Abstract
Oxytocin (OXT) suppresses food intake and lack of OXT leads to overconsumption of sucrose. Taste bud cells were recently discovered to express OXT-receptor. In the present study we tested whether administering OXT to wild-type mice affects their licking behavior for tastants in a paradigm designed to be sensitive to taste perception. We injected C57BL/6J mice intraperitoneally (i.p.) with 10 mg/kg OXT and assayed their brief-access lick responses, motivated by water deprivation, to NaCl (300 mM), citric acid (20 mM), quinine (0.3 mM), saccharin (10 mM), and a mix of MSG and IMP (100 mM and 0.5 mM respectively). OXT had no effect on licking for NaCl, citric acid, or quinine. A possible effect of OXT on saccharin and MSG+IMP was difficult to interpret due to unexpectedly low lick rates to water (the vehicle for all taste solutions), likely caused by the use of a high OXT dose that suppressed licking and other behaviors. A subsequent experiment focused on another preferred tastant, sucrose, and employed a much lower OXT dose (0.1 mg/kg). This modification, based on our measurements of plasma OXT following i.p. injection, permitted us to elevate plasma [OXT] sufficiently to preferentially activate taste bud cells. OXT at this low dose significantly reduced licking responses to 0.3 M sucrose, and overall shifted the sucrose concentration – behavioral response curves rightward (mean EC50saline = 0.362 M vs. EC50OXT = 0.466 M). Males did not differ from females under any condition in this study. We propose that circulating oxytocin is another factor that modulates taste-based behavior.
Keywords: licking, brief-access test, gustation, oxytocin, sweet
1. Introduction
Oxytocin (OXT) is a hypothalamic peptide known for facilitating parturition, lactation, and prosocial behaviors [1]. OXT also serves as a satiety peptide [2-4] in the central nervous system. Intracerebroventricular (i.c.v, 1-10 μg) and intraperitoneal (i.p., 0.4-6 mg/kg) injections of OXT in fasted rats reduced, in dose-dependent fashion, the amount of food consumed, the number of feeding bouts, and the time spent feeding, and also increased the latency to initiate feeding after food was reintroduced [5, 6]. The same doses of OXT also reduced food consumption in non-fasted rats [7]. These effects were blocked by an OXT receptor antagonist, and in some studies an oxytocin receptor antagonist by itself increased intake of food or nutritive solutions [5-9]. OXT was equally effective in male and female rats [10].
Further evidence for the role of OXT in feeding behavior comes from study of OXT-deficient (OXT-/-) mice. Such OXT-/- mice overconsume sucrose and carbohydrate solutions (such as starch and Polycose), but not isocaloric lipids [11-14]. Normally, rodents find polysaccharides such as starch palatable but discriminable in taste from simple sugars such as sucrose and glucose [15, 16]. OXT-/- mice also overconsume the artificial sweetener, saccharin [12]. Pharmacologically blocking the OXT receptor had a similar effect, with several sugars and other carbohydrates being overconsumed [8]. That is, OXT influences consumption in a taste-quality selective manner. Sclafani and coauthors [14] reported that in spite of overconsuming sucrose, OXT-/- mice will not exert additional effort to obtain it. This finding, using a progressive ratio paradigm, suggested that OXT alters only satiety rather than the hedonic valuation of sweet-tasting sucrose.
Potential central sites of action include the nucleus of the solitary tract, where OXT is necessary for the satiety hormones leptin and cholecystokinin to be fully effective [17-21], and areas of the hypothalamus known to be involved in feeding behavior, such as the ventromedial hypothalamus and supraoptic nucleus. The ventromedial hypothalamus contains subpopulations of neurons, some of which may be OXT responsive and may reduce feeding when activated [22, 23]. OXT is known to be released from the dendrites of magnocellular neurons in the nearby supraoptic nucleus [24]. Indeed, a recent report concluded that OXT microinjected into the ventromedial hypothalamus was sufficient to suppress feeding while also increasing energy expenditure of Sprague-Dawley rats [25].
We previously reported that OXT receptor is expressed in a subset of murine taste bud cells and that OXT elicits calcium responses from those same cells [26]. Other investigators have shown OXT receptor expression (and OXT-elicited calcium responses) in a cell line derived from human fungiform papillae [27]. While peripheral receptors are unlikely to play a role in behavioral responses following i.c.v. injection, we considered it possible that in the physiological state, central and peripheral OXT may work together to reduce feeding behavior following endogenous OXT elevation. If so, peripheral effects via taste signaling may have contributed to any reduction in feeding behavior following i.p. injection of exogenous OXT and to increased consumption of sweet solutions in OXT-/- mice. More specifically, OXT may depress the perceived intensity of sweet-tasting solutions, thereby reducing its hedonic appeal, and assisting central satiety mechanisms to decrease consumption.
As a first attempt to investigate potential OXT-induced alterations in gustatory responsiveness, we injected mice with either vehicle or OXT and then studied immediate responses to small volumes of taste solutions in a brief-access assay [28-32]. While this procedure does not discriminate the site of action of OXT (i.e., central or peripheral), the behavioral task is considered to be minimally influenced by post-ingestive signals and guided substantially by orosensory processes because mice are only briefly engaged with the taste solutions [28, 33-37]. Because circulating OXT is rapidly metabolized [38], the brevity of this assay also minimizes the possibility that serum levels of OXT will dramatically decrease over the course of the behavioral session.
2. Materials and Methods
2.1 Animals
Fifty-seven adult, C57BL/6J mice of both sexes were used in 3 experiments (reported below). Mice were obtained directly from Jackson Laboratory (Bar Harbor, ME) or bred in-house from these mice. All mice were under the husbandry of the Division of Veterinary Resources of the University of Miami School of Medicine (and housed in AAALAC certified facilities) or the Rollins College Animal Colony. All procedures on mice were approved by the Institutional Animal Care and Use Committees of the University of Miami School of Medicine or Rollins College, and conformed to NIH guidelines for the ethical treatment of experimental animals.
2.2 Behavioral testing apparatus
Behavioral experiments were carried out in a Davis MS-160 lickometer (DiLog Instruments, Tallahassee, FL) similar to those previously described [29, 30, 36]. Briefly, the Davis Rig [36] consists of a rodent cage with an opening in one wall permitting access to a sipper tube housed on a motorized tray. The opening can be blocked by a computer-controlled shutter door limiting access between trials, and the motorized tray can be automatically moved while the shutter is closed to position any of several sipper tubes opposite the opening in successive trials. During trials, licks are detected by a contact circuit and the onset time of each lick stored for later analysis. Trials were 5 s in duration beginning from the mouse's first lick. That is, all trials consisted of one or more licks.
2.3. Experiment 1: Multi-tastant panel
Twelve mice (6 of each sex) took part in the first experiment. Twenty-four hours before the first training session, water bottles were removed from the home cages and were not returned until after the entire experiment was completed. 1 mL of supplemental water was provided in the home cage daily after sessions.
