Abstract
Background
Posterior reversible encephalopathy syndrome (PRES) comprises clinical and radiologic findings with rapid onset and potentially dire consequences. Patients experience hypertension, seizures, headache, visual disturbance, and/or altered mentation. Magnetic resonance imaging shows edematous changes in brain (especially parietal and occipital lobes). We report PRES associated with anti-GD2 monoclonal antibody (MoAb) immunotherapy which is now standard for high-risk neuroblastoma but has not previously been implicated in PRES.
Methods
Successive clinical trials using the anti-GD2 MoAb 3F8 for neuroblastoma patients involved multiple cycles of standard-dose 3F8 (SD-3F8) (20 mg/m2/day, x5 days/cycle) or two cycles of high-dose 3F8 (HD-3F8) (80 mg/m2/day, x5 days/cycle) followed by cycles of SD-3F8.
Results
PRES was diagnosed in 5/215 (2.3%) patients, including 3/160 (1.9%) patients receiving SD-3F8 and 2/55 (3.6%) patients receiving HD-3F8 (p=0.6). All five patients had a rapid return to clinical-radiologic baseline. PRES occurred in 3/26 (11.5%) patients whose prior treatment included external-beam radiotherapy to the brain (2/6 patients status-post total body irradiation plus 1/20 patients status-post craniospinal irradiation) compared to 2/189 (1.1%) patients without prior brain irradiation (p=0.01). Hypertension, which is strongly linked to PRES, reached grade 3 toxicity in 12/215 (5.6%) patients, including the five patients with PRES and seven patients without PRES.
Conclusions
Patients receiving anti-GD2 MoAb immunotherapy should be closely monitored for, and undergo urgent treatment or evaluation of, symptoms (e.g., hypertension or headaches) that might herald PRES. Prior brain irradiation may be a predisposing factor for PRES with this immunotherapy.
Keywords: immunotherapy, neuroblastoma, PRES, monoclonal antibodies, hypertension
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) comprises striking clinical and radiologic findings with rapid onset and potentially dire consequences.1–3 The clinical features are variable but can include hypertension, seizures, headache, visual disturbance, and/or altered mentation. The radiologic hallmark is magnetic resonance imaging (MRI) of the brain showing edematous changes best visualized with fluid-attenuated inversion recovery (FLAIR) sequences. Parietal and occipital lobes are predominantly involved possibly because their relative lack of sympathetic innervation translates into greater susceptibility to adverse effects of hypertension.3
When first reported,4 this acutely developing clinico-radiologic phenomenon was called reversible posterior leukoencephalopathy. The name was modified5 because not only subcortical white but also cortical gray matter is often involved. Despite the alarming symptomatology and extensive radiologic abnormalities, optimal treatment typically results in a return to pre-PRES clinical and radiologic status within weeks, although exceptions occur, including in children.6–8
The underlying pathophysiology leading to the vasogenic edema without infarction and MRI appearance of PRES remains speculative.2,3 Etiologic considerations take into account hypertension and injury to vascular endothelium and the blood-brain barrier. Associated clinical disorders include ecclampsia, cancer, and autoimmune disease. Associated medications include immunosuppressive, chemotherapeutic, and anti-angiogenic agents. PRES has never, to our knowledge, been reported with immunotherapy mediated by anti-GD2 monoclonal antibody (MoAb). This treatment is now standard for high-risk neuroblastoma, based on favorable results in a landmark randomized study with the anti-GD2 chimeric ch14.18 MoAb,9 which followed phase I and II trials.10–14 We have used the anti-GD2 murine 3F8 MoAb in phase I and II studies.15–21 We now report PRES with 3F8.
PATIENTS AND METHODS
At Memorial Sloan-Kettering Cancer Center (MSKCC), patients with high-risk neuroblastoma in 1st or ≥2nd complete/very good partial remission (CR/VGPR) or resistant to induction and 2nd-line chemotherapy (primary refractory disease) received standard-dose 3F8 (SD-3F8), i.e., 20 mg/m2/day, x5 days/cycle, on protocol 03–077 (NCT00072358) (Table 1). In the successor MSKCC protocols 09–158 (1st CR/VGPR, post-stem cell transplantation [SCT]; NCT01183416); 09–159 (1st CR/VGPR, no prior SCT; NCT01183429); 09–160 (≥2nd CR/VGPR; NCT01183884); and 09–161 (primary refractory disease; NCT01183897), the initial two cycles used high-dose 3F8 (HD-3F8), i.e., 80 mg/m2/day, x5 days/cycle; subsequent cycles used SD-3F8 (Table 1). Granulocyte-macrophage colony stimulating factor (GM-CSF) was injected subcutaneously at least one hour before 30-minute intravenous infusions of SD-3F8 or HD-3F8.
