Considering the toxicity profiles of these two regimens and the restrictions of using cisplatin in certain patient populations including patients with renal failure, the combination of gemcitabine and 5-FU could be a valid option in unresectable BTC. This analysis is limited by three different fluoropyrimidine agents (fluorouracil, capecitabine and S-1) being merged into one group and that different doses and schedules were utilized. Whether gemcitabine in combination with 5-FU should be considered as a first-line choice in BTC remains to be determined in Phase III clinical trials. A randomized Phase III study comparing gemcitabine and cisplatin with gemcitabine and capecitabine was initiated in Canada but stopped early secondary to poor accrual (clinicaltrials.gov NCT00658593). There are no randomized Phase III trials currently comparing gemcitabine and cisplatin versus gemcitabine in combination with a fluoropyrimidine agent, but such trials are in the planning Phase [94].
Although 45% of the patients who progress on first line treatment are eligible for further treatments [12] no consensus on the standard treatment has been established [95] in this setting. This is due to a scarcity of prospective trials in the second-line setting in BTC. We identified only five trials that were exclusively second-line trials, treating a total of 164 patients. Overall, in our pooled analysis cholangiocarcinoma patients who received a second line chemotherapy had a median RR of 6.9%, median PFS of 2.5 months and a median OS of 5.9 months (data not shown). In concordance with our findings, a large retrospective study in a second-line setting found a median PFS of 2.8 months and median OS of 7.5 months [96]. In this study only 25% of the patients with BTC received second-line treatment and in the ABC trial the number was approximately 18% [10,96]. Accordingly, there is a great need for randomized trials in the second-line setting to establish an evidence-based standard of care.
Our analysis has potential limitations. First, although our study includes 83 clinical trials, some prospective studies were not included or full data from abstracts were published after our meta-analysis was completed. Since results of these trials support our findings this limitation should not have a significant impact on our conclusions. Second, comparisons across trials can be difficult due to the heterogeneity of regimens used, deriving from the different platinum or fluoropyrimidine agents, and different doses and schedules. Third, only 25 treatment arms in the analyzed trials specified whether the enrolled patients were diagnosed with ICC or ECC versus GBC neoplasm. Twelve out of these 25 trial arms limited the eligibility criteria to ICC/ECC, and the remaining 13 trial arms treated patients with GBC. These limited data preclude us from performing subgroup analysis. Finally, the trials included in our study were not restricted to randomized trials and the lack of uniformity between the included trials may have affected the results of our pooled analysis.
Our findings suggest that gemcitabine remains the backbone chemotherapy agent for future studies in cholangiocarcinoma. There is a need for randomized clinical trials that compare the current standard of care with other combinations in the first line as well as therapeutic interventions in the second-line setting. Likewise, there is a need for Phase III randomized clinical trials in order to perform more valid subgroup analyses between the different types of BTC. Nonetheless, the main challenge in conducting clinical trials in this disease remains its low incidence. Future efforts should focus on multicenter and cooperative group clinical trials approach in order to overcome this challenge, in addition to identifying new biomarkers predictive of response and exploring novel agents that could improve outcomes.
Future perspective
The increased incidence of cholangiocarcinoma and the unmet needs for treatment in this devastating disease will require future global collaborative efforts in the next decade. These efforts should focus on identifying novel biomarkers that could predict prognosis and treatment efficacy. New treatment modalities, such as immunotherapy, should be also studied and incorporated in the future treatment paradigm if found to be effective.
