Abstract
Glioblastoma Multiforme (GBM) is a rapidly progressing brain tumor. Despite the relatively low percentage of cancer patients with glioma diagnoses, recent statistics indicate that the number of glioma patients may have increased over the past decade. Current therapeutic options for glioma patients include tumor resection, chemotherapy, and concomitant radiation therapy with an average survival of approximately 16 months. The rapid progression of gliomas has spurred the development of novel treatment options, such as cancer gene therapy and oncolytic virotherapy. Preclinical testing of oncolytic adenoviruses using glioma models revealed both positive and negative sides of the virotherapy approach. Here we present a detailed overview of the glioma virotherapy field and discuss auxiliary therapeutic strategies with the potential for augmenting clinical efficacy of GBM virotherapy treatment.
Keywords: Adenovirus, Brain tumor, Glioma, Self-replicated vector, Stem cells
List of abbreviations: GBM, glioblastoma multiforme; Ad, Adenovirus; DNA, Deoxyribonucleic Acid; RNA, Ribonucleic acid; Wt, wild type; CSC, Cancer stem cells; GSC, Glioma stem cells; IFN, interferon; FACS, Fluorescent Assisted Cell Sorting; PCR, Polymerase chain reaction; RT-PCR, Reverse Transcriptase Polymerase Chain Reaction; mRNA, Messenger mRNA; MLP, Major Late Promoter; CMV, cytomegalovirus
Glioma as a target for gene therapy
Glioblastoma Multiforme (GBM) is the most common primary brain cancer in humans. In the cancer hierarchy, patients with brain tumors represent a relatively small cohort with an estimated 500,000 total cases diagnosed, and 20,000 – deaths reported annually. The incidence of GBM has risen1 lately in Europe and North America with 3.19 cases per 100,000 patients diagnosed yearly2 in the US alone. While current GBM diagnostic techniques have improved tumor detection sensitivities, the average survival is a dismal 16–20 months, and less than 20% of GBM patients survive more than 5 years after diagnosis.3
Histologically GBM can be defined as a tumor of astrocytes, which represent 80% of normal brain tissue. Astrocytomas are characterized based on several parameters, such as tumor localization in the brain, molecular features and invasiveness. According to the WHO classification, there are 4 different stages of brain cancer progression (where stage 4 is the most advanced), based largely on cell differentiation markers. Transition of astrocytoma grade 2–grade 4 (GBM) is associated with changes in cellular signaling pathways such as TP53, EGFR, PTEN, etc. Also, it is well documented that during this transition astrocytomas incur genomic deletions (IDH1, PTEN), which activate various signaling pathways responsible for new aggressive phenotypes. Based on such genomic rearrangements Verhaak et al. grouped gliomas into mesenchymal, classical, neural, and proneural subtypes.4 Each glioma subtype is characterized by a specific gene expression pattern that ultimately determines the tumor behavior.
Another constituent of glioma tumors is glioma stem cells (GSCs), or cancer stem cells, which demonstrate the ability to form tumors upon intracranial injection. Cancer stem cells are capable of forming spherical structures in vitro, called neurospheres, which may account for both chemo- and radioresistance of glioma tumors in patients.5, 6 Stem cell properties have been ascribed to those cells based on their capability to maintain the tumor cell population, which implicates them in tumorigenesis and tumor recurrence mechanisms.7, 8 It remains unclear whether the differentiation of cancer stem cells into a tumor requires environmental factors to accelerate tumorigenesis. However, scientific reports in the last 10 years suggest that one of the most devastating human cancers, such as glioma, originates from neural progenitor cells with a strong proliferative capability. Moreover, infection of progenitor cells with cytomegalovirus (CMV) significantly promotes progression of glioma in mouse experimental models of the disease.9 Additionally, a growing body of evidence suggests that both immunotherapeutic10 and chemotherapeutic11 approaches targeting CMV improve overall patient survival. This data points towards CMV as a new potential etiological factor of GBM progression, representing an ideal target for gene therapy.
Gene therapy is an alternative approach for glioma therapy
Treatment of extremely vascularized tumors, such as gliomas is very challenging. The standard of GBM patient care includes surgical resection, radiation, and chemotherapy. Although, temozolomide, bevacizumab and carmustine provide longer survival times, neither drug prevents glioma recurrences, mainly due to the activation of a mechanism that enables immune evasion and causes drug resistance. For instance, a recent study suggests that bevacizumab treatment promotes tumor invasion via activation of MMP2,12 while other scientific reports implicate activation of the mTOR pathway.13, 14 This is one of the key pathways responsible for the induction of cellular autophagy, which negatively affects glioma cells and triggers an inflammatory response. The fact that glioma stem cells (GSCs) cannot be targeted and destroyed by chemotherapy and radiation implicates them in the observed resistance of gliomas to traditional therapies, which makes treatment of the disease extremely challenging. Therefore, there is an urgent need for a new therapeutic approach with an improved efficacy that would target both the tumor cell, and the stem cell components of gliomas. Such new therapeutic approaches should target GSCs, while simultaneously comprising the existing therapeutic options, such as ionizing radiation and temozolomide. Since conventional chemotherapeutic agents exhibit strong toxicity towards cancer cells, and in most cases do not spare normal cells, cancer gene therapy seems promising with regard to its higher potential specificity and efficacy. Cancer gene therapy, therefore, is a unique approach capable of utilizing a multifunctional platform for tumor targeting, imaging, and gene delivery. This approach is based on the design of vectors capable of delivering any payload to the tumor cells using various injection routes. Viral vectors exhibit great advantages over non-viral means of gene delivery owing to their natural capability of highly efficient cell attachment and entry (perfected in the course of viral evolution) as a crucial part of gene delivery mechanism, and provide the highest level of transgene expression as part of the viral replication cycle, resulting from high amplification of transgene expression (for replication-competent vectors).
Adenoviral vectors: Exclusive and not exclusive for glioma therapy
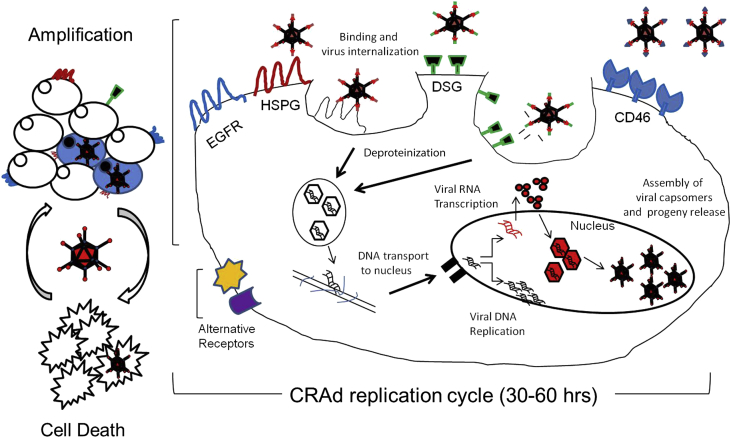
In the late 1950's Levy and Rowe discovered a new agent capable of passing through bacteria retention filters and infecting mammalian cells.15, 16 It took more than 40 years after discovery of adenovirus (Ad) to accumulate knowledge about adenoviral biology critical for the development and advancement of the Ad-based vector technology for tumor targeting. Today, human Ad-based vectors have been recognized as a major tool for gene therapy with more than 100 various adenoviral vectors developed for glioma targeting. The attractiveness of adenoviruses, especially the most studied human serotypes 2 and 5, for glioma targeting applications is largely based on the knowledge that some parts of the viral genome (implicated in modulation of the host immune and inflammatory responses), can be omitted without affecting viral replication, assembly, and can be replaced with a gene of interest for therapeutic purposes. Furthermore, currently available capsid-modified Ad vectors can recognize a large variety of cell surface molecules as primary and secondary receptors allowing efficient infection of both quiescent and rapidly proliferating tumor cells independently from the expression of the Ad native primary coxsackie-adenovirus receptor (CAR). The ability to use complementing and non-complementing cells of mammalian origin allowing human Ad propagation to high titers in culture, represents an important biotechnological advantage of using these vectors for gene and cancer gene therapy (Fig. 1). Given that many self-amplifying, or “replication-competent” Ad vectors with cancer-selective replication properties, also known as Conditionally Replicative Adenoviruses vectors (CRAds), exhibit strong oncolytic anti-glioma effects, those vectors are the primary focus of our review.
Figure 1.
CRAd replication cycle resulting in target cell oncolysis. A schematics illustrating the basic mechanism of CRAd-mediated cell killing starting with binding of a CRAd particle to a tumor-specific cell surface receptor(s). This is followed by viral internalization via the endosome pathway and subsequent capsid disintegration and trafficking of the released genomic DNA (still complexed with core proteins) to the nucleus, where the recombinant genomic DNA is transcribed to produce mRNAs coding for viral proteins. Following mRNA transport into the cytoplasm and its translation into virus-specific proteins, adenoviral progeny particles are assembled from capsomers in the nucleus, following nuclear import of the Ad structural proteins. Ad progeny is then released form the infected cells via a replication-dependent (onco)lytic mechanism.
