Abstract
Intrastriatal injection of recombinant adeno-associated viral vector serotype 2/1 (rAAV2/1) to overexpress the neurotrophic factor pleiotrophin (PTN) provides neuroprotection for tyrosine hydroxylase immunoreactive (THir) neurons in the substantia nigra pars compacta (SNpc), increases THir neurite density in the striatum (ST) and reverses functional deficits in forepaw use following 6-hydroxydopamine (6-OHDA) toxic insult. Glial cell line-derived neurotrophic factor (GDNF) gene transfer studies suggest that optimal neuroprotection is dependent on the site of nigrostriatal overexpression. The present study was conducted to determine whether enhanced neuroprotection could be accomplished via simultaneous rAAV2/1 PTN injections into the ST and SN compared with ST injections alone. Rats were unilaterally injected in the ST alone or injected in both the ST and SN with rAAV2/1 expressing either PTN or control vector. Four weeks later, all rats received intrastriatal injections of 6-OHDA. Rats were euthanized 6 or 16 weeks relative to 6-OHDA injection. A novel selective total enumeration method to estimate nigral THir neuron survival was validated to maintain the accuracy of stereological assessment. Long-term nigrostriatal neuroprotection and functional benefits were only observed in rats in which rAAV2/1 PTN was injected into the ST alone. Results suggest that superior preservation of the nigrostriatal system is provided by PTN overexpression delivered to the ST and restricted to the ST and SN pars reticulata and is not improved with overexpression of PTN within SNpc neurons.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurological disorder with motor symptoms resulting from degeneration of dopamine (DA)-producing neurons in the substantia nigra pars compacta (SNpc) and a concomitant loss of DA in the striatum (ST). Neurotrophic factor gene therapy offers significant therapeutic promise for PD in that it may enhance survival of DA-producing neurons thereby slowing disease progression while alleviating motor symptoms by elevating DA in the ST. To date, a majority of gene therapy clinical trials have restricted therapeutic delivery to the terminal fields of DA neurons in the ST, and a single trial has targeted both the ST and SN.1–4 Striatal targeting in clinical trials was driven by animal studies utilizing glial cell line-derived neurotrophic factor (GDNF) or neuturin (NTN) that demonstrated delivery to striatal terminal fields is both necessary and sufficient for symptomatic treatment and affords protection to nigral DA neurons.5–11 However, in conditions of impaired axonal transport and degeneration, such as occurs in PD patients, direct delivery of trophic factors to the nigral DA neuron bodies may be of increased benefit. Nigral expression can boost neuron survival at the level of the cell body and therefore may be complimentary to striatal administration.12 To date, two studies have been conducted in neurotoxin rodent models comparing the neuroprotective effects of AAV neurotrophic factor ST to ST and SN delivery, both reporting increased neuroprotection of nigral neurons with SN delivery.10,13
The trophic factor pleiotrophin (PTN) is intricately involved in the development of the nigrostriatal DA system and encourages survival, differentiation and outgrowth of ventral mescencephalic neurons in vitro.14,15 We have previously demonstrated that intrastriatal recombinant AAV delivery to overexpress PTN before 6-hydroxydopamine (6-OHDA) is protective of both nigral neurons and terminals and can be functionally restorative;16 however, neuroprotection was not complete. PTN transduction patterns following intrastriatal delivery indicated significant expression in the striatonigral direct pathway resulting in robust PTN immunoreactivity within terminals in the SN pars reticulata (SNpr), whereas transduction of SNpc DA neurons following intrastriatal vector injection was limited and only observed in unlesioned rats.
In the present study, we sought to determine whether additional PTN expression in the SNpc results in superior neuroprotection and functional recovery by directly comparing the effects of simultaneous injection of rAAV2/1 PTN injection to both the ST and SN with rAAV2/1 PTN injected into only the ST. Our findings reveal that over shorter transduction intervals (10 weeks), co-transduction of both the nigrostriatal and striatonigral pathways with PTN display equivalent levels of neuroprotection from intrastriatal 6-OHDA compared with PTN transduction of the striatonigral pathway alone. However, over longer transduction intervals (20 weeks) only striatonigral PTN overexpression (intrastriatal injection) provided neuroprotection to nigral DA neurons and DAergic terminals in the ST. Our results suggest that to achieve long-term neuroprotection of the nigrostriatal pathway intrastriatal rAAV PTN delivery alone should be utilized.
RESULTS
Selective total enumeration (TE) versus stereology to quantify surviving SNpc tyrosine hydroxylase immunoreactive (THir) neurons
Stereological assessment, while accurate, is time consuming. In an effort to establish a more time efficient method of determining the percentage of remaining SNpc THir neurons for the current studies, stereological quantification was directly compared with a selective TE counting method. To validate this method, estimates of surviving THir neurons within the SNpc ipsilateral and contralateral to the intrastriatal 6-OHDA injections were achieved using either TE of the three coronal sections in closest proximity to the medial terminal nucleus of the accessory optic tract (Figure 1a) or traditional stereological assessment. On average, the time required to perform the selective TE method was approximately 1/3 the time required for traditional stereological assessment. Both counting methods revealed that 6-OHDA resulted in significant THir neuron death at 4 and 6 weeks compared with 2 weeks post-lesion (P ≤0.002). The percentage of unilateral lesion determined by the selective TE counting method resulted in complete concordance with the percentage of unilateral lesion revealed by traditional stereological estimates at 2-, 4- or 6-week post-6-OHDA time points (P>0.05, Figure 1b). Therefore, the selective TE method was used for subsequent estimations of SNpc THir neurons in the remainder of the study.
Figure 1.
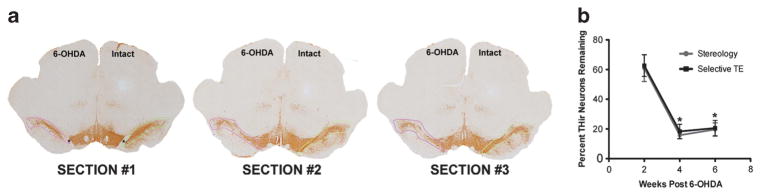
Validation of selective TE counting method for quantification of 6-OHDA lesion severity. (a) Three representative sections identified by the presence and proximity to the medial terminal nucleus (MTN) of the accessory optic tract (*MTN, SECTION #1) approximately − 5.04 mm, − 5.28 mm and − 5.52 mm relative to bregma. Contours drawn around the lesioned (pink) and contralateral intact (green) SNpc demarcate the area in which TE of THir neurons was conducted at × 20. Total THir neuron numbers in the intact or lesioned SN were averaged for the three MTN sections counted. (b) Percentages of THir nigral neurons at 2, 4 and 6 weeks after intrastriatal 6-OHDA injection quantified by either stereology or selective TE of the three MTN sections. No significant differences were detected between counting methods at any time point (P>0.05). Both counting methods revealed that 6-OHDA resulted in significant THir neuron death at 4 and 6 weeks compared with 2 weeks postlesion (*P ≤0.002).