Footnotes
Financial & competing interests disclosure
Funding was received from the Intramural Research Program of the NIH National Cancer Institute. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ciombor KK, Goff LW. Current therapy and future directions in biliary tract malignancies. Curr. Treat. Options Oncol. 2013;14(3):337–349. doi: 10.1007/s11864-013-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J. Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, Mcglynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J. Hepatol. 2004;40(3):472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J. Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Deoliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Groen PC, Gores GJ, Larusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N. Engl. J. Med. 1999;341(18):1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 7.Perpetuo MD, Valdivieso M, Heilbrun LK, Nelson RS, Connor T, Bodey GP. Natural history study of gallbladder cancer: a review of 36 years experience at M. D. Anderson Hospital and Tumor Institute. Cancer. 1978;42(1):330–335. doi: 10.1002/1097-0142(197807)42:1<330::aid-cncr2820420150>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Sharma A, Dwary AD, Mohanti BK, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J. Clin. Oncol. 2010;28(30):4581–4586. doi: 10.1200/JCO.2010.29.3605. [DOI] [PubMed] [Google Scholar]; •• Clinical trials establishing 5-fluorouracil, gemcitabine and platinum-based agents in the treatment of biliary tract carcinomas.
- 9.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]; •• Clinical trials establishing 5-fluorouracil, gemcitabine and platinum-based agents in the treatment of biliary tract carcinomas.
- 10.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]; •• Clinical trials establishing 5-fluorouracil, gemcitabine and platinum-based agents in a treatment of biliary tract carcinomas.
- 11.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br. J. Cancer. 2007;96(6):896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandi G, DiGirdamo S, deRosa F, et al. Second-line chemotherapy in patients with biliary tract cancer. ASCO Abstract J. Clin. Oncol. 2011;29(suppl e14590) [Google Scholar]
- 13.Ducreux M, Van Cutsem E, Van Laethem JL, et al. A randomised Phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur. J. Cancer. 2005;41(3):398–403. doi: 10.1016/j.ejca.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Furuse J, Okusaka T, Boku N, et al. S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter Phase II study. Cancer Chemother. Pharmacol. 2008;62(5):849–855. doi: 10.1007/s00280-007-0673-7. [DOI] [PubMed] [Google Scholar]
- 15.Chen JS, Yang TS, Lin YC, Jan YY. A Phase II trial of tegafur-uracil plus leucovorin (LV) in the treatment of advanced biliary tract carcinomas. Jpn J. Clin. Oncol. 2003;33(7):353–356. doi: 10.1093/jjco/hyg070. [DOI] [PubMed] [Google Scholar]
- 16.Chen JS, Jan YY, Lin YC, Wang HM, Chang WC, Liau CT. Weekly 24 h infusion of high-dose 5-fluorouracil and leucovorin in patients with biliary tract carcinomas. Anticancer Drugs. 1998;9(5):393–397. doi: 10.1097/00001813-199806000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Choi CW, Choi IK, Seo JH, et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am. J. Clin. Oncol. 2000;23(4):425–428. doi: 10.1097/00000421-200008000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda M, Okusaka T, Ueno H, Morizane C, Furuse J, Ishii H. A Phase II trial of Uracil-tegafur (UFT) in patients with advanced biliary tract carcinoma. Jpn J. Clin. Oncol. 2005;35(8):439–443. doi: 10.1093/jjco/hyi131. [DOI] [PubMed] [Google Scholar]