Adenovirus generation (“rescue”) systems
Various strategies have been proposed to design replication-competent Ad vectors. To generate or “rescue” a replication-competent vector, a two-phase approach is commonly used. In the first phase, all necessary Ad genetic modifications/alterations are made in the viral genome. This includes initial genetic manipulations within one or more genomic regions (typically E1, E3, E4, hexon, or Fiber genes) in the context of a small “shuttle” plasmid using conventional DNA cloning technologies, followed by sequential transfer of the resulting modifications to a large size intermediate (“backbone construct”) and/or a full-size genome (“rescue vector”) by homologous recombination (HR) in mammalian cells or bacteria (E. coli strain BJ5183). Those modifications typically involve mutations in Ad capsid (structural) proteins, replacement or incorporation of promoter elements (constitutive or tumor-specific), along with the transgene(s) of interest. In the second phase, a linearized form of recombinant full-size genomic DNA is transfected into mammalian (helper HEK293) cells, where the Ad genome termini, formed upon restriction digestion and release of the vector's plasmid (bacterial) portion, create a replication fork to initiate DNA replication (doubling), followed by intracellular production of viral mRNAs, proteins, and the assembly of viral particles. Most recently, Stanton et al. proposed to utilize a high throughput AdZ rescue system that allows a direct, single-step insertion of PCR products or synthesized sequences into the Ad genome and obviates the need in vector linearization prior to transfection into packaging cells.17
Glioma-associated alterations in signaling pathways offer molecular strategies for engineering anti-glioma CRAds
The rapidly growing body of knowledge on signaling pathways activated in glioma cells offers an important insight into potential molecular strategies for increasing antitumor efficacy of CRAd vectors. Genetic analysis of clinical samples demonstrates aberrations in the PTEN, p16INK4A, EGFR, and P53 signaling pathways. About 80% of glioblastoma specimens presented in The Cancer Genome Atlas (TCGA) possess aberrations in CDKN2A and Rb pathways. The latter regulate astrocytoma survival and tumor cell proliferation.18, 19 Furthermore, deletions of the PTEN gene are observed in ∼50% GBM specimens, while 30% of clinical samples exhibit EGFR amplification, and about 11% of samples reveal mutations in P53 and IDH1 genes.20
Ad capability for selective replication in gliomas is determined by genetic information encoded by the self-amplifying Ad genome. The first anti-glioma CRAds were designed using deletion of Immediate Early (E1) viral genes such as E1B-55K, which blocked vector replication in normal, but not in cancer cells. The glioma-specific oncolytic vector, referred to as ONYX105 or dl1520, was designed to replicate in the p53 deficient tumor cells with functional defects in p53 tumor suppressor signaling21 and induce non-apoptotic cell death during viral infection.22 However, over the past several decades, multiple scientific reports have evidenced that dl1520 can also replicate in normal (non-cancer) cells, suggesting involvement of a p53-independent replication mechanism.23, 24
The delta24 CRAd vector (also known as dl922-947, or ONYX-838) was constructed by introducing a 24 bp deletion in the E1A gene. In the course of Ad infection, the E1A-encoded protein binds to the cellular tumor suppressor protein retinoblastoma (pRb) to displace transcription factor E2F from the intracellular E2F/Rb complex, thereby controlling the intracellular pool of free E2F. The release of E2F results in entry of the infected cell into the S-phase, a prerequisite for Ad DNA replication. The delta24 Ad mutant can replicate in actively dividing cells that have an aberrant G1-S checkpoint.25 While normal cells do not support replication of the delta24 CRAd, the virus is effective against U251 and U87 glioma cells at doses of 10 infectious units per cell (iu) in vitro, and 100 iu per cell in vivo.26 Recently, Gomez-Manzano et al. reported a new vector E1A-E1B (CB1), which combines both delta24, and E1B-55K deletions. Although the CB1 vector demonstrates a more robust replication, resulting in greater cytotoxicity in vitro than delta24, intracranial injection of the double mutant vector into mice results in the same animal survival rates (p = 0.28, Mean percent survival is 59 vs. 51 days) as those found for delta24 CRAd.27
Clinical use of dl1520, delta24, or the double mutant CB1 as individual vectors (monotherapy) for gene therapy applications demonstrated limitations for each of those agents. For instance, Geoerger et al demonstrated that 5 consecutive intratumoral injections of human xenografts with dl1520 are not sufficient to prevent tumor progression in mice. This observation suggests that additional modifications are required to create a more specific and efficacious CRAd agent. Therefore, combinations of various strategies based on utilization of molecular features of glioma tumors are needed to design a potent anti-glioma therapeutic CRAd.
Improving Ad targeting and internalization
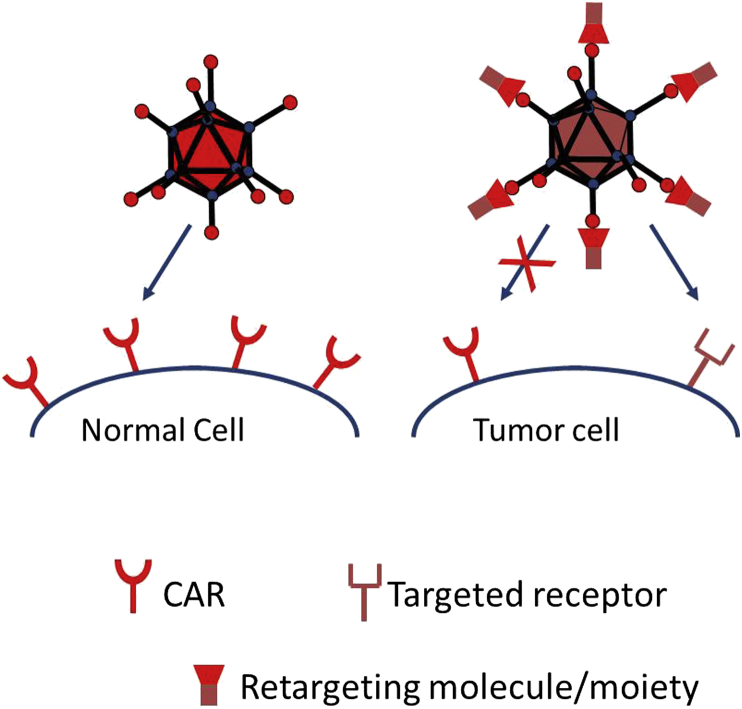
It is unclear if incorporation of capsid modifications into recombinant Ad genomes that could potentially affect therapeutic potency of the vector is always justified, i.e. whether those modifications are really necessary to achieve successful gene targeting. For example, to treat prostate cancer Freytag and collaborators used a capsid-unmodified oncolytic adenovirus for successful delivery of cytokines and two suicide genes.28 On the contrary, given that glioma cells express low levels29 of primary Ad5 receptor (Coxsackie-and-adenovirus receptor, CAR), payload delivery to the tumor cells via capsid-unmodified viral particles might be inefficient, and could induce normal cell toxicity due to CAR expression on healthy cells (Fig. 2). This evidence exposes one of the major limitations of Ad vectors, i.e. the intrinsically low efficiency of tumor cell transduction.
Figure 2.
Retargeting of adenoviral particles to an alternate receptor improves targeting specificity of replication-competent adenoviral vectors.
To increase Ad vector specificity several strategies have been developed. One of them involves Ad serotype chimerism. Currently, over 100 types/serotypes of the Adenoviridae family have been characterized. Those comprise 5 genera, capable of infecting humans and a large number of animal species. Human Ad species belong to the Mastadenovirus genus comprising 57 characterized serotypes (Ad1–Ad57) and 7 distinct species/groups (A-G) based on the variety of serotype-specific and group-specific characteristics. An important group-specific characteristic is the ability to recognize common (group-specific) receptor(s) located on the surface of target cells, such as a glioma cells. Adenovirus type 5 of group C has been a predominant vector used for gene therapy applications.
Adenoviral particles transduce target cells by a mechanism involving a direct initial interaction between the fiber protein of the Ad capsid, and the primary Ad receptors on the surface of tumor cells. It has been suggested that Desmoglein 2 (DSG2) and CD46 molecules represent such native primary receptors of the Ad group B2 serotypes (Ad11, 14, 34 and 35 and others), or group B1 serotypes (Ad3, 7, 16, 21, 50 and others), respectively. Therefore, replacing the Ad5 fiber knob (C-terminal) domain, or the knob-shaft region of the wild type Ad5 with those of other serotypes allows potential retargeting of adenoviral particles from CAR (Ad5 native receptor) to alternate (other serotype-specific) receptors such as DSG2, CD46 etc. In line with this, Nandi et al.,30 Wohfahrt et al.31 and Li et al.32 independently demonstrated that pseudotyping Ad5 particles with fibers from serotypes 3, 35 or 11 significantly improved transduction of glioma cells compared to the wild type (WT) Ad5 both in vitro, and in vivo.