PTN, LacZ and green fluorescent protein (GFP) transgene expression in the nigrostriatal system
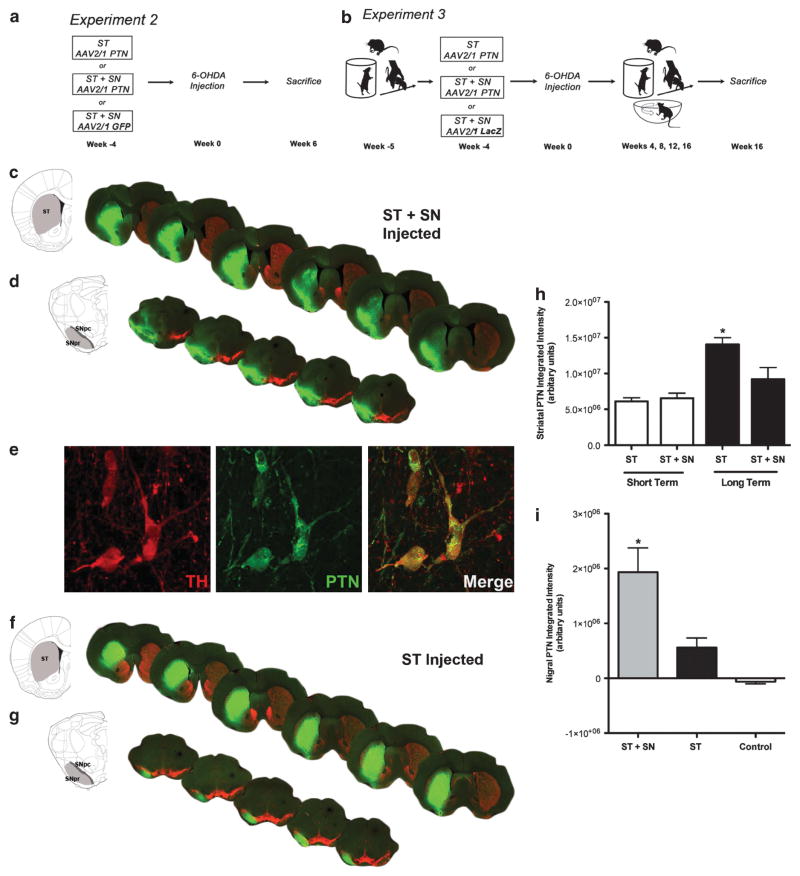
PTN and TH double immunofluorescence revealed robust striatal PTN expression in all rAAV2/1 PTN-injected animals (Figure 2). Striatal PTN transduction patterns were similar for the ST+SN- and ST-only-injected groups from both 10- and 20-week experiments (Figures 2c and f). In contrast, patterns of PTN expression in the SN were highly dependent on injection site. rAAV2/1 PTN-injected directly into the SN resulted in robust PTN expression in the surviving THir neurons of the SNpc, THir fibers in the SNpr and limited expression within neurons of the ventral tegmental area (Figures 2d and e). PTN expression was also observed in the SN of the ST-only-injected group; however, expression was confined to neurites within the SNpr, suggesting anterograde transport of PTN protein from transduced neurons in the ST (Figure 2g). Anterograde transport of PTN protein following intrastriatal rAAV2/1 PTN injection was also observed within the entopeduncular nucleus. We have previously observed retrograde viral transport following ST rAAV2/1 PTN injection to neuron bodies of the SNpc in the intact nigrostriatal system;16 however, in the 6-OHDA-lesioned rats in the current study PTN expression was not observed within neurons of the SNpc. In contrast, transduction of SNpc DA neurons was readily apparent following intranigral rAAV2/1 PTN injections (Figure 2e).
Figure 2.
PTN expression in the nigrostriatal and striatonigral pathways following rAAV2/1-PTN injection. (a) Timeline of short-term Experiment 2. Rats were unilaterally injected with rAAV2/1 PTN in the ST or ST+SN or with rAAV2/1 GFP in the ST+SN. Four weeks later, all rats received unilateral intrastriatal injections of 6-OHDA. Rats were euthanized and postmortem analysis occurred 6 weeks post-6-OHDA injection. (b) Timeline of long-term Experiment 3. Rats were unilaterally injected with rAAV2/1 PTN in the ST or ST+SN or rAAV2/1 LacZ in the ST+SN. Four weeks later, all rats received unilateral instrastriatal injections of 6-OHDA. Behavioral assessment in the cylinder task, adjusting steps and bilateral tactile stimulation test was conducted before vector injection and 4, 8, 12 and 16 weeks post-6-OHDA injection. Amphetamine-induced rotational asymmetry was analyzed 4, 8, 12 and 16 weeks post-6-OHDA injection. Rats were euthanized, and postmortem analysis occurred 16 weeks post-6-OHDA injection. (c) An example of PTN expression (green) and TH expression (red) in the ST 20 weeks following rAAV2/1 PTN injection into the ST in ST+SN injected animals. (d) PTN expression 20 weeks following direct injection of rAAV2/1 PTN into the SN. PTN immunoreactivity is robust in the SNpc, SNpr and the ventral mesencephalon following intranigral injection. (e) Confocal image (×126) of THir (red) neurons of the SNpc co-expressing PTN (green) 20 weeks following SN injection of rAAV2/1 PTN. (f) Striatal PTN expression 20 weeks following intrastriatal injection of rAAV2/1 PTN. (g) PTN expression in the SNpr 20 weeks following intrastriatal injection of rAAV2/1 PTN. Robust PTN expression is confined to the SNpr, indicative of anterograde striatonigral pathway transport of PTN protein. (h) Quantitative analysis of levels of PTN immunoreactivity indicates that striatal PTN expression was significantly greater 20 weeks after vector injection compared with 10 weeks after vector injection in ST and ST+SN-injected rats and compared with ST+SN-injected rats at the same 20 weeks after vector injection interval (*P ≤0.014). (i) Nigral PTN expression 20 weeks after vector injection was significantly greater in ST+SN-injected rats compared with ST-only-injected rats and controls (*P ≤0.007). Values are reported as mean ± s.e.m.
Analysis of levels of PTN expression in the ST confirmed that 20 weeks after vector injection rats in the ST only injection group possessed significantly increased PTN levels compared with rats in all the other treatment groups (F(1,12) = 16.142, P = 0.002, Figure 2h). Further, PTN expression 20 weeks after vector injection was significantly elevated in the ST group compared with the ST+SN-injected group (P = 0.014, Figure 2h). No significant differences in striatal PTN expression were detected between the treatment groups 10 weeks after vector injection (P ≥0.05). Striatal PTN expression was not significantly different between the ST+SN-injected rats at 20 weeks post-vector injection (Experiment 3) and either treatment group at 10 weeks post-vector injection (Experiment 2, P ≥0.05). Measurement of PTN levels in the SN revealed that 20 weeks after transduction the ST+SN treatment group had significantly higher amounts of PTN in the SN than in the ST-only-injected group (Experiment 3, P ≤0.007, Figure 2i). ST injection of rAAV2/1 PTN resulted in PTNir in neurons in the ST whose size, shape and abundance suggested that medium spiny neurons had been transduced. Transduction patterns of rAAV2/1 GFP and rAAV2/1 LacZ, used in the 10- and 20-week studies, respectively, followed similar transduction patterns as rAAV2/1 PTN as previously reported.16 In summary, rAAV2/1 PTN injection resulted in robust PTN expression at the site of injection, with long-term (20 weeks) ST delivery producing the highest level of PTN expression in the ST.