For the first two training sessions (“spout training”), the lickometer was equipped with a single stationary bottle of water. The purpose of these sessions was to allow mice to become familiar with the testing environment and learn to access fluid in the Davis Rig lickometer. Mice had access to water for a single, 20 min trial. If a mouse initiated fewer than 50 licks, it was retrained later the same day.
For the next two training sessions (“trial training”), the lickometer was equipped with 6 bottles, all containing water only. The purpose of these sessions was to allow the mice to become familiar with the noises and movements of the lickometer and to learn to sample repeatedly from bottle spouts presented in brief (5 s), successive trials. Mice could initiate up to 24 trials (with each of the 6 bottles presented in a randomized block design) during a 20 min session. In between trials, the shutter door remained closed for 10 s.
The day after the fourth training session, experimental testing began. These six sessions (“test sessions”) occurred over a span of 6 consecutive days. Thirty minutes before each session, mice were given an intraperitoneal (i.p.) injection of either Hank's balanced salt solution (“saline”) or 10 mg/kg OXT in saline, using an injection volume of 10 μL/g body weight. We used i.p. injection as a route for delivery as it achieves prolonged elevation of circulating OXT levels. The dose of OXT was selected based on a previous study showing it to be effective in modifying a range of behaviors [30]. Saline and OXT sessions alternated over the six days, with half of the mice of each sex beginning the series with saline and the other half beginning the series with OXT.
The six test sessions were identical to the last two training sessions except the six bottles were filled with 300 mM NaCl, 20 mM citric acid, 0.3 mM quinine-HCl, 10 mM Na-saccharin, 100 mM monosodium glutamate (MSG) + 0.5 mM inosine monophosphate (IMP), and distilled water. Concentrations were chosen from previous work as those that elicit approximately the half-maximal behavioral response in mice [25-29]. Each tastant was presented exactly once before it was presented again in a new random sequence for a maximum of 24 trials per session.
2.4. Experiment 2: Quantification of plasma oxytocin
To estimate the effect of our injections on circulating plasma oxytocin levels, 18 mice (3 per dose) were injected i.p. with OXT (0, 0.1, 0.3, 1, 3, or 10 mg/kg). Thirty minutes later 50 - 100 μL of blood was drawn from the facial vein. Blood was collected in EDTA and aprotinin-coated capillaries and placed on ice. Tubes were centrifuged for 15 minutes at 1600g at 4 °C, and plasma was recovered and either assayed immediately or stored at -20 °C.
OXT concentration in plasma was measured with an Oxytocin ELISA kit by AssayDesigns according to the manufacturer's instructions. Optical density was measured using a Wallac Victor 1420 multilabel counter (PerkinElmer, Waltham, MA).
2.5. Experiment 3: Sucrose concentration panel
A second behavioral experiment with 27 mice focused on sucrose. All mice were naive to sweet tastants before the first test session. Because thirsty mice lick all concentrations of sucrose at near-maximal lick rates (c.f., behavior toward saccharin in Experiment 1), it was necessary to test mice after only minimal fluid restriction. As described below, we used the partial food/partial water restriction scheme of Glendinning and colleagues to motivate sampling of the taste solutions [30-32].
Davis Rig training was identical to Experiment 1, except that 7 water bottles were used during trial training rather than 6, to accommodate the slightly larger stimulus array to be used during the test sessions. During test sessions, each mouse was presented six concentrations of sucrose (0.03, 0.1, 0.2, 0.3, 0.6, and 1 M) and distilled water in a randomized block design. Mice could initiate an unlimited number of trials in these 30 min sessions. The inter-presentation interval was 7.5 s.
During these test sessions, mice were divided into two groups (referred to as Experiment 3A consisting of 12 mice, 7 of which were male and Experiment 3B consisting of 15 mice, 8 of which were male). Both sets of mice were tested in 3 test sessions over a span of 6 days with one “off day” preceding each test day in order to implement the partial food and water restriction schedule. Twenty-four hours prior to each test session, mice were restricted to 1 g of chow and 2 mL of water.
For Experiment 3A mice, all three test sessions were identical. The purpose of this experiment was to assess whether multiple exposures to sucrose produced changes in behavioral responses over the 3 test sessions. For Experiment 3B mice, test sessions were preceded by i.p. injection of either saline or 0.1 mg/kg OXT dissolved in saline 30 minutes prior to the beginning of the test session (this lower dose was guided by the results of Experiment 2). Session 1 and 3 were preceded by saline injections; Session 2 by OXT injection. Injection volume was 10 μL/g body weight.
2.6. Chemicals
All tastants and buffer components were purchased from Sigma-Aldrich (St. Louis, MO). Taste stimuli were made in distilled water. OXT was purchased from Tocris Bioscience (Minneapolis, MN).
2.7. Data Analyses
For Experiment 1, the average licks to a tastant over the 3 sessions in a particular condition (saline or OXT) were divided by the average licks to water in those sessions on an individual mouse basis. In thirsty animals, this taste/water ratio typically ranges from near zero (complete avoidance) to 1 (maximal licking), and the standardization to water licking provides a means of controlling for differences in maximal lick rate and motivation across animals. Paired t-tests were used to explore differences in the taste/water ratio between saline and OXT conditions. A preliminary analysis tested for possible differences between males and females using a 2-way analysis of variance (ANOVA) with Sex and Tastant (5 levels) as factors.
For Experiment 3, average lick rates for each concentration of sucrose under each session (saline, OXT, or no injection in the case of control mice) were standardized using the standardized lick ratio (SLR) as described by Glendinning and colleagues [30]. The average licks to a given concentration of sucrose for a given condition was divided by each mouse's maximal 5-s lick rate, as determined by multiplying the reciprocal of the average interlick interval recorded on the first day of training by 5. The SLR serves the same standardization function as the taste-water ratio from Experiment 1. Taste/water ratios are appropriate for the standardization of lick rates to aversive stimuli in thirsty animals (where water represents the maximal lick rate), whereas the SLR is appropriate for the standardization of lick rates to preferred stimuli under conditions of no or partial fluid restriction (where a water denominator would be low and not reflective of the animal's maximal lick rate) [30, 31, 39]. A two-way analysis of variance (ANOVA) was used to test for main effects and interactions of the independent variables Treatment and Concentration.
Dose-response relationships for plasma OXT (Experiment 2) were fit with a linear regression using Prism (GraphPad Software, LaJolla, CA), and behavioral data (Experiment 3B) were fit with a nonlinear regression in Sigma Plot 12.5 (Systat Software Inc., Chicago, IL) based on the Hill Equation:
where b is a parameter representing the slope, c (hereafter, EC50) is the concentration for which the SLR is midway between the lower and upper asymptote, d is a parameter representing the lower asymptote, and x is concentration of sucrose. The upper asymptote was set to 1.0. The lower asymptote was modeled because the SLR has no theoretical lower value (it must be higher than zero but cannot be zero because a trial requires at least one lick), whereas the upper asymptote has a theoretically specified value (1.0 at the mouse's maximal lick rate) which can be achieved in practice.