The lack of the CAR receptor on glioma cell surface is the reason for the poor gene transfer in those cells by recombinant Ad vectors with an unmodified fiber. Therefore, it would be valuable to improve Ad target cell transduction for therapeutic uses. Retargeting of particles to alternate receptors abundant on the surface of glioma cells may circumvent their intrinsically low CAR expression. One group of such surface molecules characteristically expressed on glioma cells is represented by integrins. It has been shown that insertion of RGD-4C ligand (cyclic peptide) into the fiber protein of the adenoviral capsid allows interaction of virions with cellular αv intergrins, enhancing glioma transduction.33 Moreover, combining the integrin targeting of Ad vectors with their transcriptional targeting by placing the E1 genes under transcriptional control of tumor-specific (survivin34 or telomerase35) promoters (TSPs), or mutations (delta24) in E1 genes that abrogate their binding to Rb or p53 can further improve specificity and efficacy of glioma targeting.36, 37, 38, 39, 40, 41, 42
Another type of genetic modification that redirects Ad particles to alternate receptors is incorporation of a polylysine motif at the C-terminus of the fiber protein. While this modification does not ablate CAR-mediated binding and internalization of viral particles, it improves Ad infectivity through a positively-charged heparan sulfate proteoglycan (HSPG) molecules abundant on the surface of cancer cells.33 Zheng et al. tried to determine which of the HSPG receptors is needed for transduction using pK7-modified Ad vectors. Treatment of glioma cells with pK7-modified Ad vector in the presence of neutralizing antibodies against syndecan 1 and perlecan decreased efficiency of the cells transduction by 30–50%, implicating those molecules in attachment to the Ad5pK7 virus. The first Ad vector targeted to HSPGs through incorporation of 7 lysine amino acids (heptalysine) into the C-terminal domain of the wild type Ad5 fiber was designed by Wickham et al.43 The attachment of Ad particles to the target cells can be significantly augmented given that the viral capsids retain capability of binding to CAR, typically expressed by rapidly proliferating cells, as well as to cellular integrins through an RGD motif in the penton base. However, these receptors are also expressed on muscle cells, macrophages, and endothelial cells, making them a less desirable transduction targets.43 Although most cancer cell lines are highly permissive for Ad vector transduction, its efficiency in patient-derived primary tumor cells is rather poor. To further improve gene transfer of Ad5 vectors enhanced by RGD-4C or pK7 fiber modifications, the viral tropism could be expanded to additional set of receptors, not used by group C species. This can be achieved by using a small peptide/ligand (RGD-4C) modification in the context of Ad3 fiber pseudotyping.44 Although studies using the replication deficient (ΔE1) Ad5/3-RGD vector showed a great promise for gliomas,44 the benefit of this combined fiber modification for GBM oncolytic virotherapy is yet to be determined.
Expression of EGFR localized to the cell surface is upregulated in 40–60% of gliomas.45 Transductional targeting of the EGFR receptor was first proposed by Grill et al., in 2001.46 Ad5 particles can be re-directed to this receptor by a bi-specific single chain antibody (scFv) expressed from either E1 or E4 regions of the Ad5 genome that would bridge the fiber knob and the EGFR receptor on the target cell surface. However, retention of the CAR-binding site within the genetically-unmodified (WT) Ad5 fiber knob could interfere with the cancer specificity of transduction due to the potential ability of the scFv-complexed Ad5 to simultaneously recognize CAR receptor on the surface of non-cancer cells. Although, incorporation of 425-S11 single chain antibody into the fiber knob domain improves transduction of CAR-negative tumors by 2- to 11-fold,46 further ablation of the native CAR tropism is required. For instance, redirection of adenoviral particle to EGFR with simultaneous ablation of CAR and αv integrin binding ability provides selective gene transfer to glioma cells.47 These promising results led investigators to design an 435-S11 scFv-modified CRAd48 to target and destroy CAR-deficient tumors. The mutant version of EGFR (EGFRvIII) is present on the majority of glioma cells as well as breast and ovarian cancer cells49 and regulates pro-survival pathways, which makes it a promising candidate for cancer gene therapy.50
In conclusion, the data published by Nandi et al.30 suggest that retargeting Ad particles by genetic pseudotyping of fiber can greatly improve CRAd cytotoxicity for tumors. However, in many cases tumor-specificity of such Ad vectors remains poor since the alternate receptors such as CD133 (expressed on the surface of glioma stem cells and neural stem cells) and CD46 they are often retargeted to, are also found on the surface of non-cancer cells. Therefore, further capsid modifications are needed to optimize CRAds for cancer gene therapy.
Limiting adenovirus transcription to glioma cells
Genetic incorporation of tumor-associated gene expression control elements into the Ad genome for regulating its early (E1) gene expression (transcriptional or post-transcriptional), improves CRAd vector specificity by restricting viral replication to tumor cells, and thereby preventing unfavorable vector toxicity in normal cells. To achieve this specificity, incorporation of transcription factor recognition motifs and/or microRNA binding elements upstream of the Ad5 E1 genes has been proposed. The rationale behind this approach was to provide conditional (selective) expression of the E1A protein, crucial for triggering Ad early gene transcription and genomic DNA replication. A prototypical tumor-specific promoter (TSP) is known from the literature to selectively regulate/activate Ad early gene expression in tumor cells, yet remain inactive in healthy tissues, such as liver, involved in efficient uptake of most Ad5 entering the circulation. The “tumor on/liver off” expression ratio is a commonly accepted tumor-specificity characteristic of a TSP.
Recently, Guvenc et al., showed that tumor cells highly resistant to therapy exhibit high expression level of the survivin gene.51 The latter codes for an anti-apoptotic protein that governs spindle formation and chromatin separation during tumor cell mitosis.51, 52, 53 The high level of survivin protein correlates with high level of its mRNA, suggesting activity of the survivin promoter and stability of its mRNA in those cells. A ∼200 bp fragment of the survivin promoter (short version) was found to be sufficient to confer Ad replication specificity to glioma cells.34, 54 Moreover, since the survivin promoter contains radiation-inducible elements, ionizing radiation can sensitize exposed glioma cells to infection by survivin-E1 bearing CRAd vectors.55
Midkine (MK) is a heparin-binding growth factor encoded by the MDK gene. Induced during ontogenesis and inflammation, syndecan 1 is the midkine receptor regulating cell proliferation, angiogenesis, fibrinolysis, and mRNA expression in several cancers including glioblastoma and neuroblastoma. Since MK is implicated in cancer cell proliferation, it has become a target for gene therapy. However, there are also effects of MK on genesis of normal cells such as fibroblasts, myoblasts, and renal cells,56 and therefore cancer specificity of CRAd vectors with MK promoter-controlled replication is questionable. Nevertheless, an oncolytic vector with a ∼600 bp MK promoter element, driving expression of adenoviral E1A, has been demonstrated to eradicate MK-positive glioma cells in vitro and in vivo.57
The Promoter of the telomerase related gene (TERT) is also active in glioma cells. According to scientific literature, more than 85% of cancers express telomerase, which is required for cell proliferation. A 455 base pair (bp) promoter of the human gene, coding for the telomerase catalytic subunit, was successfully used for construction of OBP-301 CRAd (also known as Telomelysin) transductionally-retargeted to intergrins on glioma cell lines U87, U373, U251, and patient-derived MDC-01.35 However, not all glioma cells exhibit high telomerase activity. For instance, according to studies by Jafri et al., only 26.1% of high-grade glioma specimens exhibit high telomerase activity.58, 59 Under certain conditions, normal cells, such as fibroblasts, possess very low levels of telomerase activity, which, however, could be induced by drugs, such as HDAC.60, 61 This data points to some limitations for targeting primary tumors in clinical settings.
Another study conducted by Hoffmann et al evaluated potential glioma-specific promoters for oncolytic virotherapy including those of VEGF, GFAP, FGF, Ki-67, Nestin, and Midkine gene alone, or in combination with an SV40 promoter/enhancer.62 According to this data, elevated activity of promoters was manifested by expression of the Luciferase reporter. Based on this assay conducted in several patient-derived and established glioma cell lines (D54, U251, and U87), the top 7 promoters included: MK, hTERT, VEGFlong, VEGFshort, Ki67, GFAP, and E2F/SV40. Furthermore, in vitro promoter activity testing showed that the long version of the human GFAP promoter restricts replication of the CRAd5/35 vector to glioma cells with both high and low level of proliferation capability. Those in vivo data corroborate the ones in vitro and suggest that GFAP promoter-controlled CRAd prolongs survival of mice harboring fast growing U251 xenografts. A study performed by Horst et al.63 showed that rapidly dividing glioma cells can be targeted by a CRAd vector with GFAP promoter-controlled replication. Since GBM cells are sensitive to ionizing radiation and temozolomide treatment, virotherapy with the GFAP promoter-controlled CRAd could be used to augment current therapeutic modalities.
Tumor selective transcription: is CMV promoter the best reference?
Several studies have compared efficiency of various promoters against that of CMV. A study by Zheng et al.64 demonstrated that replacement of the E1A gene promoter with the CMV major late promoter (MLP) failed to provide cancer specificity to Ad replication. Furthermore, utilization of the CMV promoter to control CRAd replication (E1 transcription) resulted in lower replication efficiency as compared to the native (E1) promoter-bearing CRAd in OE33 and OsACL cancer cell lines. In contrast, equal levels of CRAd replication resulted from the CMV-driven E1 expression and the one controlled by the native promoter in A549 (lung adenocarcinoma cells), or WI-38 non-tumor cells. These observations suggest that the CMV promoter does not confer tumor-specificity to CRAd replication. Similar results were also obtained in other studies.65 To date, experimental evidence suggests that most tumors,66, 67 including brain tumors,68, 69, 70 contain cytomegalovirus proteins, DNA and RNA transcripts, particularly the ones of the early and immediate early CMV genes. Besides its robust activity in tumors, the CMV MLP promoter is also active in normal cells, which reduces specificity of CRAd vectors utilizing this promoter to control expression of the E1 genes.