Long-term (20 weeks) striatal PTN overexpression prevents 6-OHDA-induced functional deficits
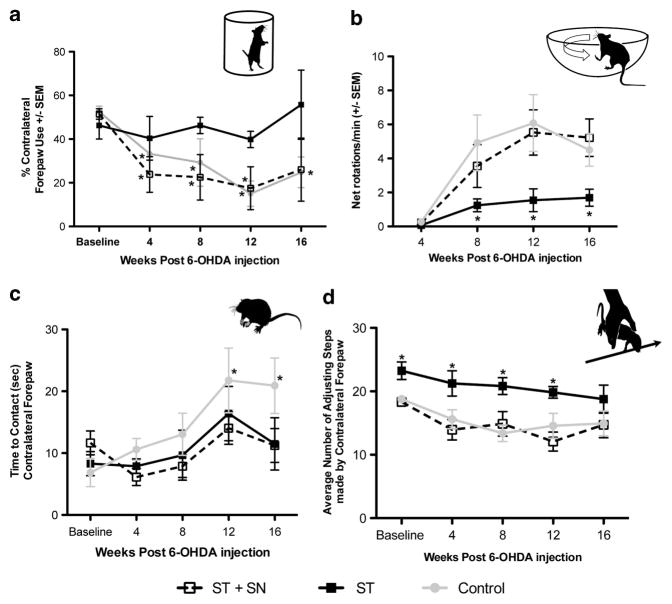
Following 6-OHDA injection, rats in Experiment 3 injected with rAAV2/1 LacZ developed significant, progressive contralateral forelimb deficits compared with baseline at 8, 12 and 16 weeks post-lesion (F(3,75) = 7.376, P<0.031, Figure 3a). Similarly, rats injected with rAAV2/1 PTN into both the ST+SN exhibited impairments at 4, 8 and 12 weeks post-lesion (P<0.005). In contrast, rats injected with rAAV2/1 PTN in the ST alone displayed no functional deficit in contralateral forelimb use over the 16-week period (P>0.05). Further, no significant differences in amphetamine-induced rotational asymmetry were observed between rAAV2/1 LacZ control rats and the rAAV2/1 PTN ST+SN group at any time point (P>0.05). Compared with both of these groups, rats in the rAAV2/1 PTN ST only treatment group exhibited significantly fewer ipsilateral rotations at 8, 12 and 16 weeks post-lesion (F(3,60) = 3.321, P <0.029, Figure 3b). Whereas the control and rAAV2/1 PTN ST+SN rats exhibited more rotations at 8, 12 and 16 weeks compared with 4 weeks (P<0.001), no significant changes in net ipsilateral rotations were detected in the ST-only-injected group over time (P>0.05). In the bilateral tactile stimulation test, rAAV2/1 LacZ control rats took significantly more time to contact the contralateral, affected paw at 12 and 16 weeks post-6-OHDA lesion compared with baseline measurements (P<0.008, Figure 3c). Neither of the rAAV2/1 PTN-injected groups displayed impairments in time to remove the adhesive sticker from their affected forepaws compared with baseline (P>0.05). In the adjusting steps tasks, no differences were observed between rAAV2/1 LacZ control rats and the rAAV2/1 PTN ST+SN group at any time point (P>0.05). Compared with both of these groups, rats in the rAAV2/1 PTN ST only treatment group exhibited significantly more contralateral steps at all time points tested except at 16 weeks (P<0.006, Figure 3d), with the rAAV2/1 LacZ control and the rAAV2/1 PTN ST+SN groups exhibiting decreased steps from baseline at all time points (P<0.006). In contrast, rats in the rAAV2/1 PTN ST only group did not exhibit decreased contralateral steps at any point after lesion (P>0.05). In summary, PTN overexpression targeted to the ST alone resulted in improved functional recovery from DA denervation in all of the motor tests examined.
Figure 3.
Instrastriatal rAAV2/1 PTN transduction prevents motor deficits. Analysis of behavior occurred between weeks 8–20 after vector injections corresponding to weeks 4–16 post-6-OHDA injection. (a) Cylinder test; rats injected with rAAV2/1 PTN in the ST only sustained no impairment in motor function over 16 weeks. Rats injected in the ST+SN and control rats showed significant motor impairments compared with baseline beginning 4 weeks after 6-OHDA injection that continued through the duration of the study (*P ≤0.031; indicates significant differences between time points and baseline measurements within each group). (b) Amphetamine-induced ipsilateral rotations; AAV2/1 PTN ST-injected rats demonstrated significantly fewer rotations compared with control AAV2/1 LacZ and AAV2/1 PTN ST+SN rats at 8, 12 and 16 weeks post-6-OHDA injection (*P ≤0.029; indicates significant differences between AAV2/1 PTN ST+SN and AAV2/1 LacZ combined compared with AAV2/1 PTN ST at each time point). (c) Bilateral tactile stimulation; neither the AAV2/1 PTN ST only nor the AAV2/1 PTN ST+SN-injected rats demonstrated a sensorimotor deficit for the duration of the study. Significant sensorimotor deficits developed in the final time points in control rats (*P ≤0.008; indicates significant differences between baseline and time points within the control group). (d) Adjusting steps task; rats in the rAAV2/1 PTN ST only treatment group exhibited significantly more contralateral steps at all time points tested except at 16 weeks (*P<0.006, indicates significant differences between AAV2/1 PTN ST+SN and AAV2/1 LacZ combined compared with AAV2/1 PTN ST at each time point). Values are reported as mean ± s.e.m.
Short-term (10 weeks) PTN overexpression in either the striatonigral pathway alone or combined striatonigral and nigrostriatal pathways is protective for SNpc DA neurons
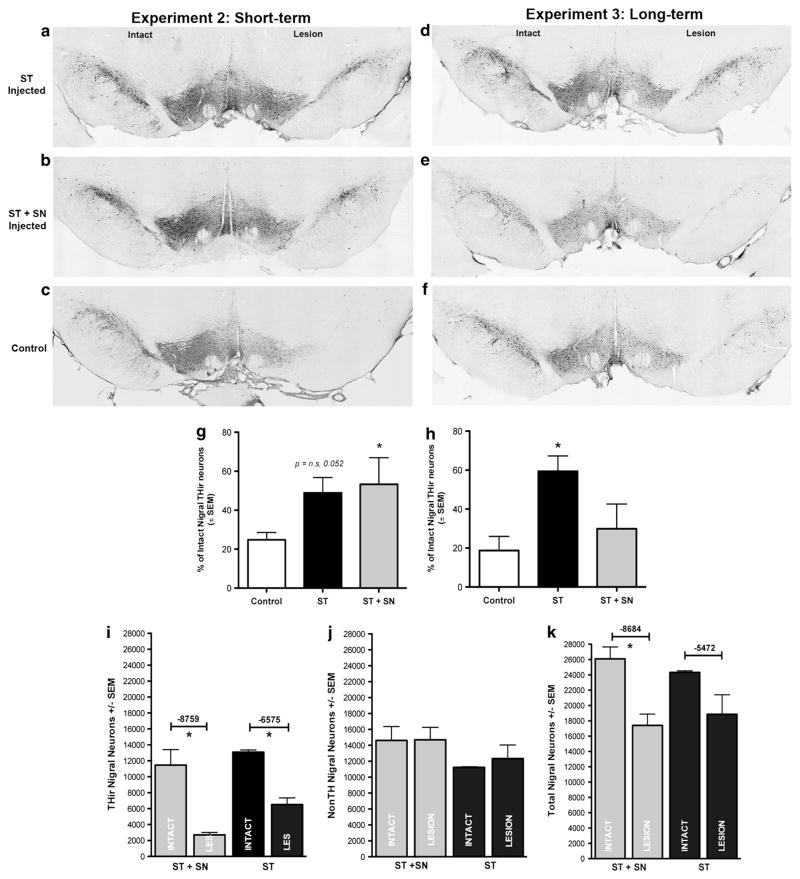
The selective TE counting method was used to determine the percentage of SNpc THir remaining in rAAV2/1 PTN-injected and rAAV2/1 GFP control rats six (Experiment 2) or between rAAV2/1 PTN-injected and rAAV2/1 lacZ control rats 16 (Experiment 3) weeks following 6-OHDA. Six weeks post-lesion (10 weeks postvector), THir neuron survival was twofold greater in the ST and ST+SN groups compared with controls and in the ST+SN-injected group; this increase achieved significance (F(2,12) = 6.148, P = 0.033, Figures 4a–c and g). An average of 197.2 ± 16.5 or 194.5 ± 22.2 THir neurons remained in the three coronal sections quantified in the intact SNpc and 96.9 ± 17.3 or 98.1 ± 16.7 remained in the lesioned SNpc of the ST-only- or ST+SN-injected groups, respectively. GFP-injected rats maintained an average of 226.2 ± 13.7 and 53.1 ± 6.5 THir neurons in the intact or lesioned SNpc, respectively. The magnitude of degeneration in the GFP control group paralleled results of vehicle injections to 6-OHDA-lesioned rats reported in a previous study by our laboratory.17 THir neuron survival in the ST only group was elevated compared with controls but did not achieve significance (P = 0.052). Survival was not different between ST+SN- and ST-only-injected groups (P>0.05). In summary, survival of nigral THir neurons 6 weeks following 6-OHDA in both the ST+SN and ST rAAV2/1 PTN treatment groups was ≈50% compared with ≈23% survival in the rAAV2/1 GFP control group.