- 19.Malik IA, Aziz Z. Prospective evaluation of efficacy and toxicity of 5-fu and folinic acid (Mayo Clinic regimen) in patients with advanced cancer of the gallbladder. Am. J. Clin. Oncol. 2003;26(2):124–126. doi: 10.1097/00000421-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Takashima A, Morizane C, Ishii H, et al. Randomized Phase II study of gemcitabine plus S-1 combination therapy vs S-1 in advanced biliary tract cancer: Japan Clinical Oncology Group Study (JCOG0805) Jpn J. Clin. Oncol. 2010;40(12):1189–1191. doi: 10.1093/jjco/hyq110. [DOI] [PubMed] [Google Scholar]
- 21.Ueno H, Okusaka T, Ikeda M, Takezako Y, Morizane C. Phase II study of S-1 in patients with advanced biliary tract cancer. Br. J. Cancer. 2004;91(10):1769–1774. doi: 10.1038/sj.bjc.6602208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang MEA. Randomized Phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in advanced biliary tract adenocarcinoma. ASCO Abstract J. Clin. Oncol. 2010;28(15S):4029. doi: 10.3109/0284186X.2012.682628. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi K, Tsuji A, Morita S, Horimi T, Shirasaka T, Kanematsu T. A Phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive (Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer. 2006;6:121. doi: 10.1186/1471-2407-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taieb J, Mitry E, Boige V, et al. Optimization of 5-fluorouracil (5-FU)/cisplatin combination chemotherapy with a new schedule of leucovorin, 5-FU and cisplatin (LV5FU2-P regimen) in patients with biliary tract carcinoma. Ann. Oncol. 2002;13(8):1192–1196. doi: 10.1093/annonc/mdf201. [DOI] [PubMed] [Google Scholar]
- 25.Nehls O, Klump B, Arkenau HT, et al. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective Phase II trial. Br. J. Cancer. 2002;87(7):702–704. doi: 10.1038/sj.bjc.6600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SEA. Oxaliplatin, 5-FU, and leucovorin (FOLFOX) in advanced biliary tract cancer. ASCO Abstract J. Clin Oncol. 2011;29(Suppl. 15):4106. [Google Scholar]
- 27.Hong YS, Lee J, Lee SC, et al. Phase II study of capecitabine and cisplatin in previously untreated advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2007;60(3):321–328. doi: 10.1007/s00280-006-0380-9. [DOI] [PubMed] [Google Scholar]
- 28.Kim TW, Chang HM, Kang HJ, et al. Phase II study of capecitabine plus cisplatin as first-line chemotherapy in advanced biliary cancer. Ann. Oncol. 2003;14(7):1115–1120. doi: 10.1093/annonc/mdg281. [DOI] [PubMed] [Google Scholar]
- 29.Nehls O, Oettle H, Hartmann JT, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre Phase II trial. Br. J. Cancer. 2008;98(2):309–315. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YJ, Im SA, Kim HG, et al. A Phase II trial of S-1 and cisplatin in patients with metastatic or relapsed biliary tract cancer. Ann. Oncol. 2008;19(1):99–103. doi: 10.1093/annonc/mdm439. [DOI] [PubMed] [Google Scholar]
- 31.Oh SEA. Phase II trial of S-1 in combination with oxaliplain in previously untreated patients with recurrent or inoperable biliary tract cancer. ASCO Abstract J. Clin. Oncol. 2008;26(15S):15611. doi: 10.1159/000159624. [DOI] [PubMed] [Google Scholar]
- 32.Morizane C, Okada S, Okusaka T, Ueno H, Saisho T. Phase II study of cisplatin, epirubicin, and continuous-infusion 5-fluorouracil for advanced biliary tract cancer. Oncology. 2003;64(4):475–476. doi: 10.1159/000070310. [DOI] [PubMed] [Google Scholar]
- 33.Takezako Y, Okusaka T, Ueno H, Ikeda M, Morizane C, Najima M. Phase II study of cisplatin, epirubicin and continuous infusion of 5-fluorouracil in patients with advanced intrahepatic cholangiocellular carcinoma (ICC) Hepatogastroenterology. 2008;55(85):1380–1384. [PubMed] [Google Scholar]
- 34.Rao S, Cunningham D, Hawkins RE, et al. Phase III study of 5FU, etoposide and leucovorin (FELV) compared with epirubicin, cisplatin and 5FU (ECF) in previously untreated patients with advanced biliary cancer. Br. J. Cancer. 2005;92(9):1650–1654. doi: 10.1038/sj.bjc.6602576. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Clinical trials establishing 5-fluorouracil, gemcitabine and platinum-based agents in a treatment of biliary tract carcinomas.