Recent reports have indicated that glioma resistance to conventional treatment modalities may be determined by expression of therapy-resistant proteins, such as the Y-box protein YB-1, a cellular transcription factor implicated in GBM cell survival.71, 72 Treatment of cells with UV radiation and chemotherapy translocates YB-1 from the cytoplasm to the nucleus, indicating its possible role in DNA repair.73, 74 Since expression of multidrug-resistance genes correlates with YB-1 activity, it is logical to expect a YB-1-dependent CRAd to selectively replicate in chemo-resistant glioma cells. In line with this expectation a YB-1-dependent CRAd dl520 (ΔE1A-13S) demonstrated oncolytic activity in glioma cells, resistant to Irinotecan and Trichostatin A.75 Most recently, a dl520-derivate Ad-Delo3-RGD, carrying an additional E1B gene deletion and the integrin binding motif in the fiber protein, also demonstrated a selective replication in chemo-resistant glioma stem cells.76
The other genetic alteration in gliomas that impacts cell division is p16INK4a, leading to phosphorylation of Rb and activation of E2F1 transcriptional factor.77 To restrict expression of the adenoviral E1A protein to target (glioma) cells deficient in the Rb pathway, authors placed E1A transcription under control of endogenous E2F1 transcription factor by cloning an E2F1 response element in place of the E1 promoter region.42 As a result, replication of such CRAd (ICOVIR5) in normal cells with low level of free E2F1 (trapped in the form of Rb/E2F1 inactive complex) was suppressed. On the contrary, an excessive amount of E2F1 in glioma activated ICOVIR5 vector's replication in target cells. Thus, replication activity of this CRAd directly correlates with the E2F1 expression in the virus-infected cells.78 However, since high level of E2F1 expression is a feature characteristic for any rapidly dividing cells,79 the E2F1-controlled CRAd vector cannot discriminate between rapidly dividing normal cells and malignant cells.
Suppression of endogenous gene expression on post-transcriptional level involving microRNA (miRNA), found in gliomas, can also be utilized in gene therapy approaches. MicroRNAs are small non-coding RNAs complimentary to target cellular mRNAs. MicroRNA binding to target mRNA leads to specific repression of regulatory genes either on transcriptional or translational levels. It is well documented that gliomas exhibit diverse microRNA expression patterns or “signatures”. Given that miRNAs can regulate cell proliferation, invasion and angiogenesis, incorporation of miRNA coding sequences into CRAds could aid to CRAd's cytotoxic activity. It was recently shown that glioma cells express high levels of microRNA 124,-128,-146B and 218,80 and therefore incorporation of microRNA recognizing elements (MREs) into the Ad genome could inhibit CRAd replication in tumor tissues. It remains to be investigated whether the microRNA-mediated CRAd targeting approach will be efficient in human tissues with regard to patient's age, and the course of treatment as those might affect miRNA expression.
Analysis of scientific literature suggests that cancer specificity of CRAd agents can be achieved by proper selection of transcription regulatory elements and their genetic incorporation in CRAd genomes to control viral E1 transcription. However, despite strong antitumor effects, incorporation of a given TSP may not prevent E1A transcription leakage from the inverted terminal repeat (ITR) sequences in the Ad genomic DNA.81 For instance, previous studies have shown that multiple enhancer elements and cryptic promoter elements exist within the Ad ITRs, which can contribute to undesired “leaky” E1 transcription in healthy cells.64, 82, 83
Strategies to improve anti-glioma efficacy of CRAds
As it was mentioned above, glioma cell populations responsible for tumor recurrence exhibit strong resistance to conventional treatment modalities. Therefore, it is critical to find a new and more effective therapeutic approach devoid of cytotoxicity caused by the conventional anti-glioma treatments. Besides, a growing body of evidence suggests that intratumoral injection of CRAds activates antiviral immune response. To circumvent the immune response problem various Ad shielding methods, such as coating with polyethylene glycol (PEG) or using stem cells as Ad delivery vehicle, have been developed.
-
a)
Strategies to improve CRAd-mediated toxicity: RT and chemotherapeutic drugs.
In has been shown that chemotherapeutic agents activate cellular pathways that contribute to CRAd-mediated toxicity. We54, 84, 85 and others86, 87, 88, 89, 90, 91 have shown that CRAds induce cell death via two main mechanisms: apoptosis, and autophagy. Despite activation of pro-apoptotic genes, such as BAX2, BIM, and BIK, CRAd infection does not trigger caspase-dependent apoptosis. Several reports suggest that CRAds induce autophagy, which involves the formation of double-membrane phagosomes. Moreover, recent evidence suggests that the E4 region of the Ad genome is required to induce autophagy upon the viral infection. Augmentation of CRAd-induced autophagy is one strategy to boost CRAd toxicity. Ionizing radiation,36, 40, 55, 89 RAd00135, 39 and clinically approved temozolomide (TMZ)35, 38, 76, 92 have been utilized to promote CRAd-mediated autophagy. However, it is, still unclear whether this effect can be directly attributed to CRAd, or is merely a result of CRAd-induced cell defense mechanism that requires more inhibition to improve CRAd cytotoxicity.
-
b)
Sensitization of cells to the CRAd-mediated toxicity using TRAIL and CD gene expression
To successfully design and develop effective treatments for GBM, combinational approaches to targeting several molecular pathways may be necessary. Despite our advancing knowledge of the genetic alterations involved in this disease, identification of new therapeutic combinations may prove advantageous. To augment CRAd-mediated toxicity, expression of pro-apoptotic molecules, such a TRAIL (TNF-related apoptosis-inducing ligand), represents a new anti-glioma approach. TRAIL represents an extracellular carboxy-terminal portion of the type II transmembrane protein that sensitizes tumor cells to apoptosis via binding to the DR4 (DR5) receptor. This, in turn, activates caspase 8/10 or the intrinsic cytochrome C release pathway, which subsequently activates SMAC/DIABLO to translocate pro-apoptotic BIK, BID and BAX.93, 94, 95, 96 The common activation of BAX and suppression of anti-apoptotic BCL-2 apparently provides a mechanistic link for the additive effect between CRAd and TRAIL expression.97, 98, 99 Wolhardt et al.31 first proposed targeting glioma cells with the CRAd-TERT-5/35 vector encoding TRAIL as a therapeutic transgene, while expressing proteins of the E1 region under control of the human telomerase promoter. In addition, the capsid of this vector was retargeted to an alternate receptor by replacing the wild type fiber knob domain with that of serotype 35. Later, this approach was elaborated by Li et al.,32 who used a delta24-5/11 backbone, and by Tsamis et al.,100 who used a delta24 backbone with the WT fiber. In all cases, expression of TRAIL in the context of an oncolytic vector resulted in strong anti-cancer effect compared to the unarmed CRAd.
In 2005 Conrad et al.101 attempted to combine an oncolytic Ad vector and pro-drug therapy to suppress gliomas. In addition to the oncolytic effect the virus elicited cytotoxicity owing to the expression of the delivered “suicide” gene (humanized form of yeast cytosine deaminase, hyCD) converting 5-FC substrate to a toxic metabolite 5-FU in tumor cells.102 Given the limitation in achieving an effective therapeutic dose without hepatotoxicity in the U87 intracranial glioma mouse model, delivery of pro-drug therapy in the context of an oncolytic vector holds a great promise.
-
c)
Modification of CRAd genome to improve CRAd-mediated cytotoxicity.
The adenoviral genome encodes immediate early and early genes transcribed before the onset of DNA replication, as well as late genes transcribed after DNA replication. The functions of the Ad5 early proteins include: controlling cell division (E1), containment of host immune responses to Ad infection, preventing apoptosis (E3) and activation of Ad replication91 (E2). The E3 genes encode 7 proteins, including adenoviral death protein (ADP), which is exclusively expressed during the late stage of infection103 and is responsible for efficient cell lysis and progeny release.104 In efforts to improve adenoviral oncolysis Yun et al. designed an anti-glioma CRAd that harbors a 55Kda-E1B deletion and expresses ADP under the control of the adenoviral Major Late Promoter (MLP) or CMV promoter.91 The vector expressing a CMV-driven ADP exhibited a strong cytotoxicity towards human U343 glioma cells. Although, in vitro data suggest that ∼40% of glioma cells were sensitive to infection with ADP-overexpressing CRAd, this observation has not been confirmed in vivo. Taken together, all this data suggest that overexpression of ADP facilitates CRAd-mediated oncolytic effect.
-
d)
Improvement of CRAd-mediated toxicity by indirect activation of viral replication in the presence of hypoxia.
One of the disadvantages of using adenoviral vectors in cancer gene therapy is uneven dissemination of the vector across the tumor mass.105 One study showed that hypoxia may compromise blood supply to certain regions of tumor tissue, which ultimately limits intratumoral distribution of CRAds.106 Given that hypoxia contributes to GBM invasion and proliferation by maintaining its CSC component,107, 108, 109 targeting GBM CSCs via accelerating CRAd replication under hypoxic conditions may improve anti-glioma therapy. It has been shown that hypoxia affects tumor progression through the blood, and regulates activity of target genes via binding of hypoxia induced-transcription factors to hypoxia response elements (HRE). These transcriptional regulators allow cells to survive hypoxia by activating proliferation.110 Therefore, CRAds designed to aggressively replicate in hypoxic environment by utilizing hypoxia-inducible factors (HIF) to control their replication may be effective in suppressing gliomas108 In line with the above Post et al demonstrated that incorporation of HRE in the E1 region improved CRAd replication in hypoxic areas of tumors.111
-
e)
Strategies to modulate the anti-Ad immune response?