Figure 4.
Neuroprotection of SNpc THir neurons 20 weeks following intrastriatal rAAV2/1 PTN transduction. (a–c) THir neurons in the intact and lesioned SNpc of rats from Experiment 2 (10 weeks after vector, 6-weeks after 6-OHDA) injected with AAV2/1 PTN in the ST (a), in the ST+SN (b) or with AAV2/1 GFP in the ST+SN (c). (d–f) THir neurons in the intact and lesioned SNpc of rats in Experiment 3 (20 weeks after vector, 16-week after 6-OHDA) injected with AAV2/1 PTN in the ST (d), ST+SN (e) or in the ST+SN with AAV2/1 LacZ (f). (g, h) THir neuron survival determined by the selective TE counting method. (g) PTN overexpression was protective to SNpc THir neurons in the AAV2/1 PTN ST+SN-injected groups compared with controls (*P =0.033) over 6 weeks in Experiment 2. Neuroprotection in the AAV2/1 PTN ST-injected group did not achieve significance (P = 0.052). (h) Only ST AAV2/1-PTN injection resulted in neuroprotection 16 weeks after 6-OHDA injection in Experiment 3 (*P ≤0.002). ST AAV2/1 PTN-injected rats possessed significantly more THir SNpc neurons than those injected with AAV2/1 PTN in both the ST+SN and controls. (i–k) THir, non-THir and total neuron population estimates calculated by stereology to determine whether long-term PTN overexpression within SN THir neurons downregulated TH phenotype. (i) Stereological counts of THir neurons in the intact and lesioned SNpc. AAV2/1 PTN ST+SN-injected rats lost an average of 8759 THir neurons in the lesioned side compared with the intact, AAV2/1 PTN ST only rats lost an average of 6575 THir neurons. In both the groups, 6-OHDA injection resulted in significant reduction of THir neurons in the lesioned SNpc (*P<0.05). (j) No difference was detected between intact and lesioned non-THir neuron counts (P>0.05). (k) Total THir and non-THir neuron counts in the intact and lesioned SNpc. Significant neuronal loss is observed between intact and lesioned SNpc hemispheres in the AAV2/1 PTN ST+SN treatment group (P<0.05). In both the groups, total neuron loss parallels the loss of THir neurons in panel (i), demonstrating decreased THir neuron survival independent of phenotypic loss. Values are reported as mean ± s.e.m.
Long-term (20 weeks) PTN overexpression in the direct striatonigral, but not additional overexpression in the nigrostriatal pathway, is protective against 6-OHDA-mediated toxicity
In contrast to the results in the shorter Experiment 2, THir neuron survival 16 weeks after 6-OHDA in Experiment 3 in the ST only group was threefold higher than the control group and twofold higher than in the ST+SN group (Figures 4d–f and h). In the ST rAAV2/1 PTN-injected group, 274.7 ± 19.3 and 159.6 ± 12.3 THir neurons remained in the three coronal sections quantified in the intact and lesioned SNpc, respectively. In contrast, in the ST+SN rAAV2/1 PTN group there were 193.73 ± 20.9 surviving THir neurons in the intact SNpc compared with 57.7 ± 22.9 in the lesioned SNpc. In the control rAAV LacZ-injected rats, there were 229.6 ± 16.68 THir neurons in the intact and 39.3 ± 13.8 in the lesioned SNpc. Survival of THir neurons in the ST-injected group was significantly greater compared with either the ST+SN (F(2, 11) = 12.316, P = 0.001) or the control (P = 0.002) groups. Importantly, no significant difference in THir neuron survival was observed in the ST+SN rAAV2/1 PTN injected groups between the 6-week and 16-week post-6-OHDA euthanasia intervals, indicating that PTN expression in the SN over extended periods of time did not produce an added detrimental effect on SNpc THir neuron survival (P = NS, compare Figures 4g and h). In summary, 16 weeks following 6-OHDA, survival of nigral THir neurons in the ST rAAV2/1 PTN treatment group was ≈58% compared with ≈30% survival in the rAAV2/1 PTN ST+SN treatment group and (≈17%) survival in the control LacZ group.
Phenotype determinations for SNpc THir neurons
In an effort to ensure that loss of THir neurons in our selective TE counts reflected actual neuron loss and not a downregulation of TH in the 20-week Experiment 3, nigral sections in both the rAAV2/1 PTN ST and PTN ST+SN groups were counterstained with cresyl violet, and THir and non-THir neurons were quantified using unbiased stereology. In both the rAAV2/1 PTN ST and the PTN ST +SN treatment groups, intrastriatal 6-OHDA resulted in significant deficits in THir neurons (P<0.05). Total neuron counts indicated that loss of neurons within both the treatment groups could be completely attributed to the loss of THir neurons, indicating that loss of THir neurons reflected true neuronal loss and that PTN overexpression did not downregulate TH expression (Figures 4i–k).