Significant changes in these parameters were assessed by paired t-test (following a log transform in the case of the EC50). One mouse was removed from the regression analysis for an outlying low r2 value indicating a poor curve fit (r2 = 0.68; r2 > 0.95 for the remaining mice). (This mouse was included in the ANOVA.) The outcome of statistical tests of curve fit parameters or the ANOVA were not altered by presence or removal of this one case. Because data were variable during Session 1 in Experiment 3A, we do not report the results of the curve-fitting in that experiment (one mouse's data could not be fit and several remaining mice had poor goodness-of-fit values in Session 1 only).
The statistical rejection criterion (a) was set to 0.05 for all analyses (post-hoc tests, where used, were Bonferroni-corrected for multiple comparisons).
3. Results
3.1. Experiment 1: OXT does not modulate lick responses to aversive tastants
OXT, injected i.p. decreases food intake [5, 6] and OXT-/- mice overconsume sweet solutions. Thus, we asked if circulating OXT at levels similar to previous studies affects taste responses to aversive (bitter, sour, salty) and appetitive (sweet, umami) stimuli. Lick rate to tastants was normalized to the lick rate for water.
Males and females did not differ in their responses to tastants, whether they were injected with saline (Sex: F(1,10) = 3.94, p = 0.075; Tastant, F(4,40) = 105.11, p < 0.0001; Sex × Tastant, F(4,40) = 0.63, p = 0.64) or OXT (Sex: F(1,10) = 0.36, p = 0.56; Tastant, F(4,40) = 42.68, p < 0.0001; Sex × Tastant, F(4,40) = 0.38, p = 0.82). Hence, data from males and females in this series were pooled.
As assessed by taste/water lick ratios, licking behavior to NaCl (t(11) = -0.686, p = 0.507), citric acid (t(11) = -0.475, p = 0.644), and quinine (t(11) = 1.266, p = 0.232) was unaffected by OXT injection (Fig. 1A). In contrast, OXT appeared to increase the attractiveness of saccharin (t(11) = -3.691, p = 0.0036) and MSG+IMP (t(11) = -2.468, p = 0.031), which are both normally preferred substances (Fig. 1B). Rather than reflecting an increase in licking these preferred tastants, however, the elevated ratios were actually caused by a greatly reduced lick rate to water (Fig. 1C). If assessed by raw lick rates, when OXT-injected, mice reduced licking water (t(11) = 11.425, p < 0.00001), saccharin (t(11) = 5.580, p = 0.00017), and MSG+IMP (t(11) = 6.174, p = 0.000070). Because lick rates to water were reduced somewhat more substantially than lick rates to the preferred stimuli, saccharin or MSG-IMP, an apparent opposite effect of OXT on these substances was seen than when assessing taste/water ratios (compare Fig. 1B and 1C).
Figure 1. Oxytocin (OXT) does not alter taste responses to aversive tastants.
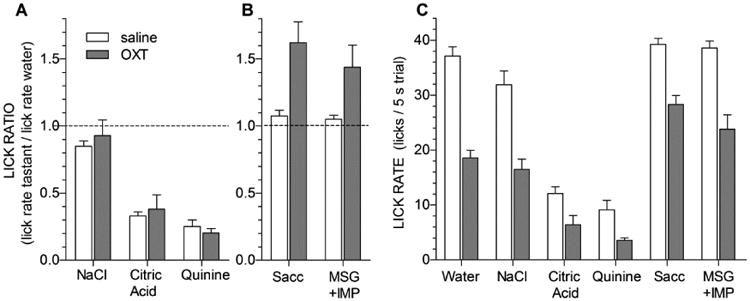
In Experiment 1, 12 water-deprived mice were injected intraperitoneally (i.p.) with saline (open bars) or 10 mg/kg OXT (gray bars) 30 minutes prior to behavioral testing in a lickometer. Data are combined across 3 identical test sessions for each injection condition. Values represent mean (+ s.e.m.) of lick ratios (i.e. lick rates for tastants relative to water lick rates in A, B) or raw lick rates during 5 s trials (in C). The dashed line in A and B represents a tastant/water ratio of 1.0. Mice were randomly presented with NaCl (300 mM), citric acid (20 mM), quinine (0.3 mM), sodium-saccharin (10 mM), and mixtures of monosodium glutamate (100mM) plus inosine monophosphate (0.5 mM) during 20-min test sessions. A, There were no differences in standardized lick ratios between OXT and saline conditions for NaCl, citric acid, and quinine. B, However, OXT increased licking of saccharin and MSG+IMP relative to water. This effect, however, may have been due to OXT significantly reducing licking for water, which is the denominator of the taste/water ratio, rather than an effect on taste perception per se. C, Raw Lick rates for water and all taste stimuli were depressed following OXT injection i.p.
Given the thirst-reducing effect of OXT, we considered whether injected OXT persistently alters drinking behavior across the time-frame of our experiment. Comparing lick rates to water across consecutive test sessions (Fig. 2), we found that the lick rate to water was similar in all three of the sessions that mice were injected with saline. And, all mice showed similarly depressed lick rates for water in all 3 sessions where OXT was injected. These conclusions were supported by a Drug × Session repeated measures ANOVA; there was a significant effect of Drug (F(1,7) = 47.993, p = 0.00023) but no effect of Session (F(2,14) = 0.084, p = 0.92) and no Drug × Session interaction (F(2,14) = 0.168, p = 0.847; degrees of freedom reflect 4 cases missing due to lack of any behavior during some OXT sessions). Thus, as expected from its short life-time in blood, the OXT-induced reduction of licking was entirely reversed within one day and did not carry over from one session to the next.
Figure 2. Behavioral effects of OXT injections wash out within one day.
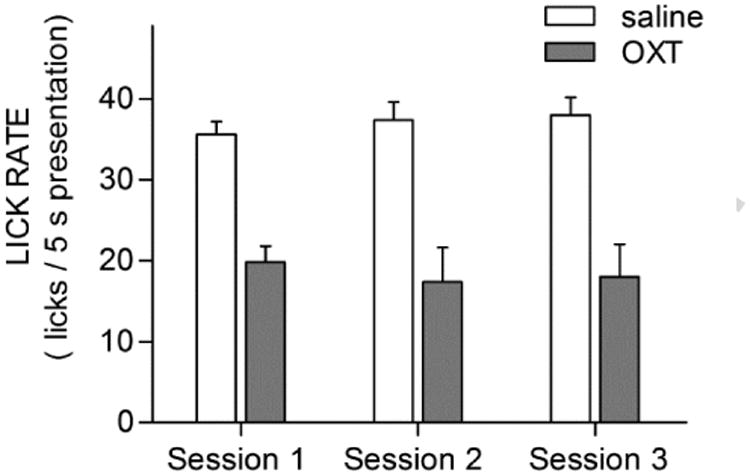
Values are mean + s.e.m. of raw lick rates for water broken down by successive test sessions for saline or OXT injections in Experiment 1. The lick rate for water was similar in all three of the sessions that mice were injected with saline (34 ± 2, 35± 3, 35± 4). This is in spite of the fact that most saline-injected sessions occurred one day after an OXT injection session. All mice also showed similarly depressed lick rates for water in all three session where OXT was injected (19± 3; 18± 4; 18± 5). That is, the licking behavior of mice related to the injection condition (saline vs. OXT) on the day of the test and there was no carry-over of an earlier day's OXT injection.