It is known that patients with GBM develop a strong immunosuppression resulting from chemotherapeutic drugs, ionizing radiation, accumulation of cancer stem cells, etc. This evidence has initiated the debate as to whether brain tumor patients might benefit from immune system stimulation through experimental therapies, such as gene therapy with CRAds, which have been shown to induce a proinflammatory response in glioma mouse models. Since the mechanism of antitumor effect achieved by glioma virotherapy is still unclear, it cannot be ruled out that modulation of the patient's immune response might strongly interfere with the effectiveness of the treatment. A recent publication by Liikanen et al.112 reignited discussions about the role of immune system activation in CRAd-mediated tumor oncolysis. Considering that immune response to Ad vector could significantly compromise the efficacy of CRAd-mediated tumor oncolysis by rapid clearing of the viral particles, the overall impact of immune response on the clinical outcome of glioma virotherapy treatment is still in question.
The immunological responses to CRAd infection has been investigated by several research groups. One study using immunosuppressed hamsters suggested that the host immune response neither significantly contributes to CRAd clearance, nor to the antiviral immune response.113 Moreover, steady levels of the virus were detected in immunosuppressed hamsters, similar to those found in mouse xenografts. In immunocompetent animals, the level of CRAd dropped 22 days after tumor implantation, suggesting activation of viral clearance. In this regard, of interest is a recent data from Klejin et al.114 demonstrating that the immune response to delta24-RGD affects therapeutic efficacy in the rodent immunocompetent glioma model. In fact, a local production of proinflammatory cytokines in response to intratumoral vector injection increased along with the number of infiltrating CD4+ and CD8+ lymphocytes and macrophages. It still needs to be determined whether the observed immune response was activated due to the host's protective response against CRAd replication, or was a result of the viral mechanism contributing to its replication.
The main role of stem cell-based vectors is to preserve and deliver CRAds to tumors in tumor-specific fashion, while avoiding activation of anti-Ad immune responses. Delivery of CRAd payloads to tumors and passing those payloads on to neighboring cancer cells within the tumor mass by means of GSCs is based on their intrinsic tumor homing properties. Glioma cells release chemokines and angiogenic factors, such as TGF-β,115 PDGFβ,116 VEGF,117 which attract stem cells administered via various routes. Indeed, the bone-marrow-derived mesenchymal stem cells (MSC) have been reported to improve CRAd persistence and dissemination in vivo.118, 119 Moreover, a delta24 CRAd MSC-delivered to glioma xenografts, significantly prolonged mice survival in glioma xenografts.120 Of note, regardless of the delivery route, intravenous,115 or intracranial (delivery of payload from one brain hemisphere to another hemisphere bearing a tumor), MSC successfully targeted glioma xenografts. Similarly, we have shown that HB1.F3.CD NSCs, which lack HLA class I antigen, when loaded with the CRAd-S-pK7 vector, exhibit a robust anti-glioma effects in vitro and in a U87 intracranial mouse model121 A side-by-side comparison between the MSC (mesenchymal stem cells)- and NSC (neural stem cells)-based CRAd delivery cell vectors showed that the NSC delivery system is more advantageous.122 One significant disadvantage of using NSCs for targeted CRAd delivery is their sensitivity to CRAd infection, which prevents effective migration and delivery of CRAd payload to other sites within the tumor due to CRAd leakage. To circumvent this drawback Kim et al.123 utilized N-acetylcysteine (NACA) for treatment of NSCs loaded with CRAd-S-pK7 to attenuate the CRAd-induced apoptosis. The NACA-treated loaded NSCs lived longer and maintained properties necessary to deliver their payload. Although these results suggest that stem cells improve CRAd distribution in vivo, the use of immunocompromised animal models makes it difficult to assess the induction of an antiviral immune response.
Safety concerns of using CRAds: Should we care?
Although the efficacy of CRAd delivery to glioma is a critical factor of the experimental therapy, safety of the treatment is of high importance too, especially since ongoing investigations emphasize the role of new etiological agents in glioma progression. CRAd safety testing has been performed in vitro using human culture of healthy adult astrocytes30, 124, 125 or cultured fibroblasts63, 75, 91, 126 and in vivo, using animal models for neurotoxicity testing. As can be seen from the data summarized in Table 1, human non-malignant cells exhibit various sensitivities to CRAd infection, which is important to assess prior to in vivo CRAd testing in glioma animal models. A preliminary survival experiment in the form of a brain neurotoxicity test, using CRAd intracranially implanted into mice brain at lowest and highest doses, is recommended to determine the maximum tolerated dose (MTD) of the viral vector. Since mice are not permissive for human Ad replication as opposed to hamsters or cotton rats, those rodents represent better animal models for neurotoxicity testing.
Table 1.
Comparative toxicity of CRAd vectors.
| Cell type/sell type system | Vendor | Method to detect CRAd specificity/toxicity | Cytotoxic dose of CRAd/effect | Reference | |
|---|---|---|---|---|---|
| Human astrocytes | Adult primary astrocytes | Lonza | LDH | 10 vp per cell/∼25% dead cells | 30 |
| Lonza | Crystal violet toxicity test and CRAd replication | 10 vp per cell/∼90% toxicity | 124 | ||
| Lonza | Progeny titration | 10 MOI per cell/CRAd replication from 1.9 × 102 to 1 × 106 | 125 | ||
| Human fibroblasts | Fetal lung fibroblasts MRC5 | ATCC | Ad replication ratio to AdWT | 0.1 MOI per cell/0.2-0.5 | 63 |
| Dermal fibroblasts Hs68 | ATCC | Cytopathic effect, light microscopy | 50 MOI per cell | 75 | |
| Normal skin fibroblasts BJ | ATCC | Crystal violet toxicity test | Various toxicity from 10 to 0.01 MOI per cell | 91 | |
| Normal skin fibroblast BJ; fung fibroblasts IMR90; lung fibroblasts WI38 | ATCC | Crystal violet toxicity test | Various toxicity from 100 till 10 MOI | 126 |
The use of NSCs to deliver a CRAd payload requires safety testing as well. An important study by Aboody et al. showed that HB1.F3.CD NSCs (which lack HLA class I antigen) are non-tumorigenic after activating CD gene expression by pro-drug 5-fluorocytosine (5 FC).127 Considering that cytomegalovirus was found to persist in NSCs,128 it is important to assess the risk of stem cells application for patients. In case of high permissiveness, addition of 5FC or any other cytotoxic agent would eliminate the stem cells but may not kill cytomegalovirus harbored by those cells. Moreover, recent data suggest that the presence of cytomegalovirus may impact activation of pro-tumorigenic adenoviral regions, such as E1A.129 Of note, it has been shown that E1A genes under certain conditions may elicit formation of oncogenic fusions130 and form tumors in newborn hamsters131, 132 Therefore, it is highly unlikely, but still possible that CMV-mediated protein expression (IE1) can trans-activate E1A to produce tumorigenic phenotype in stem cells. From a therapeutic standpoint, it remains unclear whether NSC passage can affect efficacy of CRAd delivery, as well as whether aged stem cells are capable of inducing tumor formation.
Concluding remarks and future directions
Obtaining an FDA approval for the use of CRAd vectors in human clinical studies requires significant time and efforts from investigators. Although in many cases in vitro data obtained from tumor clinical samples and in vivo data from mouse xenograft models look very promising, a single modality treatment is often less effective than a combination of other therapeutic approaches. Moreover, recent studies suggested that inflammation and immune response might affect efficacy and specificity of CRAds as anti-glioma agents. Although various immunomodulation strategies have been suggested, which involve either genetic modifications of the Ad genome to suppress anti-Ad immune response, or shield viral particles from the immune system by means of coating with molecular polymers or loading inside GSC as vector delivery vehicles, they all require additional experimental evaluation. Finally, the established role of autophagy in promoting Ad-mediated oncolysis and suppression of the host immune response to Ad will help determining the marker of cell resistance controlling antiviral response at the cellular and organismal levels. It remains unclear whether delivery of anti-angiogenic or immunomodulatory factors by Ad vectors actually improves oncolytic effect in patients with brain tumors. This data need to be analyzed in the future. Although oncolytic adenoviruses alone demonstrate a substantial anti-glioma potency in vitro and in vivo, recent studies suggest that the combination of virotherapy with chemotherapy and/or immunotherapy may provide greater therapeutic benefit. Therefore, tackling glioma progression from different directions, i.e. by utilizing a combination of immunotherapy, angiogenic therapy, oncolytic virotherapy, radiotherapy, and chemotherapy could provide the most benefits for patient survival.
Conflicts of interest
All authors have no conflicts of interest.
Acknowledgments
Supported in part by National Cancer Institute (Bethesda, MD) grants R01 NS070289 (I.U., Charles Cobbs-PI), 5R03DE021758 (A.B.) and, generous support from Russian Fund of Fundamental Research (#No 11 411.0008700. 13.082 and No 13 411. 1008799.13.120 (A.Y.B.). We thank Dr. Ramon Alemany (Gene and Viral Therapy Group, IDIBELL-Catalan Institute of Oncology (ICO), L'Hospitalet de Llobregat, Barcelona, Spain) for valuable advices.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
I.V. Ulasov, Email: ulasov75@yahoo.com, Ilya.Ulasov@Swedish.org.
A.V. Borovjagin, Email: aborov@uab.edu.
B.A. Schroeder, Email: schro248@msu.edu.
A.Y. Baryshnikov, Email: baryshnikov_anat@mail.ru.