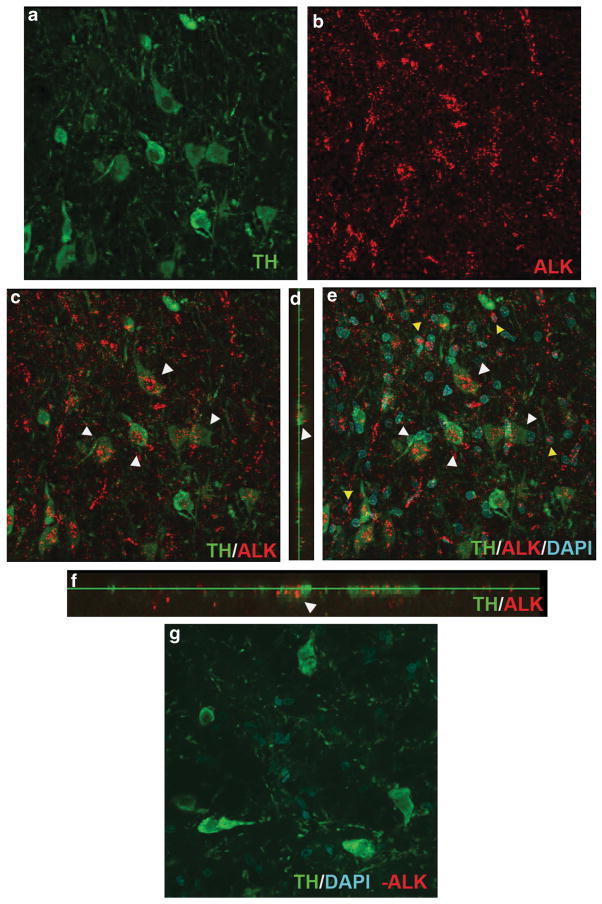
Additionally, previous studies have established that SNpc THir neurons express the PTN receptors RPTPβ/ζ and N-syndecan.18,19 However, no study has previously examined whether nigral neurons express the ALK receptor, and only the ALK receptor has been definitively linked to PTN’s trophic effects.20 Therefore, in an effort to determine whether PTN could potentially exert protective abilities through ALK receptors on DA neurons, SNpc neurons were immunofluorescently double labeled for TH and ALK and visualized using confocal microscopy. Punctate, granular staining of ALK was detected throughout the mescencephalon localized to both THir neurons and non-THir neurons within the SNpc (Figures 5a–f). Granular staining was not observed in primary antibody delete-stained tissue (Figure 5g). These results demonstrate that nigral THir neurons, as well as other cells within the SN, possess ALK receptors.
Figure 5.
ALK receptors are expressed by SNpc THir neurons. Double immunolabeling in the SNpc for TH (a, green) and ALK (b, red), showing co-localization of ALK on THir neurons (c, d, f, white arrowheads). Vertical (d) and horizontal (f) cross-sections of TH- and ALK-stained sections from image (c), showing ALK localized within THir neurons (white arrowheads). Triple labeling for TH, ALK and DAPI (e, cyan), showing that ALK is also expressed on non-THir cells (yellow arrow heads) within the SNpc. (g) Primary antibody delete resulted in no ALK staining in THir (green) and non-THir cells in the SNpc.
Long-term (20 weeks) striatal PTN overexpression following ST injection is protective to THir neurites
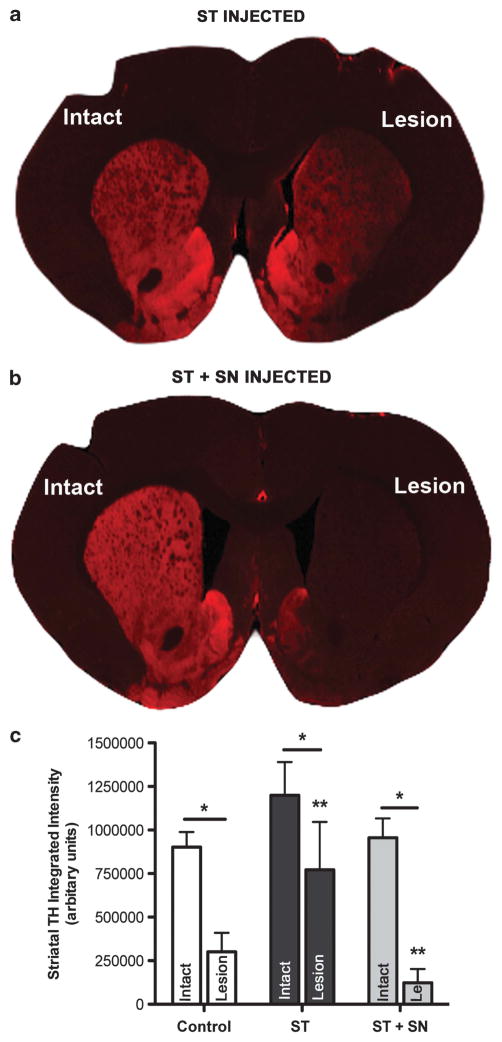
6-OHDA resulted in a significant depletion in THir optical density in the ipsilateral ST in all the groups 16 weeks after 6-OHDA in Experiment 3 (F(1,25) = 58.169, P ≤0.016, Figure 6c). However, intrastriatal rAAV2/1 PTN injection resulted in a level of TH immunoreactivity twofold higher than controls and threefold higher than ST+SN-injected rats (Figures 6a–c). THir signal intensity in the ST-only-injected group was significantly increased compared with the ST+SN-injected group (P = 0.038), and there was no significant difference between striatal THir signal intensity in rAAV LacZ control and ST+SN groups (P>0.05). In summary, 16 weeks following 6-OHDA significantly higher THir signal intensity was observed in the ST rAAV2/1 PTN treatment group compared with both the rAAV2/1 PTN ST+SN treatment group and the control LacZ group.
Figure 6.
Intrastriatal but not simultaneous intranigral/intrastriatal rAAV2/1 PTN injection preserves dopaminergic terminals in the ST over the 20-week transduction interval. (a) TH expression (red) 16 weeks post-6-OHDA in the intact and lesioned ST of a rat injected with rAAV2/1 PTN only in the ST in Experiment 3. (b) TH expression 16 weeks post-6-OHDA in the intact and lesioned ST of a rat injected with rAAV2/1 PTN in both the ST+SN. (c) 6-OHDA lesion significantly depleted striatal TH compared with the intact ST in all the groups (*P ≤0.0016). Significantly more TH immunoreactivity was observed in the lesioned ST of the rAAV2/1 PTN ST-injected group compared with the lesioned ST of the rAAV2/1 PTN ST+SN-injected group (**P =0.016). Values are reported as mean ± s.e.m.
DISCUSSION
Our results demonstrate that PTN-mediated neuroprotection against 6-OHDA-induced toxicity is dependent upon both site of administration and duration of expression. Intrastriatal delivery of rAAV2/1 PTN resulted in transduction of striatal medium spiny neurons and their projections to the SNpr (direct pathway). Intranigral rAAV2/1 PTN injection resulted in PTN expression in dopaminergic and nondopaminergic neurons within the SNpc and ventral mesencephalon. At 6 weeks following 6-OHDA (10 weeks post vector), PTN overexpression in the striatonigral pathway (intrastriatal injection) alone or in combination with nigrostriatal overexpression (intranigral injection) provided neuroprotection for nigral DA neurons against of 6-OHDA. However, over longer intervals (16 weeks postlesion, 20 weeks postvector) this neuroprotection was only maintained in the case of intrastriatal rAAV2/1 PTN delivery alone and, this protection was absent in rats that also received intranigral rAAV2/1 PTN. Neuroprotection in the 20-week intrastriatal rAAV2/1 PTN treatment group was associated with elevated PTN expression in the ST and direct pathway terminals in the SNpr. Despite increased PTN expression in the SN and transduction of nigral SNpc THir neurons, over 20 weeks this autocrine delivery of PTN ceased to be beneficial, and neuroprotection was maintained only by paracrine PTN delivery via transduction of the direct striatonigral pathway. Further, 20-week intrastriatal rAAV2/1 PTN delivery provided the greatest level of protection from 6-OHDA-induced motor impairments. Collectively, our results identify the ST as the optimal target for rAAV2/1 PTN injection and suggest that the resulting transduction of the striatonigral direct pathway is able to provide trophic support for nigrostriatal DA neurons.