- 35.Lee MA, Woo IS, Kang JH, Hong YS, Lee KS. Epirubicin, cisplatin, and protracted infusion of 5-FU (ECF) in advanced intrahepatic cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2004;130(6):346–350. doi: 10.1007/s00432-003-0534-7. [DOI] [PubMed] [Google Scholar]
- 36.Park SH, Park YH, Lee JN, et al. Phase II study of epirubicin, cisplatin, and capecitabine for advanced biliary tract adenocarcinoma. Cancer. 2006;106(2):361–365. doi: 10.1002/cncr.21621. [DOI] [PubMed] [Google Scholar]
- 37.Park KH, Choi IK, Kim SJ, et al. The efficacy of epirubicin, cisplatin, uracil/tegafur, and leucovorin in patients with advanced biliary tract carcinoma. Cancer. 2005;103(11):2338–2343. doi: 10.1002/cncr.21041. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki T, Isayama H, Nakai Y, et al. A randomized Phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2013;71(4):973–979. doi: 10.1007/s00280-013-2090-4. [DOI] [PubMed] [Google Scholar]
- 39.Eng C, Ramanathan RK, Wong MK, et al. A Phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am. J. Clin Oncol. 2004;27(6):565–569. doi: 10.1097/01.coc.0000135924.94955.16. [DOI] [PubMed] [Google Scholar]
- 40.Gallardo JO, Rubio B, Fodor M, et al. A Phase II study of gemcitabine in gallbladder carcinoma. Ann. Oncol. 2001;12(10):1403–1406. doi: 10.1023/a:1012543223020. [DOI] [PubMed] [Google Scholar]
- 41.Okusaka T, Ishii H, Funakoshi A, et al. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2006;57(5):647–653. doi: 10.1007/s00280-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 42.Park JS, Oh SY, Kim SH, et al. Single-agent gemcitabine in the treatment of advanced biliary tract cancers: a Phase II study. Jpn J. Clin. Oncol. 2005;35(2):68–73. doi: 10.1093/jjco/hyi021. [DOI] [PubMed] [Google Scholar]
- 43.Penz M, Kornek GV, Raderer M, et al. Phase II trial of two-weekly gemcitabine in patients with advanced biliary tract cancer. Ann. Oncol. 2001;12(2):183–186. doi: 10.1023/a:1008352123009. [DOI] [PubMed] [Google Scholar]
- 44.Tsavaris N, Kosmas C, Gouveris P, et al. Weekly gemcitabine for the treatment of biliary tract and gallbladder cancer. Invest. New Drugs. 2004;22(2):193–198. doi: 10.1023/B:DRUG.0000011797.09549.53. [DOI] [PubMed] [Google Scholar]
- 45.Lin MH, Chen JS, Chen HH, Su WC. A Phase II trial of gemcitabine in the treatment of advanced bile duct and periampullary carcinomas. Chemotherapy. 2003;49(3):154–158. doi: 10.1159/000070622. [DOI] [PubMed] [Google Scholar]
- 46.Von Delius S, Lersch C, Schulte-Frohlinde E, Mayr M, Schmid RM, Eckel F. Phase II trial of weekly 24-hour infusion of gemcitabine in patients with advanced gallbladder and biliary tract carcinoma. BMC Cancer. 2005;5:61. doi: 10.1186/1471-2407-5-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahfouf H. Chemotherapy using gemcitabine plus cisplatin in locally in stage III and IV gallbladder and biliary tract. ASCO Abstract J. Clin. Oncol. 2010;28(15S):e14654. [Google Scholar]
- 48.Meyerhardt JA, Zhu AX, Stuart K, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig. Dis. Sci. 2008;53(2):564–570. doi: 10.1007/s10620-007-9885-2. [DOI] [PubMed] [Google Scholar]
- 49.Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann. Oncol. 2005;16(2):279–281. doi: 10.1093/annonc/mdi046. [DOI] [PubMed] [Google Scholar]
- 50.Doval DC, Sekhon JS, Gupta SK, et al. A Phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br. J. Cancer. 2004;90(8):1516–1520. doi: 10.1038/sj.bjc.6601736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am. J. Clin. Oncol. 2006;29(2):127–131. doi: 10.1097/01.coc.0000203742.22828.bb. [DOI] [PubMed] [Google Scholar]