Taking these data into account, it appears that aversive tastants were not affected by OXT (as licking to these tastants was reduced in proportion to licks to water). It is more difficult to interpret whether OXT affected licking to saccharin and MSG+IMP, particularly because the lick rate for saccharin and MSG+IMP in saline-injected animals may have been constrained by a ceiling effect. If so, then the fact that licking to these compounds was reduced less by OXT than licking to water could simply be an artifact of this ceiling effect.
In addition to these effects on lick rate, OXT also significantly reduced the total number of trials initiated over the 3-days of testing (34.333 ± 2.664) relative to the saline condition (66.667 ± 2.853; t(11) = 9.274, p = 0.0000020), indicated that OXT may have had a general suppressive effect on behavior. These concerns prompted us to study whether lower injection doses of OXT might be used which did not produce a general inhibition on behavior (see Section 3.2) and may reveal any taste-selective effects of OXT if they exist.
3.2. Experiment 2: Circulating [OXT] varies predictably with injected dose
In Experiment 2, we injected various doses of OXT and measured [OXT] available peripherally after 30 min (the same time-frame as in our behavioral tests). Plasma [OXT] increased linearly with injected dose (r2 = 0.87, Fig. 3). The plasma [OXT] for mice injected with vehicle only was ∼0.1 nM, with a range of 0.01 to 0.49 nM, similar to the 0.02 to 0.1 nM value reported in other rodent studies [38, 40, 41]. On the other hand, the EC50 for central OXT-responsive neurons is ∼22 nM and ∼33 nM for OXT-responsive taste cells [26, 41]. Thus, we selected an i.p. dose of 0.1 mg/kg for our second behavioral experiment, with evoked plasma oxytocin concentrations of ∼10 nM (c.f., Fig. 3). The 10 mg/kg dose used in in our first experiment, similar to earlier studies [5], resulted in plasma OXT concentrations well above the physiological range for peripheral effects.
Figure 3. Oxytocin (OXT) injections elevate plasma OXT levels in a dose-dependent manner.
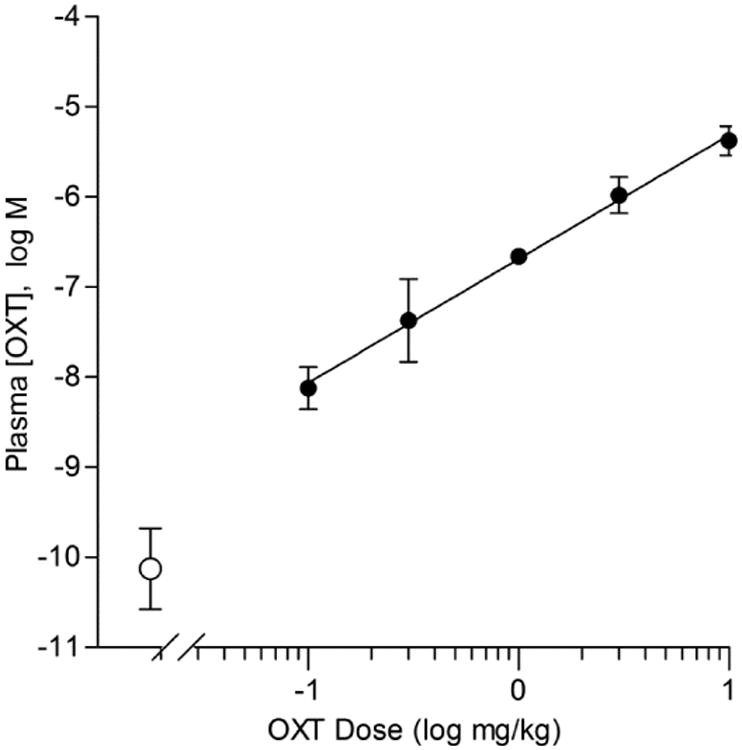
Mean (± s.e.m.) plasma OXT, measured 30-min following i.p. injections of OXT at 5 doses (filled symbols). Each point is a mean of 3 mice. Open symbols are values obtained from uninjected control mice. The solid line is a linear regression (r2 = 0.87, slope = 1.375 ± 0.145, y-intercept = -6.692 ± 0.103) for logarithmically-transformed concentrations of [plasma OXT] vs. dose of OXT injected.
3.3. Experiment 3A: Licking for sucrose increases with repeated exposures
In Experiment 3, mice were tested behaviorally after partial food- and-water restriction to avoid the ceiling effect for palatable tastants encountered above. This paradigm does not permit using water licking as a gauge; instead, we used “standardized lick ratios, SLR” (see Methods) to compare across the population. We found that an uninjected control group altered their behavior to a sucrose-concentration series over 3 consecutive sessions (Fig. 4). In all sessions, mice increased licking for sucrose in a concentration-dependent manner, but the concentration-response function became steeper, and the maximal lick rate higher, during Sessions 2 and 3 relative to Session 1. This was supported by a significant Concentration × Session interaction (with water included as a concentration: F(12,132) = 5.392, p <0.0001; without water included as a concentration: F(10,110) = 5.745, p <0.0001). These interactions remained highly significant when comparing Session 1 to either Session 2 or 3 in separate ANOVAs, but there were no differences between Session 2 and 3 for Session (F(1,11) = 1.203, p = 0.296) or the Concentration × Session interaction (F(6,66) = 0.647, p = 0.692). A possible explanation is that mice develop greater contrast between sucrose concentrations after the first session; i.e., they anticipate higher, sweeter concentrations of sucrose and sample less from lower, less sweet concentrations. By Session 2, behavior is stable.
Figure 4. Mice naive to sucrose modify their taste responses after the first exposure.