Table 1.
Immunotherapy schema
| Priming doses of scGM-CSF | scGM-CSF + 3F8 by 30-minute iv infusion | |
| 5 days (Wednesday–Sunday) | → | 5 days (Monday–Friday) |
sc, subcutaneous; iv, intravenous
GM-CSF: 250 μg/m2/day for the priming doses and first 2 days of 3F8, then 500 μg/m2/day
3F8: standard-dose regimen: 20 mg/m2/day; high-dose regimen: 80 mg/m2/day
Rest periods: 2–4 weeks from end of one cycle to start of 3F8 in next cycle, through 4 cycles after complete response; then 6–8 week rest periods, through 24 months from the first dose of 3F8. Cycles were deferred only for HAMA-positivity.
For these protocols, eligibility requirements included less than grade 3 toxicity of major organs by National Cancer Institute Common Toxicity Criteria version 3.0. These criteria were also used to score toxicities of therapy. Informed written consents for treatment and tests were obtained according to institutional review board rules. In the absence of human anti-mouse antibody (HAMA), 3F8 treatments were repeated monthly x4 cycles after documentation of CR/VGPR, and then every 6–8 weeks through 24 months from the first dose of 3F8. Protocol treatment also included six cycles of 13-cis-retinoic acid 160 mg/m2/day, x14 days/cycle, following established practice.22 Before study enrollment and then at least every three months, all patients underwent extent-of-disease evaluations that included 123I-metaiodobenzylguanidine (MIBG) scan and computed tomography (CT) or MRI of the primary site and head. Imaging of the head was standard because of our concern about asymptomatic relapse in the central nervous system (CNS).23
Because of expected pain and hives, opiates and antihistamines were administered before initiating 3F8 infusions and then as needed. Vital signs (including blood pressure) were measured as follows: at two separate time points before 3F8 treatment; during the 30-minute infusion of 3F8; upon completion of the 3F8 infusion; and before discharge from the clinic.
PRES was prospectively diagnosed and studied in the protocol patients treated with 3F8/GM-CSF from July 2009, when this entity was first identified in this patient population and thereby first came to our attention as a toxicity risk, through August 2012. Of note, the herald case did not have a seizure, a hallmark symptom of PRES. Subsequent to this unexpected occurrence of PRES, the appearance of symptoms concerning for PRES prompted appropriate investigations.
Comparisons regarding the frequency of events were assessed using Fischer’s exact test (two-sided).
RESULTS
PRES was diagnosed in 5/215 (2.3%) patients, including 3/160 (1.9%) patients receiving SD-3F8 and 2/55 (3.6%) patients receiving HD-3F8 (p=0.6). PRES was associated with 5/847 (<1%) cycles of 3F8, including 3/753 (<1%) cycles of SD-3F8 and 2/94 (2.1%) cycles of HD-3F8 (p=0.10). After experiencing PRES, the five patients received no additional MoAb therapy.
Hypertension, which is strongly linked to PRES, reached grade 3 toxicity in 12/215 (5.6%) patients, including 9/160 (5.6%) patients receiving SD-3F8 and 3/55 (5.5%) patients receiving HD-3F8 (p=1.0). The 12 patients with grade 3 hypertension included all five patients with PRES and seven patients without PRES. Among the latter, three patients (including one with preexisting hypertension from nephrotoxic chemoradiotherapy) developed grade 3 hypertension in two cycles (of SD-3F8), and one patient (treated with HD-3F8) had preexisting hypertension from disease-related renal failure and had previously experienced unacceptable toxicity with ch14.189 (stopped after two of the planned five cycles). Overall, grade 3 hypertension occurred in 15/847 (1.8%) cycles, including 12/753 (1.6%) cycles of SD-3F8 and 3/94 (3.2%) cycles of HD-3F8 (p=0.23).
Other symptoms associated with PRES, including seizures, headaches, visual disturbances, and altered mentation, were not associated with the immunotherapy.