- 52.Giuliani F. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a mulitcenter Phase II study of the GOIM. Ann. Oncol. 2006;17(Suppl. 7):vii73. doi: 10.1093/annonc/mdl956. [DOI] [PubMed] [Google Scholar]
- 53.Kim ST, Park JO, Lee J, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106(6):1339–1346. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- 54.Lee J, Park SH, Chang HM, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, Phase 3 study. Lancet Oncol. 2012;13(2):181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 55.Jang JS, Lim HY, Hwang IG, et al. Gemcitabine and oxaliplatin in patients with unresectable biliary cancer including gall bladder cancer: a Korean Cancer Study Group Phase II trial. Cancer Chemother. Pharmacol. 2010;65(4):641–647. doi: 10.1007/s00280-009-1069-7. [DOI] [PubMed] [Google Scholar]
- 56.Andre T, Reyes-Vidal JM, Fartoux L, et al. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a Phase II study. Br. J. Cancer. 2008;99(6):862–867. doi: 10.1038/sj.bjc.6604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harder J, Riecken B, Kummer O, et al. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br. J. Cancer. 2006;95(7):848–852. doi: 10.1038/sj.bjc.6603334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HEA. Phase II study of gemcitabine and oxaliplatin as first-line chemotherapy in patients with inoperable biliary tract cancer. ASCO Abstract J. Clin Oncol. 2009;26(15S):15536. doi: 10.1007/s00280-008-0883-7. [DOI] [PubMed] [Google Scholar]
- 59.Sharma A, Mohanti B, Raina V, et al. A Phase II study of gemcitabine and oxaliplatin (Oxigem) in unresectable gall bladder cancer. Cancer Chemother. Pharmacol. 2010;65(3):497–502. doi: 10.1007/s00280-009-1055-0. [DOI] [PubMed] [Google Scholar]
- 60.Lim H. Phase III study of gemcitabine/oxaliplatin GEMOX with or without erlotinib in unresectable, metastatic biliary tract carcinoma. ASCO Abstract J. Clin Oncol. 2011;29(18 S):LBA4032. [Google Scholar]
- 61.Phelip JEA. Multicentric Phase II randomized trial comparing chemoradiation with 5-fluorouracil, cisplatin and 50 GY versus chemotherapy alone with gemcitabine plus oxaliplatin for locally advanced biliary tract cancer. Ann. Oncol. 2012;3(9S):730P. [Google Scholar]
- 62.Uddin M. Phase II study of gemcitabine and oxaliplatin in unresectable gall bladder cancer. Ann. Oncol. Abstract. 2010;21(S 8):798. [Google Scholar]
- 63.Verderame F, Russo A, Di Leo R, et al. Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann. Oncol. 2006;17(Suppl. 7):vii68–72. doi: 10.1093/annonc/mdl955. [DOI] [PubMed] [Google Scholar]
- 64.Malka DEA. Gemcitabine and oxaliplatin (GEMOX) alone or in combination with cetuximab as first-line treatment for advanced biliary cancer: final analysis of a randomized Phase II trial (BINGO) ASCO abstract J. Clin Oncol. 2012;30(15 S):4032. [Google Scholar]
- 65.Julka PK, Puri T, Rath GK. A Phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary. Pancreat. Dis. Int. 2006;5(1):110–114. [PubMed] [Google Scholar]
- 66.Williams KJ, Picus J, Trinkhaus K, et al. Gemcitabine with carboplatin for advanced biliary tract cancers: a Phase II single institution study. HPB (Oxford) 2010;12(6):418–426. doi: 10.1111/j.1477-2574.2010.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Clinical trials establishing 5-fluorouracil, gemcitabine and platinum-based agents in a treatment of biliary tract carcinomas.