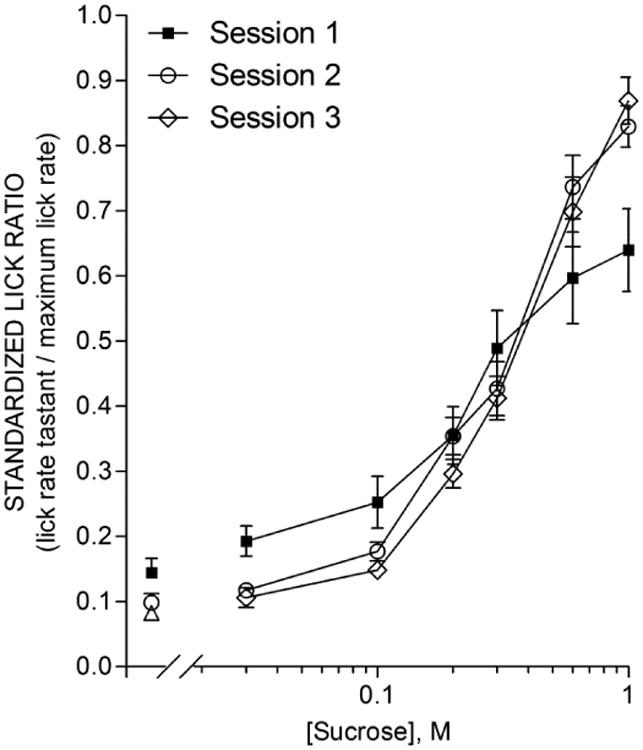
Mean (± s.e.m.) licks to sucrose of 12 mice in each of 3 consecutive sessions separated by 48 h. Sucrose concentrations (0, 0.03, 0.1, 0.2, 0.3, 0.6, and 1.0 M) were presented in random 5-s trials. Lick rates are presented as the standardized lick ratio (see Methods). The Session 1 lick-concentration function was flatter than that for Session 2 and 3, as supported by a significant Session × Concentration interaction (F(12,132) = 5.39, p <0.0001) which also appeared when comparing just Session 1 and 2 or just Session 1 and 3 but disappeared when comparing just Session 2 and 3.
As in the first behavioral experiment, we confirmed that there were no differences between males and females when each session was examined separately (all p-values > 0.23 for main effect of Sex and Sex × Concentration interaction).
3.4. Experiment 3B: OXT reduces licking to sucrose
Experiment 3A above demonstrated that, under control conditions, licking behavior to sucrose changes from Session 1 to Session 2, but not from Session 2 to Session 3. In Experiment 3B, therefore, we compared the behavior in Session 2 (OXT injection) to Session 3 (saline injection) with the expectation that any differences in behavior between these sessions could be attributed to the type of injection, rather than time- or experience-based effects. In addition, we used a lower OXT dose than in Experiment 1, to activate any influence upon the peripheral taste system that may occur physiologically. Our expectation was also that using a lower OXT dose might not cause the indiscriminate reduction of licking seen in Experiment 1.
OXT-injected mice consumed 1.0 M sucrose at near-maximal lick rates (Figure 5; licking near an SLR of 1.0), suggesting that they were capable of licking at rates as high as saline-injected mice. In addition, OXT-injected mice actually initiated more (t(14) = 5.459, p = 0.000084) total taste trials (52.667 ± 2.441) than saline-injected mice (41.600 ± 1.817), reinforcing the notion that the lowered OXT dose used in Experiment 3 did not suppress licking behavior as the higher dose did in Experiment 1.
Figure 5. Oxytocin (OXT) decreases licking for sucrose.
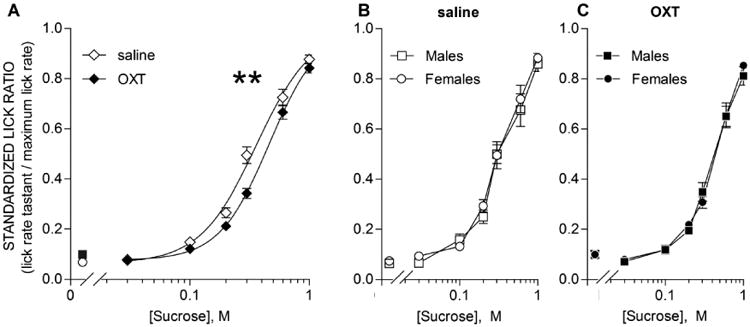
Mean (± s.e.m.) licks to sucrose of 15 mice injected (i.p.) with saline or 0.1 mg/kg OXT 30-min prior to behavioral sessions. Sucrose concentrations (0, 0.03, 0.1, 0.2, 0.3, 0.6, and 1.0 M) were presented in random 5-s trials. Lick rates were standardized using the standardized lick ratio (see Methods). A, relative to saline (open symbols), licking for sucrose was significantly reduced by OXT (closed symbols). ** denotes main effect of Treatment: F(1,14) = 20.10, p = 0.00052; Treatment × Concentration interaction: F(6,84) = 8.60, p <0.0001). Post-hoc t-tests (Bonferroni correction) between OXT and saline at each concentration reveal a significant difference at 0.3 M (t(14) = 5.13, p = 0.0011). To better understand effects across the full concentration range, 3-parameter sigmoidal functions (solid lines) were fit to the concentration-response data for each animal. The lower asymptote and slope were unaffected by OXT, but the EC50 was significantly shifted by 0.109 log units (t(13) = -4.290, p= 0.00088; one mouse removed for poor curve fit). B, C, male (squares) and female (circles) mice are compared for the saline session and OXT session, respectively. No differences were found.
Licking for sucrose (Fig. 5A) was slightly but significantly reduced by OXT injection (main effect of Treatment: F(1,14) = 20.10, p = 0.00052; Treatment × Concentration interaction: F(6,84) = 8.60, p <0.0001). Post-hoc t-tests (with a Bonferroni correction for multiple comparisons) between OXT and saline at each concentration revealed a significant reduction in sucrose licking at 0.3 M (t(14) = 5.13, p = 0.0011) and a non-significant trend at 0.2 M.
To better appreciate the effects across the entire concentration range, sigmoidal curves were fit to the concentration-SLR functions for each mouse in each condition (OXT or saline). The b-parameter (slope) and d-parameter (lower asymptote) were unaffected by OXT. The EC50, however, was significantly right-shifted by 0.109 log units (t(13) = -4.290, p= 0.00088), a finding that reinforces the results of the ANOVA. Average curve fit parameters are shown in Table 1.
Table 1. Mean (± s.e.m.) values of parameters of sigmoidal curve fits of sucrose concentration-SLR data from Experiment 3B1.
| Parameter | Saline | Oxytocin | t(13)2 | p |
|---|---|---|---|---|
| log EC50 | -0.4413 (0.0291) | -0.3318 (0.0192) | -4.290 | 0.00088 |
| EC503 | 0.362 M | 0.466 M | ||
| Slope (b) | 2.1096 (0.1753) | 2.3292 (0.2044) | -0.918 | 0.376, n.s. |
| Lower (d) | 0.0683 (0.0114) | 0.0800 (0.0077) | -0.831 | 0.421, n.s. |
See Method for details of the nonlinear regression.
Results of a paired t-test (saline vs. oxytocin condition) with 13 degrees of freedom.
The mean EC50 in given in linear units for reference; t-test was conducted on log-transformed values for concentration.
Subsidiary analyses revealed that males and females did not differ in their responses to sucrose, whether they were injected with saline (Fig. 5B) or OXT (Fig. 5C).