Patients with PRES: clinical characteristics and prior therapy
The five patients (3 male, 2 female) were 1.9–7.9 (median 5.3) years old when diagnosed with neuroblastoma, and 4.9–11.1 (median 9.3) years old when diagnosed with PRES (Table 2). The time from diagnosis of neuroblastoma to PRES was 1.1–4.3 (median 3.1) years. These five patients were receiving 3F8/GM-CSF as treatment for primary refractory disease (n=2) or to consolidate ≥2nd CR (n=3). Prior oncologic treatment included 8–15 (median 10) cycles of chemotherapy, completed 1–21 (median 6) months before PRES.
Table 2.
PRES patients: Clinical profiles
| Patient no./gender | Age (years) at Dx of NB | ---------------------------Prior treatment------------------------ | --------------------PRES----------------- | ||||
|---|---|---|---|---|---|---|---|
| No. of cycles of chemoRxa | Radiation to brain | Anti-GD2 MoAb | Age (years) | Disease status | Months from Dx/chemoRx | ||
| Protocol 03–077 | |||||||
| 1/F | 4.2 | 9 | none | HD-3F8 x2 cycles SD-3F8 x3 cycles |
5.3 | 1O refractory | 13/6 |
| 2/F | 7.9 | 8b | 131I-MIBGx2, TBI | SD-3F8 x6 cycles | 9.4 | 1O refractory | 17/6 |
| 3/M | 6.8 | 12 | none | SD-3F8 x11 cycles | 11.1 | 3rd CR | 51/21 |
| Protocol 09–160 | |||||||
| 4/M | 1.8 | 10c | CSI, IT-131I-8H9 | ch14.18 x5 cyclesd HD-3F8 x1 cycle |
4.9 | 2nd CR | 37/3 |
| 5/M | 5.3 | 15c | TBI | HD-3F8 x1 cycle | 9.3 | 2nd CR | 48/1 |
chemoRx, chemotherapy; CR, complete remission; CSI, craniospinal irradiation; Dx, diagnosis, IT, intrathecal; HD, high-dose; MoAb, monoclonal antibody; NB, neuroblastoma; SD, standard-dose; TBI, total body irradiation
included various combinations of high-dose cyclophosphamide, high-dose cisplatin, doxorubicin, etoposide, topotecan, and irinotecan in all patients
including myeloablative chemoradiotherapy (melphalan/TBI) with autologous stem-cell transplantation
including tandem autologous stem-cell transplantations after myeloablative chemotherapy
completed 23 months before PRES
Three patients had prior external-beam radiotherapy to brain: total body irradiation (TBI) (patient #5); TBI plus 131I-MIBG therapy (patient #2); and craniospinal irradiation (1800 cGy) plus intrathecal administration of 131I-8H9 MoAb23 as treatment for a relapse in the brain (patient #4). Overall, PRES was diagnosed in 2/6 study patients who had received TBI and in 1/20 study patients who had received craniospinal irradiation (19 also received intrathecal radiolabeled MoAb23). Thus, PRES occurred in 3/26 (11.5%) patients whose prior treatment included external-beam radiotherapy to the brain compared to 2/189 (1.1%) patients without prior brain irradiation (p=0.01). PRES was associated with 3/89 (3.4%) cycles in patients with prior brain irradiation, and with 2/758 (0.3%) cycles in patients without that prior treatment (p=0.01).
The three patients who developed PRES in association with SD-3F8 had previously received multiple cycles of 3F8: patient #1 had tolerated two cycles of HD-3F8 (in a phase I study20) plus three cycles of SD-3F8 (with grade 3 emesis between the 2nd and 3rd cycles – see below); patient #2 had been treated with five cycles of SD-3F8 without complication; and patient #3 had received 11 cycles of SD-3F8 marked by intermittent hypertension that responded to nifedipine.The two patients who developed PRES after only one cycle of HD-3F8 had not previously been treated with 3F8 although one (patient #4) had received five cycles of ch14.189 without complication. Subsequently, as noted above, this patient relapsed in brain and received multi-modality therapy23 before proceeding to consolidation with HD-3F8.
Clinical features of PRES
Patient #1, who was the first 3F8 patient diagnosed with PRES, presented with emesis after only two days of her sixth cycle of 3F8 (Table 3). (She had developed grade 3 emesis of unknown etiology 8 days after completion of an earlier cycle of SD-3F8, but had done well with the subsequent cycle.) CT showed no intestinal block, but MRI performed to rule out relapse in the brain as the cause for the emesis revealed findings indicative of PRES. This patient never had a seizure. In the four subsequently diagnosed cases of PRES (Table 3), the presenting symptoms included headache followed by a seizure <24 hours after completion of a cycle of 3F8 (patients #3 and #5); headache <24 hours after completion of a cycle with a seizure on the following day (patient #2); and a seizure five days after completion of a cycle (patient #4).