References
- 1.Zouaoui S., Darlix A., Fabbro-Peray P. Oncological patterns of care and outcomes for 265 elderly patients with newly diagnosed glioblastoma in France. Neurosurg Rev. 2014;37:415–424. doi: 10.1007/s10143-014-0528-8. [DOI] [PubMed] [Google Scholar]
- 2.Wen P.Y., Lee E.Q., Reardon D.A., Ligon K.L., Alfred Yung W.K. Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro Oncol. 2012;14:819–829. doi: 10.1093/neuonc/nos117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sant M., Minicozzi P., Lagorio S., Borge Johannesen T., Marcos-Gragera R., Francisci S. Survival of European patients with central nervous system tumors. Int J Cancer. 2012;131:173–185. doi: 10.1002/ijc.26335. [DOI] [PubMed] [Google Scholar]
- 4.Verhaak R.G., Hoadley K.A., Purdom E. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich J.N. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 6.Rich J.N., Bao S. Chemotherapy and cancer stem cells. Cell Stem Cell. 2007;1:353–355. doi: 10.1016/j.stem.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Friedman G.K., Cassady K.A., Beierle E.A., Markert J.M., Gillespie G.Y. Targeting pediatric cancer stem cells with oncolytic virotherapy. Pediatr Res. 2012;71(4 Pt 2):500–510. doi: 10.1038/pr.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sneddon J.B., Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price R.L., Song J., Bingmer K. Cytomegalovirus contributes to glioblastoma in the context of tumor suppressor mutations. Cancer Res. 2013;73:3441–3450. doi: 10.1158/0008-5472.CAN-12-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuessler A., Smith C., Beagley L. Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res. 2014;74:3466–3476. doi: 10.1158/0008-5472.CAN-14-0296. [DOI] [PubMed] [Google Scholar]
- 11.Cobbs C.S. Cytomegalovirus and brain tumor: epidemiology, biology and therapeutic aspects. Curr Opin Oncol. 2013;25:682–688. doi: 10.1097/CCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 12.Thaci B., Ulasov I.V., Ahmed A.U., Ferguson S.D., Han Y., Lesniak M.S. Anti-angiogenic therapy increases intratumoral adenovirus distribution by inducing collagen degradation. Gene Ther. 2013;20:318–327. doi: 10.1038/gt.2012.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiler M., Blaes J., Pusch S. mTOR target NDRG1 confers MGMT-dependent resistance to alkylating chemotherapy. Proc Natl Acad Sci U S A. 2014;111:409–414. doi: 10.1073/pnas.1314469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzmil M., Hemmings B.A. Overcoming resistance to rapalogs in gliomas by combinatory therapies. Biochim Biophys Acta. 2013;1834:1371–1380. doi: 10.1016/j.bbapap.2013.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Rowe W.P., Hartley J.W., Roizman B., Levy H.B. Characterization of a factor formed in the course of adenovirus infection of tissue cultures causing detachment of cells from glass. J Exp Med. 1958;108:713–729. doi: 10.1084/jem.108.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy H.B., Rowe W.P., Snellbaker F.L., Hartley J.W. Biochemical changes in HeLa cells associated with infection by type 2 adenovirus. Proc Soc Exp Biol Med. 1957;96:732–738. doi: 10.3181/00379727-96-23592. [DOI] [PubMed] [Google Scholar]
- 17.Stanton R.J., McSharry B.P., Armstrong M., Tomasec P., Wilkinson G.W. Re-engineering adenovirus vector systems to enable high-throughput analyses of gene function. Biotechniques. 2008;45:659–662. doi: 10.2144/000112993. 664-658. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.H., Lachuer J., Mittelbronn M. Alterations in the RB1 pathway in low-grade diffuse gliomas lacking common genetic alterations. Brain Pathol. 2011;21:645–651. doi: 10.1111/j.1750-3639.2011.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura M., Konishi N., Tsunoda S. Retinoblastoma protein expression and MIB-1 correlate with survival of patients with malignant astrocytoma. Cancer. 1997;80:242–249. doi: 10.1002/(sici)1097-0142(19970715)80:2<242::aid-cncr12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 20.Jha P., Suri V., Singh G. Characterization of molecular genetic alterations in GBMs highlights a distinctive molecular profile in young adults. Diagn Mol Pathol. 2011;20:225–232. doi: 10.1097/PDM.0b013e31821c30bc. [DOI] [PubMed] [Google Scholar]
- 21.Parato K.A., Senger D., Forsyth P.A., Bell J.C. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 22.Libertini S., Iacuzzo I., Ferraro A. Lovastatin enhances the replication of the oncolytic adenovirus dl1520 and its antineoplastic activity against anaplastic thyroid carcinoma cells. Endocrinology. 2007;148:5186–5194. doi: 10.1210/en.2007-0752. [DOI] [PubMed] [Google Scholar]
- 23.Geoerger B., Grill J., Opolon P. Oncolytic activity of the E1B-55 kDa-deleted adenovirus ONYX-015 is independent of cellular p53 status in human malignant glioma xenografts. Cancer Res. 2002;62:764–772. [PubMed] [Google Scholar]
- 24.Heise C., Sampson-Johannes A., Williams A., McCormick F., Von Hoff D.D., Kirn D.H. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–645. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 25.Libertini S., Abagnale A., Passaro C. AZD1152 negatively affects the growth of anaplastic thyroid carcinoma cells and enhances the effects of oncolytic virus dl922-947. Endocr Relat Cancer. 2011;18:129–141. doi: 10.1677/ERC-10-0234. [DOI] [PubMed] [Google Scholar]
- 26.Fueyo J., Gomez-Manzano C., Alemany R. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Manzano C., Balague C., Alemany R. A novel E1A-E1B mutant adenovirus induces glioma regression in vivo. Oncogene. 2004;23:1821–1828. doi: 10.1038/sj.onc.1207321. [DOI] [PubMed] [Google Scholar]
- 28.Freytag S.O., Barton K.N., Zhang Y. Efficacy of oncolytic adenovirus expressing suicide genes and interleukin-12 in preclinical model of prostate cancer. Gene Ther. 2013;20:1131–1139. doi: 10.1038/gt.2013.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuxe J., Liu L., Malin S., Philipson L., Collins V.P., Pettersson R.F. Expression of the coxsackie and adenovirus receptor in human astrocytic tumors and xenografts. Int J Cancer. 2003;103:723–729. doi: 10.1002/ijc.10891. [DOI] [PubMed] [Google Scholar]
- 30.Nandi S., Ulasov I.V., Rolle C.E., Han Y., Lesniak M.S. A chimeric adenovirus with an Ad 3 fiber knob modification augments glioma virotherapy. J Gene Med. 2009;11:1005–1011. doi: 10.1002/jgm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wohlfahrt M.E., Beard B.C., Lieber A., Kiem H.P. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res. 2007;67:8783–8790. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- 32.Li X., Mao Q., Wang D., Zhang W., Xia H. A fiber chimeric CRAd vector Ad5/11-D24 double-armed with TRAIL and arresten for enhanced glioblastoma therapy. Hum Gene Ther. 2012;23:589–596. doi: 10.1089/hum.2011.130. [DOI] [PubMed] [Google Scholar]
- 33.Zheng S., Ulasov I.V., Han Y., Tyler M.A., Zhu Z.B., Lesniak M.S. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007;9:151–160. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 34.Ulasov I.V., Zhu Z.B., Tyler M.A. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007;18:589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 35.Yokoyama T., Iwado E., Kondo Y. Autophagy-inducing agents augment the antitumor effect of telerase-selve oncolytic adenovirus OBP-405 on glioblastoma cells. Gene Ther. 2008;15:1233–1239. doi: 10.1038/gt.2008.98. [DOI] [PubMed] [Google Scholar]
- 36.Lamfers M.L., Grill J., Dirven C.M. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002;62:5736–5742. [PubMed] [Google Scholar]
- 37.de Jonge J., Berghauser Pont L.M., Idema S. Therapeutic concentrations of anti-epileptic drugs do not inhibit the activity of the oncolytic adenovirus Delta24-RGD in malignant glioma. J Gene Med. 2013;15:134–141. doi: 10.1002/jgm.2703. [DOI] [PubMed] [Google Scholar]
- 38.Holzmuller R., Mantwill K., Haczek C. YB-1 dependent virotherapy in combination with temozolomide as a multimodal therapy approach to eradicate malignant glioma. Int J Cancer. 2011;129:1265–1276. doi: 10.1002/ijc.25783. [DOI] [PubMed] [Google Scholar]
- 39.Alonso M.M., Jiang H., Yokoyama T. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther. 2008;16:487–493. doi: 10.1038/sj.mt.6300400. [DOI] [PubMed] [Google Scholar]
- 40.Lamfers M.L., Idema S., Bosscher L. Differential effects of combined Ad5-delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin Cancer Res. 2007;13:7451–7458. doi: 10.1158/1078-0432.CCR-07-1265. [DOI] [PubMed] [Google Scholar]
- 41.Jiang H., Gomez-Manzano C., Aoki H. Examination of the therapeutic potential of Delta-24-RGD in brain tumor stem cells: role of autophagic cell death. J Natl Cancer Inst. 2007;99:1410–1414. doi: 10.1093/jnci/djm102. [DOI] [PubMed] [Google Scholar]
- 42.Alonso M.M., Cascallo M., Gomez-Manzano C. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007;67:8255–8263. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 43.Wickham T.J., Tzeng E., Shears L.L., 2nd Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tyler M.A., Ulasov I.V., Borovjagin A. Enhanced transduction of malignant glioma with a double targeted Ad5/3-RGD fiber-modified adenovirus. Mol Cancer Ther. 2006;5:2408–2416. doi: 10.1158/1535-7163.MCT-06-0187. [DOI] [PubMed] [Google Scholar]