- 67.Cho JY, Nam JS, Park MS, et al. A Phase II study of capecitabine combined with gemcitabine in patients with advanced gallbladder carcinoma. Yonsei. Med. J. 2005;46(4):526–531. doi: 10.3349/ymj.2005.46.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alberts SR, Al-Khatib H, Mahoney MR, et al. Gemcitabine, 5-fluorouracil, and leucovorin in advancedbiliary tract and gallbladder carcinoma: a North Central Cancer Treatment Group Phase II trial. Cancer. 2005;103(1):111–118. doi: 10.1002/cncr.20753. [DOI] [PubMed] [Google Scholar]
- 69.Iqbal S, Rankin C, Lenz HJ, et al. A Phase II trial of gemcitabine and capecitabine in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma: Southwest Oncology Group study S0202. Cancer Chemother. Pharmacol. 2011;68(6):1595–1602. doi: 10.1007/s00280-011-1657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kanai M, Yoshimura K, Tsumura T, et al. A multi-institution Phase II study of gemcitabine/S-1 combination chemotherapy for patients with advanced biliary tract cancer. Cancer Chemother. Pharmacol. 2011;67(6):1429–1434. doi: 10.1007/s00280-010-1443-5. [DOI] [PubMed] [Google Scholar]
- 71.Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104(12):2753–2758. doi: 10.1002/cncr.21591. [DOI] [PubMed] [Google Scholar]
- 72.Bae BSE. Phase II study of fixed dose-rate infusion of gemcitabine and UFT combination chemotherapy in patients with advanced bile duct cancer: DAEGU GYEOUNGBUK oncology group. Ann. Oncol. 2010;21(Suppl. 8) Abstract. [Google Scholar]
- 73.Hsu C, Shen YC, Yang CH, et al. Weekly gemcitabine plus 24-h infusion of high-dose 5-fluorouracil/leucovorin for locally advanced or metastatic carcinoma of the biliary tract. Br. J. Cancer. 2004;90(9):1715–1719. doi: 10.1038/sj.bjc.6601796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iyer RV, Gibbs J, Kuvshinoff B, et al. A Phase II study of gemcitabine and capecitabine in advanced cholangiocarcinoma and carcinoma of the gallbladder: a single-institution prospective study. Ann. Surg. Oncol. 2007;14(11):3202–3209. doi: 10.1245/s10434-007-9539-9. [DOI] [PubMed] [Google Scholar]
- 75.Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a Phase II trial. J. Clin. Oncol. 2005;23(10):2332–2338. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 76.Lee SEA. Fixed dose rate infusion of gemcitabine and UFT combination chemotherapy in patients with advanced biliary cancer. ASCO Abstract J. Clin. Oncol. 2009;27(15S):e15581. [Google Scholar]
- 77.Nakamura K. A Phase II trial of oral S-1 combined with gemcitabine in patients with unresectable biliary tract cancer. ASCO Abstract J. Clin. Oncol. 2009;27(15S):e15527. [Google Scholar]
- 78.Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded Phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110(6):1307–1312. doi: 10.1002/cncr.22902. [DOI] [PubMed] [Google Scholar]
- 79.Santini D. Fixed dose rate FDR gemcitabine and capecitabine in patients with metastatic biliary tract cancer: Final results of Phase II trial. ASCO Abstract J. Clin. Oncol. 2009;27(15S):e15510. [Google Scholar]
- 80.Sasaki T. Multicenter Phase II study of gemcitabine plus S-1 in patients with advanced biliary tract cancer. ASCO Abstract J. Clin. Oncol. 2009;27(15S):e15516. [Google Scholar]
- 81.Ueno MEA. Randomized Phase II trial of gemcitabine plus S-1 combination therapy versus S-1 in advanced biliary tract cancer: Results for the Japan Clinicla Oncology Group study. ASCO Abstract J. Clin. Oncol. 2012;30(Suppl. 15):4031. [Google Scholar]
- 82.Wagner AD, Buechner-Steudel P, Moehler M, et al. Gemcitabine, oxaliplatin and 5-FU in advanced bile duct and gallbladder carcinoma: two parallel, multicentre Phase-II trials. Br. J. Cancer. 2009;101(11):1846–1852. doi: 10.1038/sj.bjc.6605377. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Phase II clinical trials investigating gemcitabine-based combinations with 5-fluorouracil in biliary tract carcinomas.