Table 3.
Clinical features of PRES
| Patient no. | Problem with prior MoAb Rx | Timing; initial symptoms | Hypertension | Headache | Visual disturbance | Altered mentation | Seizure | Hyponatremia |
|---|---|---|---|---|---|---|---|---|
| 1. | Emesisa | 2 days of cycle 6; emesis and hypertension | 140/110 | none | none | lethargic, agitated | none | Grade 3 |
| 2. | none | 1 day post-cycle 6; headache | 170/110 | yes | none | “not making sense” | complex, then brief sensory | none |
| 3. | Hypertensionb | 1 day post-cycle 11; headache, visión disturbance, seizure | 174/110 | yes | vision loss | confused | left-sided | none |
| 4. | none | 5 days post-cycle 1; seizure | 150s/100s | none | none | none | generalized, then partial | Grade 4 |
| 5. | not applicable (no prior anti-GD2 Rx) | 1 day post-cycle 1; headache and seizure | 150/110 | yes | none | “behavior lability” “mood swings” | generalized x2 | Grade 3 |
MoAb, monoclonal antibody; Rx, therapy
This patient experienced intractable (grade 3) emesis beginning 8 days after cycle 2 of standard-dose 3F8 but had no complication with either prior high-dose 3F8 x2 cycles or subsequent cycle 3 of standard-dose 3F8.
Grade 2 hypertension in multiple cycles of SD-3F8, responsive to nifedipine.
Symptoms of PRES included transient vision loss in one patient and transient mental status alterations in four patients. Acute management included anti-hypertensive medications in all five patients and anti-convulsant therapy in four patients. Grade 3 or 4 hyponatremia was noted in three patients during evaluation of the PRES event (serum sodium levels were normal on the preceding days); it was attributed to emesis in patient #1, and multiple factors, including transient cerebral salt wasting, in patients #4 and #5. One patient (#2) had transient grade 2 renal dysfunction attributed to hypertension and prior nephrotoxic therapies. Four patients had full recovery to baseline neurological status within one week, and one patient (#4) had a complete resolution of symptoms over five weeks.
Radiologic features of PRES
Initial MRIs showed findings typical of PRES, i.e., T2/FLAIR signal abnormality in subcortical white matter and cortex predominantly in parietal and occipital lobes (Table 4). These findings were not present in prior MRIs which had been performed as part of routine disease staging. Follow-up MRIs performed 9–36 days after the event showed complete resolution of PRES-related abnormalities. Three patients had additional abnormal radiologic findings at presentation of PRES: In patient #1, both CT and MRI showed a small focus suspicious for hemorrhage or relapse but follow-up MRI showed neither (Figure 1). In patient #4, who had previously undergone surgical resection of brain metastases, MRI showed a small focus with restricted diffusion suspicious for hemorrhage or relapse in the left parietal lobe; follow-up MRIs showed resolution of the abnormality and no relapse. In patient #5, CT and MRI revealed small lesions suspicious for hemorrhage or relapse in the frontal lobes; follow-up MRI showed evolution of one lesion to hemosiderin deposit, consistent with evolving focal hemorrhage, and absence of any other lesions.
Table 4.
Radiologic findings
| Patient no. | CT abnormalities at time of PRES | -----------------------------------------MRI at time of PRES------------------------------------- | Follow-up, time from PRES | |||
|---|---|---|---|---|---|---|
| Areas with T2/FLAIR signal abnormalitiesa | Hemorrhage | Enhancement | Restricted diffusion | |||
| 1. | ill-defined mass in R parietal lobeb | bilateral parietal and occipital lobes | small focus (as in CT) | none | none | MRI(−), day 7 |
| 2. | none | bilateral medial parietal lobes and superior frontal gyri (L>R) less prominent: medial occipital lobes |
none | none | mild cortical in some areasc | MRI(−), day 36 |
| 3. | none | bilateral parietal and occipital lobes less prominent: paremedian frontal lobes |
none | none | none | MRI(−), day 17 |
| 4. | post-surgical changesd | bilateral parietal (L>R) and frontal lobes | none | focus in L parietal lobeb | focus in L parietal lobeb | MRI(−), day 37 |
| 5. | subtle 6 mm hyperdensity in R frontal lobeb | bilateral parietal and occipital lobes less prominent: bilateral frontal and temporal lobes |
L frontal noduleb | L frontal noduleb
L parietal noduleb |
none | MRI(−), day 31 (hemosiderin in L frontal lobe) |
In all patients, there were MRI abnormalities in subcortical white matter and cortex.