4. Discussion
Given the recent discovery of OXT receptors in taste buds [26, 27], we examined the effect of peripheral OXT injection on ingestive behavior towards a variety of taste solutions in a taste-salient behavioral paradigm. Overall, OXT did not seem to alter licking behavior for NaCl, citric acid, and quinine, though the high doses of OXT used in our first experiment reduced licking to water and therefore may have had nonspecific effects on ingestion. A second behavioral experiment using lower OXT doses did not produce detectable general effects, and did reduce the licking behavior of mice for sucrose, right-shifting the lick-concentration function 0.109 log units. Thus, OXT may influence gustatory processing for sucrose, although other mechanisms of action are also consistent with our data.
In our first experiment, we used water restriction to motivate stimulus sampling. Water restriction encourages mice to lick unpalatable tastants that they otherwise would avoid, increasing the number of trials mice initiate, and decreasing variability in lick rates across sessions [28, 29]. In Experiment 1, OXT did not alter acceptability of moderate concentrations of NaCl, citric acid, and quinine. Interpretation of the potential effects of OXT on palatable stimuli, saccharin and MSG+IMP, however, were complicated by the fact that OXT reduced licking to water. Taste/water ratios for saccharin and MSG-IMP actually increased following OXT injection, whereas the raw lick rates decreased (but decreased more to water). Furthermore, because licking to saccharin and MSG+IMP in the saline-injected mice was already near maximum, due to water restriction, mice may have been prevented from licking more in these sessions to saccharin and MSG+IMP (i.e., a ceiling effect). If so, then the greater reduction of licking of water compared to the palatable stimuli may represent merely an artifact of this ceiling effect.
These results prompted us to alter our procedure in two ways: first, to attempt to find a lower dose of OXT that elevated plasma oxytocin levels only to physiological concentrations; and second, to study licking to preferred substances under conditions of lower thirst levels, to minimize the influence of OXT on physiological state and to assess behavior at lower concentrations of tastants while avoiding ceiling effects in lick rates caused by pronounced thirst.
In Experiment 2, we identified a lower OXT injection dose (0.1 mg/kg) that elevated plasma OXT levels to the low nM range which we had earlier found were effective for activating taste bud cells [26]. The lower dose could reasonably be expected to minimize or eliminate the non-specific effects on licking behavior. Indeed, in Experiment 3, mice did not exhibit the generalized depression of lick rates (to water and taste stimuli) as seen in Experiment 1. In addition, rather than motivating mice with complete removal of water, we adopted a partial water/partial food deprivation paradigm that was shown previously to be effectively for appetitive taste stimuli [30, 31]. The partial restriction encourages mice to sample the drinking spout during behavioral sessions. Licking behavior after the sampling is concentration-dependent (i.e., low lick rates to weak concentrations; higher lick rates to higher concentrations), indicating that thirst levels are mild and licking behavior is largely controlled by stimulus detection and palatability.
We assessed (Experiment 3A) how licking behavior toward sucrose changed over three consecutive sessions, 48 hours apart. The avidity of licking sucrose can also be influenced by several factors that might change over time. Examples include the elimination of neophobia [42, 43], enhancement of contrast effects between low and high concentrations as a result of learning [31, 32, 44-46], or conditioned flavor preferences established by pairing the taste of sucrose to postingestive metabolic rewards in mildly food-restricted mice [47]. Our observation that the concentration-response functions for sucrose became steeper from Session 1 to Session 2 is consistent with the development of anticipatory contrast. Regardless of the mechanism, our experiment demonstrated that, in our paradigm, licking for sucrose changed from Session 1 to Session 2, but was stable between Session 2 and Session 3. Additional experiments (data not shown) suggested that the concentration-response function for sweet stimuli remains stable in control mice for several subsequent sessions and up to 2 weeks after Session 1.
While fully counterbalancing the order of treatments would have been ideal, the stability of the concentration-response functions after an initial session set the stage for Experiment 3B. Here, we compared the lick responses of mice injected with 0.1 mg/kg OXT injection prior to Session 2 and saline prior to Session 3. The absence of an order-effect is supported by several data. As shown in Fig. 2, licking behavior of mice consistently returned to control levels within 24 h of OXT injection. In addition, the sucrose concentration-response functions of mice injected with saline after OXT (Experiment 3B, Fig. 5A) was indistinguishable from that of mice never injected with OXT (Experiment 3A, Fig. 4 Sessions 2, 3) for any parameter (lower asymptote, slope, or EC50; all paired t-test p-values in all cases > 0.129).
In Experiment 3B, OXT-injected mice reduced licking to sucrose compared with saline-injected mice, as indicated by a small but significant rightward shift in the concentration-response function [28]. The concentration that evoked an SLR midway between the lower and upper asymptote shifted from 0.36 M sucrose to 0.47 M sucrose, without a significant change in the slope or lower asymptote of the function. One interpretation of this result is that sucrose is somewhat less palatable when plasma OXT levels are elevated, with or without alterations in perceived intensity. While it is not possible to determine, from our method, whether such an alteration in the gustatory signal is central or peripheral in nature, the plasma concentration of OXT was more than 10-fold lower than those previously reported to influence central pathways [5].
The notion that oxytocin reduces gustatory palatability is broadly consistent with a number of studies involving central or peripheral injection of oxytocin or oxytocin receptor antagonists, as well as studies of oxytocin knockout mice reviewed in the Introduction. The brief-access paradigm used in the present study is considered to reflect orosensory, rather than postingestive, processes, due to the small volumes of taste solutions used and the measurement of immediate responses [28, 35, 36], although in some cases, postingestive influences have been inferred [48]. Because there was no difference in sucrose licking at the highest concentration, and because OXT-injected mice initiated more trials than saline-injected mice, there is no evidence in the current study that OXT reduced sucrose licking by, for example, reducing hunger or thirst. Instead, our observations are consistent with a reduction in the palatability of sucrose.
If oxytocin reduces the palatability of sucrose, however, one would also expect oxytocin to decrease the “break point” for lever pressing for sucrose reinforcement in a progressive ratio task (or, equivalently, OXT knockout mice would have an increase in the break point). Sclafani and colleagues [14] showed that, although OXT knockout mice overconsumed sucrose in intake tests, they did not increase their willingness to press a lever in an increasingly-demanding situation to obtain sucrose reward. These authors concluded that oxytocin's role in feeding behavior was limited to satiety mechanisms rather than including sensory mechanisms.
Of course, there are a number of procedural differences between their study and ours which might also account for the apparently discrepant results - the most obvious being that OXT knockout mice may not behave, in all respects, consistently with wild type mice given injections of oxytocin (or molecules that enhance or interfere with oxytocin receptors). A second difference was that our comparisons were made within subjects rather than between groups, which may have improved our ability to detect small effects, a possibility which is in line with the non-significant direction of the differences of means in the study of Sclafani and colleagues (c.f., their Figure 3).