- 45.Arwert E., Hingtgen S., Figueiredo J.L. Visualizing the dynamics of EGFR activity and antiglioma therapies in vivo. Cancer Res. 2007;67:7335–7342. doi: 10.1158/0008-5472.CAN-07-0077. [DOI] [PubMed] [Google Scholar]
- 46.Grill J., Van Beusechem V.W., Van Der Valk P. Combined targeting of adenoviruses to integrins and epidermal growth factor receptors increases gene transfer into primary glioma cells and spheroids. Clin Cancer Res. 2001;7:641–650. [PubMed] [Google Scholar]
- 47.van Beusechem V.W., Grill J., Mastenbroek D.C. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J Virol. 2002;76:2753–2762. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Beusechem V.W., Mastenbroek D.C., van den Doel P.B. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003;10:1982–1991. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- 49.Lorimer I.A., Lavictoire S.J. Targeting retrovirus to cancer cells expressing a mutant EGF receptor by insertion of a single chain antibody variable domain in the envelope glycoprotein receptor binding lobe. J Immunol Methods. 2000;237:147–157. doi: 10.1016/s0022-1759(99)00219-7. [DOI] [PubMed] [Google Scholar]
- 50.Piao Y., Jiang H., Alemany R. Oncolytic adenovirus retargeted to Delta-EGFR induces selective antiglioma activity. Cancer Gene Ther. 2009;16:256–265. doi: 10.1038/cgt.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Altieri D.C. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 52.Ulasov I.V., Rivera A.A., Sonabend A.M. Comparative evaluation of survivin, midkine and CXCR4 promoters for transcriptional targeting of glioma gene therapy. Cancer Biol Ther. 2007;6:679–685. doi: 10.4161/cbt.6.5.3957. [DOI] [PubMed] [Google Scholar]
- 53.Rosa J., Canovas P., Islam A., Altieri D.C., Doxsey S.J. Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Mol Biol Cell. 2006;17:1483–1493. doi: 10.1091/mbc.E05-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulasov I.V., Tyler M.A., Zhu Z.B., Han Y., He T.C., Lesniak M.S. Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. Int J Oncol. 2009;34:729–742. doi: 10.3892/ijo_00000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nandi S., Ulasov I.V., Tyler M.A. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008;68:5778–5784. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muramatsu T. Midkine: a promising molecule for drug development to treat diseases of the central nervous system. Curr Pharm Des. 2011;17:410–423. doi: 10.2174/138161211795164167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohno S., Nakagawa K., Hamada K. Midkine promoter-based conditionally replicative adenovirus for malignant glioma therapy. Oncol Rep. 2004;12:73–78. [PubMed] [Google Scholar]
- 58.Jafri A.M., Sarina S., George P.J., Nizam I.M. Presence of telomerase activity with undetectable p16 gene mutation in Malaysian patients with brain tumor. Med J Malaysia. 2004;59:480–485. [PubMed] [Google Scholar]
- 59.Kheirollahi M., Mehrazin M., Kamalian N., Mohammadi-asl J., Mehdipour P. Telomerase activity in human brain tumors: astrocytoma and meningioma. Cell Mol Neurobiol. 2013;33:569–574. doi: 10.1007/s10571-013-9923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bortolanza S., Qian C., Kramer M.G. An oncolytic adenovirus controlled by a modified telomerase promoter is attenuated in telomerase-negative cells, but shows reduced activity in cancer cells. J Mol Med (Berl) 2005;83:736–747. doi: 10.1007/s00109-005-0681-1. [DOI] [PubMed] [Google Scholar]
- 61.Won J., Chang S., Oh S., Kim T.K. Small-molecule-based identification of dynamic assembly of E2F-pocket protein-histone deacetylase complex for telomerase regulation in human cells. Proc Natl Acad Sci U S A. 2004;101:11328–11333. doi: 10.1073/pnas.0401801101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffmann D., Meyer B., Wildner O. Improved glioblastoma treatment with Ad5/35 fiber chimeric conditionally replicating adenoviruses. J Gene Med. 2007;9:764–778. doi: 10.1002/jgm.1076. [DOI] [PubMed] [Google Scholar]
- 63.Horst M., Brouwer E., Verwijnen S. Targeting malignant gliomas with a glial fibrillary acidic protein (GFAP)-selective oncolytic adenovirus. J Gene Med. 2007;9:1071–1079. doi: 10.1002/jgm.1110. [DOI] [PubMed] [Google Scholar]
- 64.Zheng X., Rao X.M., Snodgrass C. Adenoviral E1a expression levels affect virus-selective replication in human cancer cells. Cancer Biol Ther. 2005;4:1255–1262. doi: 10.4161/cbt.4.11.2137. [DOI] [PubMed] [Google Scholar]
- 65.Irving J., Wang J., Powell S. Conditionally replicative adenovirus driven by the human telomerase promoter provides broad-spectrum antitumor activity without liver toxicity. Cancer Gene Ther. 2004;11(3):174–185. doi: 10.1038/sj.cgt.7700666. [DOI] [PubMed] [Google Scholar]
- 66.Harkins L.E., Matlaf L.A., Soroceanu L. Detection of human cytomegalovirus in normal and neoplastic breast epithelium. Herpesviridae. 2010;1:8. doi: 10.1186/2042-4280-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taher C., de Boniface J., Mohammad A.A. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PLoS One. 2013;8:e56795. doi: 10.1371/journal.pone.0056795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ding D., Han S., Wang Z., Guo Z., Wu A. Does the existence of HCMV components predict poor prognosis in glioma? J Neurooncol. 2014;116:515–522. doi: 10.1007/s11060-013-1350-9. [DOI] [PubMed] [Google Scholar]
- 69.Dos Santos C.J., Stangherlin L.M., Figueiredo E.G., Correa C., Teixeira M.J., da Silva M.C. High prevalence of HCMV and viral load in tumor tissues and peripheral blood of glioblastoma multiforme patients. J Med Virol. 2014 Nov;86(11):1953–1961. doi: 10.1002/jmv.23820. [DOI] [PubMed] [Google Scholar]
- 70.Soroceanu L., Matlaf L., Bezrookove V. Human cytomegalovirus US28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011;71:6643–6653. doi: 10.1158/0008-5472.CAN-11-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayakawa H., Uchiumi T., Fukuda T. Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry. 2002;41:12739–12744. doi: 10.1021/bi0201872. [DOI] [PubMed] [Google Scholar]
- 72.Izumi H., Imamura T., Nagatani G. Y box-binding protein-1 binds preferentially to single-stranded nucleic acids and exhibits 3′→5′ exonuclease activity. Nucleic Acids Res. 2001;29:1200–1207. doi: 10.1093/nar/29.5.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koike K., Uchiumi T., Ohga T. Nuclear translocation of the Y-box binding protein by ultraviolet irradiation. FEBS Lett. 1997;417:390–394. doi: 10.1016/s0014-5793(97)01296-9. [DOI] [PubMed] [Google Scholar]
- 74.Gaudreault I., Guay D., Lebel M. YB-1 promotes strand separation in vitro of duplex DNA containing either mispaired bases or cisplatin modifications, exhibits endonucleolytic activities and binds several DNA repair proteins. Nucleic Acids Res. 2004;32:316–327. doi: 10.1093/nar/gkh170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bieler A., Mantwill K., Dravits T. Novel three-pronged strategy to enhance cancer cell killing in glioblastoma cell lines: histone deacetylase inhibitor, chemotherapy, and oncolytic adenovirus dl520. Hum Gene Ther. 2006;17:55–70. doi: 10.1089/hum.2006.17.55. [DOI] [PubMed] [Google Scholar]
- 76.Mantwill K., Naumann U., Seznec J. YB-1 dependent oncolytic adenovirus efficiently inhibits tumor growth of glioma cancer stem like cells. J Transl Med. 2013;11:216. doi: 10.1186/1479-5876-11-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ueki K., Ono Y., Henson J.W., Efird J.T., von Deimling A., Louis D.N. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996;56:150–153. [PubMed] [Google Scholar]
- 78.Parr M.J., Manome Y., Tanaka T. Tumor-selective transgene expression in vivo mediated by an E2F-responsive adenoviral vector. Nat Med. 1997;3:1145–1149. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 79.Muller H., Moroni M.C., Vigo E., Petersen B.O., Bartek J., Helin K. Induction of S-phase entry by E2F transcription factors depends on their nuclear localization. Mol Cell Biol. 1997;17:5508–5520. doi: 10.1128/mcb.17.9.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao W., Guo G., Zhang Q., Fan L., Wu N., Bo Y. The application of multiple miRNA response elements enables oncolytic adenoviruses to possess specificity to glioma cells. Virology. 2014;458-459:69–82. doi: 10.1016/j.virol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto M., Davydova J., Takayama K., Alemany R., Curiel D.T. Transcription initiation activity of adenovirus left-end sequence in adenovirus vectors with e1 deleted. J Virol. 2003;77:1633–1637. doi: 10.1128/JVI.77.2.1633-1637.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osborne T.F., Berk A.J. Far upstream initiation sites for adenovirus early region 1A transcription are utilized after the onset of viral DNA replication. J Virol. 1983;45:594–599. doi: 10.1128/jvi.45.2.594-599.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berk A.J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- 84.Ulasov I.V., Sonabend A.M., Nandi S., Khramtsov A., Han Y., Lesniak M.S. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer. 2009;100:1154–1164. doi: 10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tyler M.A., Ulasov I.V., Lesniak M.S. Cancer cell death by design: apoptosis, autophagy and glioma virotherapy. Autophagy. 2009;5:856–857. doi: 10.4161/auto.8792. [DOI] [PubMed] [Google Scholar]