Suspicious for metástasis or hemorrhage.
Hyperintensity on the diffusion sequences was likely T2 shine through.
Patient was 6 months status-post resection of brain metastases.
Figure 1.
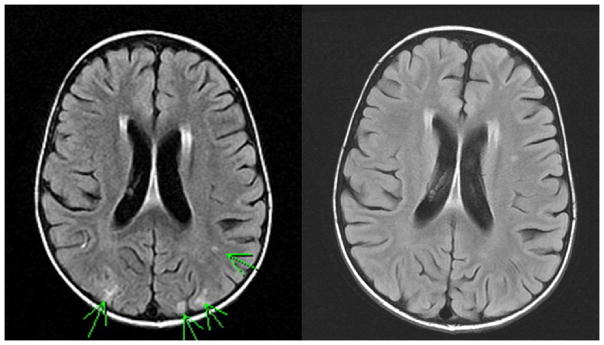
Magnetic resonance imaging (MRI; fluid-attenuated inversion recovery images) of patient #1 showing PRES (left) and return to no abnormal enhancement within one week (right). Subsequent routine follow-up MRIs also showed no abnormal enhancement.
DISCUSSION
The recognition of PRES in children with cancer appears to be increasing, probably due to wider awareness of this complex syndrome and expanded use of MRI which is essential for its diagnosis. Hypertension is the most prevalent causative factor of PRES in general and is the most commonly described inciting factor for PRES in children being treated for cancer.6–8,24 Reported cases involve leukemia and solid tumors, with associated treatments including systemic and intrathecal chemotherapy, major surgery, immunosuppressive agents, myeloablative therapy with autologous or allogeneic SCT, and anti-angiogenic agents. Notably absent is anti-GD2 MoAb-mediated immunotherapy, despite a large published experience covering this treatment in children9–21,25–30 and adults,15,31–36 and despite case reports of PRES with MoAbs that are direct immunomodulators.7,37,38 Anti-GD2 MoAbs trigger antibody-dependent cellular cytotoxicity and complement-mediated cytotoxicity,39 but it remains to be determined if these anti-neoplastic mechanisms can predispose to PRES.1–3
MRIs in each of the five 3F8 patients with PRES displayed the characteristic abnormalities which are thought to result from endothelial injury and disruption of the blood-brain-barrier with transudation of fluid and protein into brain parenchyma.2,3,40 The radiologic findings were clearly attributable to the events leading to the diagnosis of PRES, given that all five patients, as part of routine monitoring of disease status, had undergone MRI of the brain shortly before PRES, and MRI findings returned to baseline within days-to-weeks after PRES resolved clinically.
Hypertension and seizures are the predominant symptoms of PRES but are not invariably present. PRES in patient #1 was associated with emesis, as well as hypertension, but, unlike patients #2–5 who had classic PRES, this child never developed headaches, seizures or visual disturbances. The absence of these symptoms - which often prompt investigations that reveal the radiologic findings of PRES – suggests that cases of PRES can escape detection. Hence, although PRES has not previously been described in NB patients receiving anti-GD2 MoAb, undiagnosed cases likely occurred. In reports on anti-GD2 MoAb treatment, the hallmark features of PRES are rare, with significant hypertension, CNS cortical symptoms, and emesis in 0–5% of patients.9–21, 25–36 In our series, one patient failed to complete prior treatment with the anti-GD2 ch14.18 MoAb due to toxicity and, after relapse, experienced grade 3 hypertension (without PRES) with HD-3F8. This case illustrates that impaired function of major organs, especially kidneys,41,42 from prior aggressive multimodality therapy and/or tumor-related local-regional effects, can pose challenges for the safe implementation of anti-GD2 MoAb therapy in neuroblastoma patients.