A third difference was that Sclafani and colleagues used a single sucrose concentration, whereas we tested a range of concentrations. Although the concentration chosen by Sclafani and colleagues was approximately 0.3 M, which our mice treated differently when injected with OXT, the context of one vs. many concentrations may be critical. For example, Glendinning and colleagues [31] found that mice lacking α-gustducin, a subunit of a G-protein involved in taste transduction, were similar to heterozygote littermate controls in their avidity for a variety of preferred substances in a paradigm very similar to our Experiment 3. When the same mice were next tested in a paradigm in which single concentrations were offered once per day – a situation that avoids simultaneous contrast effects – relatively large differences were seen between the knockout mice and their controls. In the present case, we appear to have the reverse – we detected a small but significant difference in sucrose responsiveness after OXT injection in the context of multiple concentrations of sucrose available in a single session, whereas Sclafani and colleagues found no differences between OXT-/- mice and controls in a progressive ratio task in which a single sucrose concentration served as the reward. Thus, behavioral context, oxytocin manipulation (genetic or pharmacological), statistical power considerations, behavioral task demands (unconditioned licking vs. progressive ratio) or duration (30-min vs. 24-h), or other factors could account for the different interpretations of these two studies.
That OXT-injected mice initiated significantly more trials in Experiment 3B than saline-injected mice was an unexpected and interesting observation, particularly given that the higher dose used in Experiment 1 had the opposite effect. The behavioral suppression seen at the higher dose might have any number of causes, including general effects such as discomfort, lethargy, or sleepiness to more specific effects such as reducing thirst [7] or sensory salience of the preferred substances that might otherwise motivate trial initiation. The significant increase in trial initiation in Experiment 3B might occur if OXT (at those more physiological doses): 1) increased thirst or hunger (enhancing the influence of our partial food/water restriction on behavior), 2) elevated the animal's level of alertness, 3) decreased the animal's level of anxiety, or 4) was a secondary effect of sensory changes (e.g., the mouse was “seeking” the sucrose which was more palatable during the initial habituation session). The first explanation is implausible because, as reviewed in the Introduction, OXT has been shown to reduce food and fluid intake and delay the onset of meals [5-7, 10]. Given the well-documented role for OXT as an anxiolytic [49], the third explanation has more support than the second. Despite the fact that our mice were well-habituated to the testing schedule and apparatus by the test sessions, it may be that OXT-injected mice were more willing to engage behaviorally in the task due to a reduction in anxiety. Nevertheless, once a trial was initiated, the lick-rate recorded is expected to represent orosensory detection.
Early studies examining the effect of OXT on intake of food or preferred taste solutions employed both i.c.v. and i.p. injections [5, 7, 9, 10]. Because the highest doses of i.p. OXT (up to 6 mg/kg) resulted in a suppressive effect equivalent to the i.c.v. doses, Arletti and colleagues [5, 7] concluded that the effect of OXT on feeding was central, and that the high i.p. doses were penetrating the blood-brain barrier sufficiently to reach central oxytocin receptors. In our multi-tastant panel, we tested an i.p. dose even higher – 10 mg/kg – and found no effect on aversive taste stimuli. Thus, there is no evidence for a central or peripheral effect of OXT on avoidance of bitter, sour, or salty tastants, at least at the concentrations we studied. This conclusion is currently tentative however, given the high OXT dose used in the first experiment. OXT at high i.p. doses is thought to permeate across the blood-brain barrier to target hypothalamic neurons. Our low i.p. dose of OXT in our final experiment (0.1 mg/kg), was lower than the lowest i.p. dose used in Arletti et al [5]. This leaves open the possibility that in our study, OXT acted predominantly on peripheral targets, such as taste buds [26, 27], to produce the decrease in sucrose sensitivity we observed. This possibility, too, awaits further experimentation.
Highlights.
Brief-access lickometry reveals that i.p. oxytocin influences taste sensitivity
Circulating oxytocin depressed responsivity towards sweet stimuli.
Licking responses to aversive tastes (bitter, salty, sour) were unaffected
Circulating oxytocin was calibrated to mimic concentrations affecting taste buds
We suggest OXT damps peripheral responses to sweet, complementing central anorexia
Acknowledgments
We would like to thank Dr. John Glendinning for providing guidance with implementing partial food-and-water restriction in our lickometry experiments. We would also like to give special thanks to Shakirra Meghjee and Bennett Garfinkel for their assistance with behavioral experiments. This work was supported by grants from the National Institutes of Health (R21DC010073 and R01DC006308).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Donaldson ZR, Young LJ. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 2.Leng G, Onaka T, Caquineau C, Sabatier N, Tobin VA, Takayanagi Y. Oxytocin and appetite. In: Inga DN, Rainer L, editors. Progress in Brain Research. Elsevier; 2008. pp. 137–51. [DOI] [PubMed] [Google Scholar]
- 3.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18:158–68. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Olszewski PK, Klockars A, Schiöth HB, Levine AS. Oxytocin as feeding inhibitor: Maintaining homeostasis in consummatory behavior. Pharmacology Biochemistry and Behavior. 2010;97:47–54. doi: 10.1016/j.pbb.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-x. [DOI] [PubMed] [Google Scholar]
- 6.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides. 1991;12:113–8. doi: 10.1016/0196-9781(91)90176-p. [DOI] [PubMed] [Google Scholar]
- 7.Arletti R, Benelli A, Bertolini A. Oxytocin inhibits food and fluid intake in rats. Physiology & Behavior. 1990;48:825–30. doi: 10.1016/0031-9384(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 8.Herisson FM, Brooks LL, Waas JR, Levine AS, Olszewski PK. Functional relationship between oxytocin and appetite for carbohydrates versus saccharin. Neuroreport. 2014;25 doi: 10.1097/WNR.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 9.Lokrantz CM, Uvnäs-Moberg K, Kaplan JM. Effects of Central Oxytocin Administration on Intraoral Intake of Glucose in Deprived and Nondeprived Rats. Physiology & Behavior. 1997;62:347–52. doi: 10.1016/s0031-9384(97)00021-8. [DOI] [PubMed] [Google Scholar]
- 10.Benelli A, Bertolini A, Arletti R. Oxytocin-induced inhibition of feeding and drinking: No sexual dimorphism in rats. Neuropeptides. 1991;20:57–62. doi: 10.1016/0143-4179(91)90040-p. [DOI] [PubMed] [Google Scholar]
- 11.Amico JA, Vollmer RR, Cai HM, Miedlar JA, Rinaman L. Enhanced initial and sustained intake of sucrose solution in mice with an oxytocin gene deletion. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1798–R806. doi: 10.1152/ajpregu.00558.2005. [DOI] [PubMed] [Google Scholar]