Previous preclinical studies examining the effects of site of administration utilizing trophic factors have tested either GDNF or NTN overexpression.5–11,13 In the case of GDNF, long-term overexpression in the ST and SN was shown to be neuroprotective to nigral dopaminergic neurons; however, GDNF overexpression in the SN was detrimental to functional outcomes and associated with dopaminergic fibers turning back toward the SN.9–11 Our results demonstrating superior functional outcomes associated with intrastriatal rAAV PTN injection are in line with superior functional outcomes observed following intrastriatal GDNF over-expression. In the case of NTN, overexpression in the SN was associated with superior neuroprotection 2 weeks after 6-OHDA compared with overexpression in both the SN and the ST however, longer post-lesion intervals and functional assessments were not examined.13 Our findings that PTN overexpression in the ST and the SN is slightly more neuroprotective compared with ST overexpression alone (albeit not significantly so) in the shorter Experiment 2 is similar to what was observed with shorter post-6-OHDA lesion intervals and NTN overexpression. Without a direct comparison of the three factors within the same experiment, it is difficult to state definitive conclusions, but these results illustrate the importance of studying different sites of trophic factor overexpression within nigrostriatal circuitry as well as whether effects observed short term can be maintained long term.
Interestingly, striatal PTN levels were elevated over the 20-week interval in ST-injected rats compared with those injected in the ST+SN despite the fact that both groups received equivalent intrastriatal rAAV2/1 PTN injections. Additionally, rats injected only in the ST in the long-term study had increased density of striatal THir neurites 16 weeks postlesion compared with ST+SN-injected counterparts. Although the PTN antibody used in the present study is targeted to the human PTN transgene, some cross-reactivity with rat PTN can occur. Previous studies have reported that endogenous PTN is transiently upregulated in the nigrostriatal system following injury, including in the rodent ST following 6-OHDA administration19 and in surviving nigral DA neurons of PD patients.15 Of note, significantly increased striatal THir terminal density was observed in the ST group, and our previous and present functional analyses indicate ongoing replenishment of striatal innervation.16 It is therefore possible that the uniquely high levels of PTN detected within the ST of the ST-injected group reflects contributions from both vector-delivered PTN and host endogenous PTN. Further experiments will be required to examine this issue.
The efficacy of trophic factor delivery is dependent upon access to the signaling receptor. In the present study, neuroprotection was associated with PTN expression in striatal neurons and in their terminals in the SNpr, suggesting that this circuitry provides a source of PTN for SNpc DA neurons. Evidence for a neuroprotective role of paracrine-supplied PTN is provided by mesencephalic cell culture studies14,15 as well as by a recent study in which PTN overexpression targeted to mescencephalic astrocytes increased THir SNpc neuronal survival following 6-OHDA lesion.18 Indeed, the PTN receptor RPTPβ/ζ and (based on our present results) ALK are expressed by nigral SNpc DA neurons and are known to be localized to dendrites.21–23 The presence of ALK on striatal terminals is controversial, although ALK mRNA can be detected in the STR.19 It is possible that PTN secreted within the SNpr resulted in activation of PTN receptors located on the dendrites of SNpc neurons. Binding of PTN to its receptors has been shown to activate prosurvival Akt and ERK1/2 signaling.24–30 For unknown reasons, overexpression of PTN within SNpc neurons did not provide long-term neuroprotection, whereas long-term over-expression of GDNF within SNpc neurons has been shown to be supportive.9–11 Presumably overexpression by SNpc neurons would result in release at distant terminals in the ST. Evidence suggests that once released, GDNF is retrogradely transported to promote survival signaling.31–33 However, no such phenomenon has been suggested for PTN and not all trophic factors function via retrograde survival signaling. A difference such as this one between PTN and GDNF may explain the failure of PTN overexpression within nigral neurons to promote long-term survival. Ultimately, further experiments are necessary to elucidate which PTN receptor(s) participate in the neuroprotection provided by striatonigral PTN overexpression.
To date, the trophic factor NTN has been the sole trophic factor with available clinical trial results for efficacy in PD gene. However, in double-blind Phase II studies neither intraputaminal injections of AAV2/2 NTN nor concurrent delivery to both the putamen and the SN resulted in improved UPDRS scores.1,3 An ongoing clinical trial seeks to test the potential of intraputaminal injection of AAV2/2-GDNF, a close relative of NTN, to provide neuroprotection and functional restoration, again in advanced in PD.4
Should GDNF gene therapy, like NTN, also prove to not be disease-modifying, we will be left with questions regarding whether the trophic factor or the stage of intervention, or both, contributed to the failure. In order to optimize the predictive validity of preclinical trials, the models and timing of intervention must be carefully considered. Neurotoxin PD models have been effectively used to refine gene therapy parameters, including the optimal site of administration, dosage, transport, transduction volume and identification of side-effects.9,10,34 However, with very few exceptions, the preclinical paradigms that have been used to evaluate the neuroprotective potential of a particular trophic factor have consisted of factor delivery before, or in conjunction with, neurotoxin administration. When NTN and GDNF over-expression occurs before 6-OHDA, robust neuroprotection of both nigral neurons and terminals in the ST is observed.10,35–38 These results are markedly different if delivery of these factors is delayed until after significant nigrostriatal degeneration has occurred. In delayed paradigms, the positive effects of these therapies are essentially limited to slight increases in axonal regeneration and striatal dopaminergic tone.39–43 These findings predicted the clinical results of the NTN gene therapy trial in advanced PD patients and may also predict the results of the ongoing GDNF gene therapy trial in advanced PD patients. Our PTN results indicate that AAV-PTN delivery to the ST is clearly superior to rAAV PTN delivery to both the SN and ST in terms of providing nigrostriatal neuroprotection before insult. These findings suggest that intrastriatal rAAV PTN delivery may provide neuroprotection for early PD patients. Future studies will determine whether intrastriatal AAV-PTN delivery can halt ongoing degeneration and provide protection in different PD animal models.
MATERIALS AND METHODS
Animals
Male, Sprague Dawley rats (Harlan, Indianapolis, IN, USA) 3 months of age (n = 44) were used in these studies. All animals were given food and water ad libitum and housed in 12-h reverse light–dark cycle conditions in the Van Andel Research Institute vivarium, which is fully AAALAC approved. All procedures were conducted in accordance with guidelines set by the Institutional Animal Care and Use Committee (IACUC) of Michigan State University.
Experimental overview
Experiment 1
A cohort of rats (n = 15) was unilaterally lesioned with intrastriatal 6-OHDA and euthanized 2, 4 or 6 weeks after injection. Surviving SNpc THir neurons were quantified to establish the accuracy of a modified TE counting method as compared with standard stereological assessment.
Experiment 2
Rats were unilaterally injected with either rAAV2/1 PTN (n = 6) or rAAV2/1 GFP (n = 6) in both the ST and SN or in the ST only. Four weeks later, all rats recieved unilateral instrastriatal injections of 6-OHDA. Rats were euthanized 6 weeks post-6-OHDA injection (Figure 2a).