Differences in schedule and dosing set 3F8 protocols apart from other anti-GD2 MoAb protocols9–14, 25–36 and might influence the risk of PRES. Thus, treatments with 3F8 and other MoAbs contrast as regards infusion times (30 minutes for 3F8, versus ≥4 hours), number of cycles (dependent on HAMA for 3F8 with no limit on total number of cycles, versus five cycles), and dosage (3F8 at 20 or 80 mg/m2/day x5 days/cycle, versus ch14.18 at 20–50 mg/m2/day x4–5 days/cycle9–14,27,28). PRES occurred in three 3F8 patients after >5 cycles; it is conceivable that perturbations of the blood-brain-barrier that eventuate in PRES accrue with each cycle.
The incidence of PRES or grade 3 hypertension did not differ significantly between SD-3F8 and HD-3F8 patients. Dosing, however, merits consideration since not only are 3F8 dosages higher than those of other anti-GD2 MoAbs, but patients #4 and #5 developed PRES after only a single cycle of HD-3F8. Patient #4 may have been at increased risk for CNS problems such as seizures and/or PRES because, despite having tolerated five cycles of ch14.18 without complication, his subsequent brain relapse was treated with surgery as well as craniospinal irradiation and intrathecal radiotherapy.23 Patient #5 had no obvious predisposing clinical factors – but his prior treatment included TBI. In this regard, patient #2, who developed PRES after multiple cycles of SD-3F8, also received TBI. The occurrence of PRES in these three patients prompted us to investigate whether prior brain irradiation might be a predisposing factor to PRES in patients receiving anti-GD2 MoAb immunotherapy. Indeed, an association appeared substantiated in the ensuing analysis, with PRES in 3/26 (11.5%) patients with, versus 2/189 (1.1%) patients without, prior brain irradiation (p=0.01). It should be noted that, in the absence of anti-GD2 MoAb immunotherapy, TBI41–43 and craniospinal irradiation plus intrathecal radiotherapy23 have not been associated with PRES, though no study has specifically addressed the possibility.
PRES presents with dramatic and life-threatening events that can be controlled with proper emergent management. Yet the risk of a catastrophic outcome is self-evident. Furthermore, although a hallmark of PRES is a rapid return to clinical and radiologic baseline with no long-term sequelae (as fortunately transpired with the 3F8 patients), that scenario is not invariable.6–8 Hence, our experience indicates that patients receiving anti-GD2 MoAb immunotherapy should be closely monitored for, and undergo immediate treatment or evaluation of, symptoms (e.g., hypertension or headaches) that might herald PRES.
Acknowledgments
Supported in part by grants from the National Institutes of Health (CA10450), Bethesda, MD; the Robert Steel Foundation, New York, NY; and Katie’s Find A Cure Fund, New York, NY.
Footnotes
The authors have no financial disclosures to declare.
Contributor Information
Brian H. Kushner, Email: kushnerb@mskcc.org.
Shakeel Modak, Email: modaks@mskcc.org.
Ellen M. Basu, Email: basue@mskcc.org.
Stephen S. Roberts, Email: robertss@mskcc.org.
Kim Kramer, Email: kramerk@mskcc.org.
Nai-Kong V. Cheung, Email: cheungn@mskcc.org.
References
- 1.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: Controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29:1043–1049. doi: 10.3174/ajnr.A0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske SK. Posterior reversible encephalopathy syndrome: A review. Semin Neurol. 2011;31:202–215. doi: 10.1055/s-0031-1277990. [DOI] [PubMed] [Google Scholar]
- 4.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 5.Casey SO, Sampaio RC, Michel E, et al. Posterior reversible encephalopathy syndrome: Utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000;21:1199–1206. [PMC free article] [PubMed] [Google Scholar]
- 6.Morris EB, Laningham FH, Sandlund JT, et al. Posterior reversible encephalopathy syndrome in children with cancer. Pediatr Blood Cancer. 2007;48:152–159. doi: 10.1002/pbc.20703. [DOI] [PubMed] [Google Scholar]
- 7.Lucchini G, Grioni D, Colombini A, et al. Encephalopathy syndrome in children with hemato-onocological disorders is not always posterior and reversible. Pediatr Blood Cancer. 2008;51:629–633. doi: 10.1002/pbc.21688. [DOI] [PubMed] [Google Scholar]