- 86.Tazawa H., Kagawa S., Fujiwara T. Oncolytic adenovirus-induced autophagy: tumor-suppressive effect and molecular basis. Acta Med Okayama. 2013;67:333–342. doi: 10.18926/AMO/52006. [DOI] [PubMed] [Google Scholar]
- 87.Jiang H., White E.J., Rios-Vicil C.I., Xu J., Gomez-Manzano C., Fueyo J. Human adenovirus type 5 induces cell lysis through autophagy and autophagy-triggered caspase activity. J Virol. 2011;85:4720–4729. doi: 10.1128/JVI.02032-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gomez-Manzano C., Fueyo J. Oncolytic adenoviruses for the treatment of brain tumors. Curr Opin Mol Ther. 2010;12:530–537. [PubMed] [Google Scholar]
- 89.Kim J., Kim P.H., Yoo J.Y. Double E1B 19 kDa- and E1B 55 kDa-deleted oncolytic adenovirus in combination with radiotherapy elicits an enhanced anti-tumor effect. Gene Ther. 2009;16:1111–1121. doi: 10.1038/gt.2009.72. [DOI] [PubMed] [Google Scholar]
- 90.Jiang H., White E.J., Gomez-Manzano C., Fueyo J. Adenovirus's last trick: you say lysis, we say autophagy. Autophagy. 2008;4:118–120. doi: 10.4161/auto.5260. [DOI] [PubMed] [Google Scholar]
- 91.Yun C.O., Kim E., Koo T., Kim H., Lee Y.S., Kim J.H. ADP-overexpressing adenovirus elicits enhanced cytopathic effect by induction of apoptosis. Cancer Gene Ther. 2005;12:61–71. doi: 10.1038/sj.cgt.7700769. [DOI] [PubMed] [Google Scholar]
- 92.Kaliberova L.N., Krendelchtchikova V., Harmon D.K. CRAdRGDflt-IL24 virotherapy in combination with chemotherapy of experimental glioma. Cancer Gene Ther. 2009;16:794–805. doi: 10.1038/cgt.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang S., El-Deiry W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–8633. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 94.El-Deiry W.S. Insights into cancer therapeutic design based on p53 and TRAIL receptor signaling. Cell Death Differ. 2001;8:1066–1075. doi: 10.1038/sj.cdd.4400943. [DOI] [PubMed] [Google Scholar]
- 95.Ozoren N., El-Deiry W.S. Cell surface death receptor signaling in normal and cancer cells. Semin Cancer Biol. 2003;13:135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 96.Verhagen A.M., Ekert P.G., Pakusch M. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 97.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 98.Fulda S., Kufer M.U., Meyer E., van Valen F., Dockhorn-Dworniczak B., Debatin K.M. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20:5865–5877. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- 99.Green D.R. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 100.Tsamis K.I., Alexiou G.A., Vartholomatos E., Kyritsis A.P. Combination treatment for glioblastoma cells with tumor necrosis factor-related apoptosis-inducing ligand and oncolytic adenovirus delta-24. Cancer Invest. 2013;31:630–638. doi: 10.3109/07357907.2013.849724. [DOI] [PubMed] [Google Scholar]
- 101.Conrad C., Miller C.R., Ji Y. Delta24-hyCD adenovirus suppresses glioma growth in vivo by combining oncolysis and chemosensitization. Cancer Gene Ther. 2005;12:284–294. doi: 10.1038/sj.cgt.7700750. [DOI] [PubMed] [Google Scholar]
- 102.Miller C.R., Williams C.R., Buchsbaum D.J., Gillespie G.Y. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–780. [PubMed] [Google Scholar]
- 103.Tollefson A.E., Ryerse J.S., Scaria A., Hermiston T.W., Wold W.S. The E3-11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 104.Tollefson A.E., Scaria A., Hermiston T.W., Ryerse J.S., Wold L.J., Wold W.S. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bilbao R., Bustos M., Alzuguren P. A blood-tumor barrier limits gene transfer to experimental liver cancer: the effect of vasoactive compounds. Gene Ther. 2000;7:1824–1832. doi: 10.1038/sj.gt.3301312. [DOI] [PubMed] [Google Scholar]
- 106.Ram Z., Culver K.W., Oshiro E.M. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3:1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 107.Mohyeldin A., Garzon-Muvdi T., Quinones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 108.Bar E.E. Glioblastoma, cancer stem cells and hypoxia. Brain Pathol. 2011;21:119–129. doi: 10.1111/j.1750-3639.2010.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sgubin D., Wakimoto H., Kanai R., Rabkin S.D., Martuza R.L. Oncolytic herpes simplex virus counteracts the hypoxia-induced modulation of glioblastoma stem-like cells. Stem Cells Transl Med. 2012;1:322–332. doi: 10.5966/sctm.2011-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Z., Bao S., Wu Q. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Post D.E., Van Meir E.G. A novel hypoxia-inducible factor (HIF) activated oncolytic adenovirus for cancer therapy. Oncogene. 2003;22:2065–2072. doi: 10.1038/sj.onc.1206464. [DOI] [PubMed] [Google Scholar]
- 112.Liikanen I., Ahtiainen L., Hirvinen M.L. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thomas M.A., Spencer J.F., Toth K., Sagartz J.E., Phillips N.J., Wold W.S. Immunosuppression enhances oncolytic adenovirus replication and antitumor efficacy in the Syrian hamster model. Mol Ther. 2008;16:1665–1673. doi: 10.1038/mt.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kleijn A., Kloezeman J., Treffers-Westerlaken E. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS One. 2014;9:e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shinojima N., Hossain A., Takezaki T. TGF-beta mediates homing of bone marrow-derived human mesenchymal stem cells to glioma stem cells. Cancer Res. 2013;73:2333–2344. doi: 10.1158/0008-5472.CAN-12-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hata N., Shinojima N., Gumin J. Platelet-derived growth factor BB mediates the tropism of human mesenchymal stem cells for malignant gliomas. Neurosurgery. 2010;66:144–156. doi: 10.1227/01.NEU.0000363149.58885.2E. discussion 156–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Birnbaum T., Roider J., Schankin C.J. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 118.Ahmed A.U., Rolle C.E., Tyler M.A. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hai C., Jin Y.M., Jin W.B. Application of mesenchymal stem cells as a vehicle to deliver replication-competent adenovirus for treating malignant glioma. Chin J Cancer. 2012;31:233–240. doi: 10.5732/cjc.011.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yong R.L., Shinojima N., Fueyo J. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tyler M.A., Ulasov I.V., Sonabend A.M. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16:262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thaci B., Ahmed A.U., Ulasov I.V. Pharmacokinetic study of neural stem cell-based cell carrier for oncolytic virotherapy: targeted delivery of the therapeutic payload in an orthotopic brain tumor model. Cancer Gene Ther. 2012;19:431–442. doi: 10.1038/cgt.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim C.K., Ahmed A.U., Auffinger B. N-acetylcysteine amide augments the therapeutic effect of neural stem cell-based antiglioma oncolytic virotherapy. Mol Ther. 2013;21:2063–2073. doi: 10.1038/mt.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ulasov I.V., Tyler M.A., Rivera A.A., Nettlebeck D.M., Douglas J.T., Lesniak M.S. Evaluation of E1A double mutant oncolytic adenovectors in anti-glioma gene therapy. J Med Virol. 2008;80:1595–1603. doi: 10.1002/jmv.00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fueyo J., Alemany R., Gomez-Manzano C. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 126.Kang Y.A., Shin H.C., Yoo J.Y., Kim J.H., Kim J.S., Yun C.O. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol Ther. 2008;16:1033–1040. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 127.Aboody K.S., Najbauer J., Metz M.Z. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5(184) doi: 10.1126/scitranslmed.3005365. 184ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belzile J.P., Stark T.J., Yeo G.W., Spector D.H. Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. J Virol. 2014;88:4021–4039. doi: 10.1128/JVI.03492-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakajima T., Mukai N. Cell origin of human adenovirus type 12-induced subcutaneous tumor in Syrian hamsters. Acta Neuropathol. 1979;45:187–194. doi: 10.1007/BF00702670. [DOI] [PubMed] [Google Scholar]
- 130.Kirn D., Hermiston T. Induction of an oncogenic fusion protein by a viral gene – a new chapter in an old story. Nat Med. 1999;5:991–992. doi: 10.1038/12426. [DOI] [PubMed] [Google Scholar]
- 131.Mukai N., Kobayashi S. Undifferentiated intraperitoneal tumors induced by human adenovirus type 12 in hamsters. Am J Pathol. 1972;69:331–348. [PMC free article] [PubMed] [Google Scholar]
- 132.Hohlweg U., Hosel M., Dorn A. Intraperitoneal dissemination of Ad12-induced undifferentiated neuroectodermal hamster tumors: de novo methylation and transcription patterns of integrated viral and of cellular genes. Virus Res. 2003;98:45–56. doi: 10.1016/j.virusres.2003.08.012. [DOI] [PubMed] [Google Scholar]