- 12.Billings LB, Spero JA, Vollmer RR, Amico JA. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav Brain Res. 2006;171:134–41. doi: 10.1016/j.bbr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 13.Miedlar JA, Rinaman L, Vollmer RR, Amico JA. Oxytocin gene deletion mice overconsume palatable sucrose solution but not palatable lipid emulsions. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1063–R8. doi: 10.1152/ajpregu.00228.2007. [DOI] [PubMed] [Google Scholar]
- 14.Sclafani A, Rinaman L, Vollmer RR, Amico JA. Oxytocin knockout mice demonstrate enhanced intake of sweet and nonsweet carbohydrate solutions. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1828–R33. doi: 10.1152/ajpregu.00826.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nissenbaum JW, Sclafani A. Qualitative differences in polysaccharide and sugar tastes in the rat: a two-carbohydrate taste model. Neuroscience and Biobehavioral Reviews. 1987;11:187–96. doi: 10.1016/s0149-7634(87)80025-8. [DOI] [PubMed] [Google Scholar]
- 16.Sclafani A. The sixth taste? Appetite. 2004;43:1–3. doi: 10.1016/j.appet.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Baskin DG, Kim F, Gelling RW, Russell BJ, Schwartz MW, Morton GJ, et al. A new oxytocin-saporin cytotoxin for lesioning oxytocin-receptive neurons in the rat hindbrain. Endocrinology. 2010;151:4207–13. doi: 10.1210/en.2010-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blevins JE, Eakin TJ, Murphy JA, Schwartz MW, Baskin DG. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Research. 2003;993:30–41. doi: 10.1016/j.brainres.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Blevins JE, Stanley BG, Reidelberger RD. Brain regions where cholecystokinin suppresses feeding in rats. Brain Research. 2000;860:1–10. doi: 10.1016/s0006-8993(99)02477-4. [DOI] [PubMed] [Google Scholar]
- 20.Blevins JE, S MW, B DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 21.Bray GA. Afferent signals regulating food intake. Proceedings of the Nutrition Society. 2000;59:373–84. doi: 10.1017/s0029665100000422. [DOI] [PubMed] [Google Scholar]
- 22.King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiology & Behavior. 2006;87:221–44. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Sabatier N, Rowe I, Leng G. Central release of oxytocin and the ventromedial hypothalamus. Biochemical Society Transactions. 2007;35:1247–51. doi: 10.1042/BST0351247. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig M, Leng G. Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci. 2006;7:126–36. doi: 10.1038/nrn1845. [DOI] [PubMed] [Google Scholar]
- 25.Noble EE, Billington CJ, Kotz CM, Wang C. Oxytocin in the Ventromedial Hypothalamic Nucleus Reduces Feeding and Acutely Increases Energy Expenditure. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2014 doi: 10.1152/ajpregu.00118.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, et al. Oxytocin signaling in mouse taste buds. PLoS ONE. 2010;5:e11980. doi: 10.1371/journal.pone.0011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hochheimer A, Krohn M, Rudert K, Riedel K, Becker S, Thirion C, et al. Endogenous Gustatory Responses and Gene Expression Profile of Stably Proliferating Human Taste Cells Isolated From Fungiform Papillae. Chemical Senses. 2014;39:359–77. doi: 10.1093/chemse/bju009. [DOI] [PubMed] [Google Scholar]
- 28.St John SJ, Spector AC. 4.22 - Behavioral Analysis of Taste Function in Rodent Models. In: Masland RH, Albright TD, Albright TD, Masland RH, Dallos P, Oertel D, et al., editors. The Senses: A Comprehensive Reference. New York: Academic Press; 2008. pp. 409–27. [Google Scholar]
- 29.Boughter JD, Jr, St John SJ, Noel DT, Ndubuizu O, Smith DV. A brief-access test for bitter taste in mice. Chemical Senses. 2002;27:133–42. doi: 10.1093/chemse/27.2.133. [DOI] [PubMed] [Google Scholar]
- 30.Glendinning JI, Gresack J, Spector AC. A High-throughput Screening Procedure for Identifying Mice with Aberrant Taste and Oromotor Function. Chemical Senses. 2002;27:461–74. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- 31.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, et al. Contribution of α-Gustducin to Taste-guided Licking Responses of Mice. Chemical Senses. 2005;30:299–316. doi: 10.1093/chemse/bji025. [DOI] [PubMed] [Google Scholar]
- 32.Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial Licking Responses of Mice to Sweeteners: Effects of Tas1r3 Polymorphisms. Chemical Senses. 2005;30:601–14. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- 33.Davis JD. Some new developments in the understanding of oropharyngeal and postingestional controls of meal size. Nutrition. 1999;15:32–9. doi: 10.1016/s0899-9007(98)00109-9. [DOI] [PubMed] [Google Scholar]
- 34.Davis JD, Levine MW. A model for the control of ingestion. Psychological Review. 1977;84:379–412. [PubMed] [Google Scholar]
- 35.Smith GP. John Davis and the meanings of licking. Appetite. 2001;36:84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- 36.Smith JC. The history of the “Davis Rig”. Appetite. 2001;36:93–8. doi: 10.1006/appe.2000.0372. [DOI] [PubMed] [Google Scholar]
- 37.Smith JC, Davis JD, O'Keefe GB. Lack of an order effect in brief contact taste tests with closely spaced test trials. Physiology & Behavior. 1992;52:1107–11. doi: 10.1016/0031-9384(92)90467-g. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi T, Tadokoro Y, Honda K, Negoro H. Detailed analysis of blood oxytocin levels during suckling and parturition in the rat. Journal of Endocrinology. 1986;110:251–6. doi: 10.1677/joe.0.1100251. [DOI] [PubMed] [Google Scholar]
- 39.Dotson CD, Spector AC. The Relative Affective Potency of Glycine, l-Serine and Sucrose as Assessed by a Brief-access Taste Test in Inbred Strains of Mice. Chemical Senses. 2004;29:489–98. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- 40.Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–93. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Tang Y, Chen Z, Tao H, Li C, Zhang X, Tang A, et al. Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain. Neuropharmacology. 2014;77:277–84. doi: 10.1016/j.neuropharm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Lin JY, Amodeo LR, Arthurs J, Reilly S. Taste neophobia and palatability: The pleasure of drinking. Physiology ' Behavior. 2012;106:515–9. doi: 10.1016/j.physbeh.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monk KJ, Rubin BD, Keene JC, Katz DB. Licking microstructure reveals rapid attenuation of neophobia. Chemical Senses. 2014;39:203–13. doi: 10.1093/chemse/bjt069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grigson PS, Spector AC, Norgren R. Microstructural analysis of successive negative contrast in free- feeding and deprived rats. Physiology and Behavior. 1993;54:909–16. doi: 10.1016/0031-9384(93)90301-u. [DOI] [PubMed] [Google Scholar]
- 45.Flaherty CF. Anticipatory and simultaneous contrast. Incentive Relativity. 1996:107–34. [Google Scholar]
- 46.Flaherty CF, Turovsky J, Krauss KL. Relative hedonic value modulates anticipatory contrast. Physiol Behav. 1994;55:1047–54. doi: 10.1016/0031-9384(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 47.Sclafani A. Oral and postoral determinants of food reward. Physiology & Behavior. 2004;81:773–9. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 48.Treesukosol Y, Smith KR, Spector AC. Behavioral Evidence for a Glucose Polymer Taste Receptor That Is Independent of the T1R2+3 Heterodimer in a Mouse Model. The Journal of Neuroscience. 2011;31:13527–34. doi: 10.1523/JNEUROSCI.2179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:2259–71. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