- 83.Alberts SR, Sande JR, Foster NR, et al. Pemetrexed and gemcitabine for biliary tract andgallbladder carcinomas: a North Central CancerTreatment Group (NCCTG) Phase I and II Trial, N9943. J. Gastrointest. Cancer. 2007;38(2-4):87–94. doi: 10.1007/s12029-008-9037-8. [DOI] [PubMed] [Google Scholar]
- 84.Chung MJ, Kim YJ, Park JY, et al. Prospective Phase II trial of gemcitabine in combination with irinotecan as first-line chemotherapy in patients with advanced biliary tract cancer. Chemotherapy. 2011;57(3):236–243. doi: 10.1159/000328021. [DOI] [PubMed] [Google Scholar]
- 85.Karachaliou N, Polyzos A, Kentepozidis N, et al. A multicenter Phase II trial with irinotecan plus oxaliplatin as first-line treatment for inoperable/metastatic cancer of the biliary tract. Oncology. 2010;78(5–6):356–360. doi: 10.1159/000320462. [DOI] [PubMed] [Google Scholar]
- 86.Feisthammel J, Schoppmeyer K, Mossner J, Schulze M, Caca K, Wiedmann M. Irinotecan with 5-FU/FA in advanced biliary tract adenocarcinomas: a multicenter Phase II trial. Am. J. Clin. Oncol. 2007;30(3):319–324. doi: 10.1097/01.coc.0000258124.72884.7a. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg RM. N9741: a Phase III study comparing irinotecan to oxaliplatin-containing regimens in advanced colorectal cancer. Clin. Colorectal Cancer. 2002;2(2):81. doi: 10.1016/S1533-0028(11)70509-6. [DOI] [PubMed] [Google Scholar]
- 88.Findlay M, Cunningham D, Norman A, et al. A Phase II study in advanced gastro-esophageal cancer using epirubicin and cisplatin in combination with continuous infusion 5-fluorouracil (ECF) Ann. Oncol. 1994;5(7):609–616. doi: 10.1093/oxfordjournals.annonc.a058932. [DOI] [PubMed] [Google Scholar]
- 89.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008;358(1):36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 90.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369(18):1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen LH, Larsen O. Systemic treatment of biliary tract cancer. Ugeskr. Laeger. 2013;175(42):2478–2481. [PubMed] [Google Scholar]
- 92.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann. Oncol. 1996;7(6):593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 93.Yonemoto N, Furuse J, Okusaka T, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J. Clin. Oncol. 2007;37(11):843–851. doi: 10.1093/jjco/hym116. [DOI] [PubMed] [Google Scholar]
- 94.Sasaki T, Isayama H, Nakai Y, Koike K. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean. J. Intern. Med. 2013;28(5):515–524. doi: 10.3904/kjim.2013.28.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cereda S, Belli C, Rognone A, Mazza E, Reni M. Second-line therapy in advanced biliary tract cancer: what should be the standard? Crit. Rev. Oncol. Hematol. 2013;88(2):368–374. doi: 10.1016/j.critrevonc.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 96.Walter T, Horgan AM, Mcnamara M, et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur. J. Cancer. 2013;49(2):329–335. doi: 10.1016/j.ejca.2012.08.003. [DOI] [PubMed] [Google Scholar]