- 8.de Laat P, te Winkel ML, Devos AS, Catsman-Berrevoets CE, Pieters R, van den Heuvel-Eibrink Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol. 2011;22:472–478. doi: 10.1093/annonc/mdq382. [DOI] [PubMed] [Google Scholar]
- 9.Yu A, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handgretinger R, Anderson K, Lang P, et al. A phase I study of human/mouse chimeric anti-ganglioside GD2 antibody ch14. 18 in patients with neuroblastoma. Eur J Cancer. 1995;31A:261–267. doi: 10.1016/0959-8049(94)00413-y. [DOI] [PubMed] [Google Scholar]
- 11.Yu AL, Batova A, Alvarado C, Rao VJ, Castelberry RP. Usefulness of a chimeric anti-GD2 (ch14. 18) and GM-CSF for refractory neuroblastoma: A POG phase II study. Am Soc Clin Oncol. 1997;16:513a. (abstract) [Google Scholar]
- 12.Yu AL, Uttenreuther-Fischer MM, Huang CS, et al. Phase I trial of a human-mouse chimeric anti-disialoganglioside monoclonal antibody ch14. 18 in patients with refractory neuroblastoma and osteosarcoma. J Clin Oncol. 1998;16:2169–80. doi: 10.1200/JCO.1998.16.6.2169. [DOI] [PubMed] [Google Scholar]
- 13.Ozkaynak MF, Sondel PM, Krailo MD, et al. Phase I study of chimeric human/murine anti-GD2 monoclonal antibody (ch14. 18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: A Children’s Cancer Group study. J Clin Oncol. 2001;18:4077–85. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 14.Gilman AL, Ozkaynak MF, Matthay KK, et al. Phase I study of ch14. 18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: A report from the Children’s Oncology Group. J Clin Oncol. 2009;27:85–91. doi: 10.1200/JCO.2006.10.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung N-KV, Lazarus H, Miraldi FD, et al. Ganglioside GD2 specific monoclonal antibody 3F8: A phase I study in patients with neuroblastoma and malignant melanoma. J Clin Oncol. 1987;5:1430–40. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 16.Cheung N-KV, Kushner BH, Yeh SJ, Larson SM. 3F8 monoclonal antibody treatment of patients with stage IV neuroblastoma: A phase II study. Int J Oncol. 1998;12:1299–1306. doi: 10.3892/ijo.12.6.1299. [DOI] [PubMed] [Google Scholar]
- 17.Cheung N-KV, Kushner BH, Cheung IY, et al. Anti-GD2 antibody treatment of minimal residual stage 4 neuroblastoma diagnosed at more than 1 year of age. J Clin Oncol. 1998;16:3053–60. doi: 10.1200/JCO.1998.16.9.3053. [DOI] [PubMed] [Google Scholar]
- 18.Kushner BH, Kramer K, Cheung N-KV. Phase II trial of the anti-GD2 monoclonal antibody 3F8 and granulocyte-macrophage colony-stimulating factor for neuroblastoma. J Clin Oncol. 2001;19:4189–94. doi: 10.1200/JCO.2001.19.22.4189. [DOI] [PubMed] [Google Scholar]
- 19.Cheung N-KV, Sowers R, Vickers AJ, Cheung IY, Kushner BH, Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2006;24:2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 20.Kushner BH, Kramer K, Modak S, Cheung N-KV. Successful multi-fold dose escalation of anti-GD2 monoclonal antibody 3F8 in patients with neuroblastoma: A phase I study. J Clin Oncol. 2011;29:1168–1174. doi: 10.1200/JCO.2010.28.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung N-KV, Cheung IY, Kushner BH, Ostrovnaya I, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony stimulating factor and 13-cis-retinoic acid is effective against chemoresistant marrow MRD among high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Engl J Med. 1999;341:1165–73. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 23.Kramer K, Kushner BH, Modak S, et al. Compartmental intrathecal radioimmunotherapy: Results for treatment for metastatic CNS neuroblastoma. J Neuro-Oncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Won SC, Kwon SY, Han JW, Choi SY, Lyu CJ. Posterior reversible encephalopathy syndrome in childhood with hematologic/oncologic diseases. J Pediatr Hematol Oncol. 2009;31:505–508. doi: 10.1097/MPH.0b013e3181a71868. [DOI] [PubMed] [Google Scholar]