Experiment 3
Rats were unilaterally injected with either rAAV2/1 PTN (n = 10) or rAAV2/1 LacZ (n = 6) in both the ST and SN or in the ST alone. Baseline behavioral measurements were conducted, and 4 weeks later, all rats were intrastriatally injected with 6-OHDA. Behavioral tests were conducted at 4, 8, 12 and 16 weeks post-6-OHDA injection. Rats were euthanized 16 weeks post-6-OHDA injection (Figure 2b).
Viral vectors
Plasmid and rAAV vector production was completed as previously described.16 Human PTN was cloned from human brain cDNA and inserted into an AAV plasmid backbone downstream to a chicken beta-actin promoter/cytomegalovirus enhancer promoter hybrid. All vectors contained AAV2 terminal repeats. PTN and GFP plasmids were packaged into AAV1 capsids. rAAV2/1 GFP was injected into control rats in the short-term, 10-week study (Experiment 2). rAAV2/1 LacZ vector was purchased from Vector Biolabs (no.7026, Philadelphia, PA, USA) and used in the long-term, 20-week experiment (Experiment 3), because rAAV-GFP, at high titers and over long durations, has the potential to be toxic to nigral DA neurons.44,45 PTN and GFP vector titers were determined by quantitative PCR. The rAAV2/1 PTN titer was 5.3 × 1012 viral genomes ml −1 (vg ml−1), rAAV2/1 LacZ titer was 1.0 × 1013 vg ml−1 and rAAV2/1 GFP titer was 4.8 × 1012 vg ml −1.
rAAV2/1 PTN, rAAV2/1 GFP and rAAV2/1 LacZ injections into the nigrostriatal system
Rats were anesthetized with isoflurane and placed into a stereotaxic frame. Rats in the dual-structure transduction treatment group (SN plus ST) were injected with a total of 8 μl of rAAV2/1 PTN or rAAV2/1 GFP (Experiment 2) or rAAV2/1 LacZ (Experiment 3). Rats in the single-structure transduction treatment group (ST only) received a total of 4 μl of rAAV2/1 PTN, GFP or LacZ. Striatal coordinates from bregma were AP +1.6 mm, ML +2.4 mm, DV − 4.2 mm from dura and AP +0.2 mm, ML +2.6 mm, DV − 7.0 mm, 2μl per site. Nigral coordinates were AP − 5.3 mm from bregma, ML +2.0 mm, DV − 7.2 mm from dura and AP − 6.0 mm, ML +2.0 mm, DV − 7.2 mm with 2 μl per site. Dual injection site coordinates were chosen to deliver vector to the entire rostral–caudal axis of the nigra, as previously reported.46 Before striatal injections, all rats received 0.3 ml per 100 g of 25% Manitol solution intraperitoneally as described previously to increase hyperosmolality.47 A Hamilton syringe fitted with a pulled glass micropipette (Hamilton Gas Tight syringe 80 000, 26s/2″ needle; Hamilton, Reno, NV, USA; coated in Sigmacote, Sigma-Aldrich, St Louis, MO, USA; SL2, micropipette opening ~ 60–80 μm) was used for injection. For injections into the ST, the needle was lowered to the site, 1 min passed before the injection began, and vector was infused at the rate of 0.5 μl min−1, and 4 min following the end of injection, the needle was retracted. For each nigral injection, the needle was lowered to the site, vector injection began immediately at a rate of 0.5 μl min−1 and the needle remained in place for an additional 5 min before retraction.
Intrastriatal 6-OHDA
Four weeks following vector injection, all rats were anesthetized with isofluorane, and scalp wounds were re-opened. All rats received two intrastriatal injections of 6-OHDA (MP Biomedicals, Solon, OH, USA; 5 μg μl−1 (free base) 6-OHDA in 0.2% ascorbic acid, 0.9% saline solution).17 Striatal 6-OHDA coordinates were identical to the striatal vector coordinates with 2 μl injected per site. For each injection, the needle was lowered into the ST, 1 min passed before the injection began and 6-OHDA was infused at the rate of 0.5 μl min−1. The needle was retracted 2 min following the end of each injection.
Behavioral analysis
Behavioral analysis was conducted and analyzed by researchers blinded to the treatment groups in Experiment 3. Animals were tested before 6-OHDA injection (baseline) and 4, 8, 12 and 16 weeks following 6-OHDA. We have previously determined that rAAV2/1 PTN striatal injection into intact, unlesioned rats does not impact motor function.16
Cylinder test
Spontaneous forepaw use was measured in the cylinder test as previously described.48 Briefly, during the dark cycle rats were placed in a clear plexiglass cylinder, and behavior was videotaped until 5 min passed or 20 weight-bearing paw taps on the sides of the cylinder were made. The number of taps made by the paw ipsilateral or contralateral to 6-OHDA or both paws was recorded from the videotapes by a blinded rater. Data are reported as the percentage of contralateral (to 6-OHDA) forelimb use: ((contralateral+½ both)/(ipsilateral+contralateral+both))×100.
Adjusting steps (bracing) task
The test was performed as described previously.49–51 To gauge nigrostriatal DA loss, the number of stepping movements made with each forepaw contralateral and ipsilateral to 6-OHDA injection were assessed. Rats were held by the torso with hindlimbs such that the weight of the rat’s body was resting on a single forepaw in contact with the table. The handler moved rats side-to-side alternating which forepaw supported their weight over a fixed distance (36 inches) for a 10-s period for three trials. Self-initiated steps by each forelimb are counted and averaged across trials. Rats with a unilateral nigrostriatal lesion make fewer steps with the forepaw contralateral to the injection side. Data are presented as the average number of steps made by the contralateral forepaw for each group over time.
Bilateral tactile stimulation test
This test was performed as previously described.52–54 Phase 1: To test somatosensory asymmetry, rats were individually removed from their home cage and adhesive stickers (Tough-Spots½″ diameter, USA Scientific, Ocala, FL, USA, 9185-0504) were placed on the distal–radial aspect of both forepaws. Immediately after stimulus placement, rats were returned to their home cage. The order of and time to contact each forepaw was recorded for five trials, and trials lasted a maximum of 60 s before a trial was redone. Preference for the unaffected forepaw (ipsilateral to 6-OHDA injection) indicated a sensory bias and nigrostriatal lesion. Data are presented as the average time to contact the affected forepaw.
Amphetamine-induced rotational asymmetry
Rats were assessed for ipsilateral rotational asymmetry as previously described.17,55 Rats received intraperitoneal injections of 3.0 mg kg−1 amphetamine in 0.9% saline and placed into cylindrical bowls. Rotational behavior was quantified using the TSE LabMaster (Chesterfield, MO, USA) rotometer program. Recording began 5 min after amphetamine injections and continued for 90 min. Data are expressed as the number of ipsilateral (to 6-OHDA injection) rotations per minute.
Euthanasia and tissue preparation
Rats assessed for modified TE counting verification were euthanized at 2, 4 or 6 weeks post-6-OHDA (Experiment 1). Rats were euthanized 6 or 16 weeks post-6-OHDA injection in Experiments 2 and 3, respectively (Figures 2a and b). All animals were deeply anesthetized (60 mg kg −1 pentobarbital, intraperitoneally) and perfused intracardially with 0.9% saline solution containing 1 ml per 10 000 USP heparin. Brains were postfixed in 4% paraformaldehyde in 0.1 M PO4 buffer for 7 days and then transferred to 30% sucrose in 0.1 M PO4 until sinking. Brains were frozen on dry ice and sectioned at 40 μm using a sliding microtome (American Optical Sliding Microtome Model 860, Buffalo, NY, USA).