- 25.Handgretinger R, Baader P, Dopfer R, et al. A phase I study of neuroblastoma with the anti-ganglioside GD2 antibody 14. G2a Cancer Immunol Immunother. 1992;35:199–204. doi: 10.1007/BF01756188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost JD, Hank JA, Reaman GH, et al. A phase I/IB trial of murine monoclonal anti-GD2 antibody 14. G2a plus interleukin-2 in children with refractory neuroblastoma. Cancer. 1997;80:317–333. doi: 10.1002/(sici)1097-0142(19970715)80:2<317::aid-cncr21>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 27.Simon T, Hero B, Faldum A, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14. 18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol. 2004;22:3549–57. doi: 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 28.Simon T, Hero B, Faldum A, et al. Infants with stage 4 neuroblastoma: The impact of the chimeric anti-GD2-antibody ch14. 18 consolidation therapy. Klin Padiatr. 2005;217:147–152. doi: 10.1055/s-2005-836518. [DOI] [PubMed] [Google Scholar]
- 29.Osenga KL, Hank JA, Albertini MR, et al. A phase I clinical trial of the hu14. 18-IL2 (EMD 273063) as a treatment for children with refractory or recurrent neuroblastoma and melanoma: A study of the Children’s Oncology Group. Clin Cancer Res. 2006;12:1750–1759. doi: 10.1158/1078-0432.CCR-05-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of Hu14. 18-IL2 in patients with relapsed/refractory neuroblastoma: A Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saleh MN, Khazaeli MB, Wheeler RH, et al. Phase I trial of the chimeric anti-GD2 monoclonal antibody ch14. 18 in patients with malignant melanoma. Hum Antibody Hybridom. 1992;3:19–24. [PubMed] [Google Scholar]
- 32.Saleh MN, Khazaeli MB, Wheeler RH, et al. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992;52:4342–4347. [PubMed] [Google Scholar]
- 33.Murray JL, Cunningham JE, Brewer H, et al. Phase I trial of murine monoclonal antibody 14G2a administered by prolonged intravenous infusion in patients with neuroectodermal tumors. J Clin Oncol. 1994;12:184–193. doi: 10.1200/JCO.1994.12.1.184. [DOI] [PubMed] [Google Scholar]
- 34.Murray JL, Kleinerman ES, Jia S, et al. Phase Ia/Ib trial of anti-GD2 chimeric monoclonal antibody 14.18 (ch14. 18) and recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) in metastatic melanoma. J Immunother. 1996;19:206–217. doi: 10.1097/00002371-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Albertini MR, Hank JA, Schiller JH, et al. Phase IB trial of chimeric antidisialoganglioside antibody plus interleukin 2 for melanoma patients. Clin Cancer Res. 1997;3:1277–1288. [PubMed] [Google Scholar]
- 36.King DM, Albertini MR, Schalch H, et al. Phase I clinical trial of the immunocytokine EMD 273063 in melanoma patients. J Clin Oncol. 2004;22:4463–4473. doi: 10.1200/JCO.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastrup O, Diener HC. TNF-antagonist etanercept induced reversible posterior leukoencephalopathy syndrome. J Neurol. 2008;255:452–453. doi: 10.1007/s00415-008-0732-y. [DOI] [PubMed] [Google Scholar]
- 38.Haddock R, Garrick V, Horrocks I, Russell RK. A case of posterior reversible encephalopathy syndrome in a child with Crohn’s disease treated with infliximab. J Crohns Colitis. 2011;5:623–627. doi: 10.1016/j.crohns.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Parsons K, Bernhardt B, Strickland B. Targeted immunotherapy for high-risk neuroblastoma – The role of monoclonal antibodies. Ann Pharmacol. 2013;47:210–218. doi: 10.1345/aph.1R353. [DOI] [PubMed] [Google Scholar]
- 40.Pavlakis SG, Frank Y, Chusid R. Hypertensive encephalopathy, reversible occipitoparietal encephalopathy, or reversible posterior leukoencephalopathy: Three names for an old syndrome. J Child Neurol. 1999;14:277–281. doi: 10.1177/088307389901400502. [DOI] [PubMed] [Google Scholar]
- 41.Flandin I, Hartmann O, Michon M, et al. Impact of TBI on late effects in children treated by megatherapy for stage IV neuroblastoma. A study of the French Society of Pediatric Oncology. Int J Radiat Oncol Biol Phys. 2006;64:1424–1431. doi: 10.1016/j.ijrobp.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Trahair TN, Vowels MR, Johnston K, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007;40:741–746. doi: 10.1038/sj.bmt.1705809. [DOI] [PubMed] [Google Scholar]
- 43.Hobbie WL, Moshang T, Carlson CA, et al. Late effects in survivors of tandem peripheral blood stem cell transplant for high-risk neuroblastoma. Pediatr Blood Cancer. 2008;51:679–683. doi: 10.1002/pbc.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]