Immunohistochemistry
Immunohistochemical staining was performed using the free-floating method.
TH immunohistochemistry
Free-floating sections were blocked in 10% normal goat serum and incubated in primary antisera against TH (Chemicon, Billerica, MA, USA, MAB318, mouse anti-TH, 1:4000) with 5 mg per 100 ml sodium azide overnight at room temperature. Following primary incubation and rinses, TH-labeled sections were incubated in secondary antisera against mouse IgG (Chemicon AP124B, goat anti-mouse, 1:400) for 2 h, followed by the Vector ABC detection kit using horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA). Antibody labeling was visualized by exposure to 0.5 mg ml −1 3,3′ diaminobenzidine and 0.03% H2O2 in Tris buffer. An additional series of tissue sections from a cohort of rats in both the ST only and ST plus SN rAAV2/1-PTN treatment groups (n = 5) in Experiment 2 were counterstained with cresyl violet following TH immunolabeling in order to distinguish between loss of TH phenotype and neuronal loss. Sections were mounted on subbed slides and coverslipped with CytoSeal mounting medium (Thomas Scientific, 6705A15, Swedesboro, NJ, USA).
PTN and TH immunofluorescence for near infrared imaging and optical density analysis
To quantify transduction efficiency by rAAV2/1 PTN in the nigrostriatal system and levels of striatal TH immunoreactivity, free-floating tissue sections were blocked in Odyssey blocking buffer (LI-COR Bioscience, Lincoln, NE, USA, 927-40000) for 60 min at room temperature. Tissues were then incubated in primary antibody for PTN (R&D Systems, Minneapolis, MN, USA, AF252PB, biotinylated goat antihuman PTN, 1:25, in Odyssey blocking buffer with 0.2% Triton-X) or TH (Chemicon MAB318, Mouse anti-TH, 1:1000, in Odyssey blocking buffer with 0.2% Triton-X) overnight at room temperature. After rinsing, tissue was incubated in secondary antisera for 2 h at room temperature (PTN, LI-COR Biosciences 926-32214, IRDye 800CW donkey anti-goat, 1:250 or TH, 926-32210, IRDye 800CW goat anti-mouse, 1:250 in Odyssey blocking buffer). Sections were then rinsed in 0.1 M Tris-buffered saline and immediately mounted onto subbed slides, dehydrated and coverslipped with Cytoseal (Richard-Allan Scientific, Waltham, MA, USA). Slides were then imaged using the Odyssey infrared image system (LI-COR Bioscience) to quantify the level of PTN transduction in the ST and SN and TH protein levels in the nigrostriatal system. Rats in the PTN treatment groups were included in the behavioral and morphological analyses if they displayed nigrostriatal PTN transduction.
Immunofluorescence to verify GFP and LacZ transduction
Free-floating tissue sections were fluorescently labeled for LacZ in Experiment 3. Tissue was blocked in 10% normal goat serum for 20 min at room temperature and then incubated in chicken anti-β-galactosidase primary antisera (Abcam, Cambridge, MA, USA, ab9361, 1:1000) overnight at room temperature. Tissues were rinsed in 1.0 M Tris-buffered saline solution and incubated in goat anti-chicken IgG (Alexa Fluor 488, Life Technologies, Carlsblad, CA, USA, A11039, 1:400) secondary antisera for 3 h at room temperature. All tissues were mounted on subbed slides and coverslipped with Vectashield HardSet Mounting Medium (Vector Laboratories H1400). Fluorescence imaging was conducted on immunolabeled and GFP autofluorescence using a Nikon Eclipse 90i microscope (Nikon, Tokyo, Japan).
ALK and TH immunofluorescence for co-localization
Free-floating tissue sections were incubated in primary rabbit anti-TH (Millipore, Billerica, MA, USA, AB-152, 1:4000) at room temperature overnight. Tissues were rinsed in 1.0 M Tris-buffered saline solution, and tissue sections were incubated in donkey anti-rabbit IgG (Invitrogen, Carlsblad, CA, USA, A21206 Alexa Fluor 488, 1:500) at room temperature for 2 h. Subsequently, tissue sections were incubated in primary antisera against ALK (Santa Cruz, Santa Cruz, CA, USA, sc-6345, 1:50) overnight at room temperature, followed by incubation with donkey anti-goat secondary (Invitrogen A11057, Alexa Fluor 568, 1:500). Sections were mounted on subbed slides and coverslipped with Vectashield Mounting Medium with DAPI (Vector Biolabs, Burlingame, CA, USA, H-1200). Immunolabeling was visualized using an Olympus FluoView FV10i confocal laser scanning microscope (Olympus Corporation, Tokyo, Japan).
Quantification of TH and PTN immunoreactivity
Immunolabeled sections were scanned at a resolution of 42 μm in the 800 channel and a 3.0 (arbitrary unit) sensitivity setting on a LI-COR Odyssey scanner (LI-COR Biosciences). Integrated signal intensities were collected from vector/6-OHDA-injected hemisphere and the contralateral hemispheres. To quantify striatal TH, integrated intensities were taken from a series of 1 in 6 sections throughout the ST of each animal (+2.28 mm through − 0.60 mm relative to bregma). Integrated intensities were measured in the PTN transduced and naive striatal hemispheres of six tissue sections from approximately +1.44 mm to 0.00 mm relative to bregma. Nigral PTN measurements were taken in the PTNir SNpc and the untransduced SNpc beginning at − 4.56 mm to − 6.48 mm relative to bregma. Slides were normalized by analyzing and subtracting background staining intensity from the contralateral cortex for each animal. TH intensity data are presented as the average percentage of remaining striatal TH intensity (compared with contralateral intact striatum) for each group. PTN data are presented as the average PTN integrated intensity values for the transduced and naive striatum and SNpc for each group.
Selective TE of THir neurons in the SNpc
SNpc THir neurons from three sections, easily identified by proximity to the medial terminal nucleus of the accessory optic tract56 (-5.04 mm, − 5.28 mm and − 5.52 mm relative to bregma), were quantified. Using a Nikon Eclipse 80i microscope, Retiga 4000R (QImaging, Surrey, BC, Canada) and the Microbrightfield StereoInvestigator software (Microbrightfield Bioscience, Burlingame, V, USA), selective TE THir neuron quantification was completed by drawing a contour around the SNpc borders at × 4. Virtual markers were then placed on THir neurons at × 20 and quantified. Total THir neuron numbers in the intact or lesioned SN were averaged for the three medial terminal nucleus sections counted (Figure 1a).
Stereological assessment of THir neurons in the SNpc
Stereology utilizing the optical fractionator principle was completed using every sixth serial section throughout the entire SNpc as described previously.16 The anterior–posterior axis measurements for sections counted using stereology were − 4.56, − 4.80, − 5.04, − 5.28, − 5.52, − 5.76, − 6.00, − 6.24 and − 6.48 mm relative to bregma. The same cohort of rats utilized for selective TE quantification were analyzed. In addition, THir and cresyl violet-counterstained sections from a cohort of rats from Experiment 2 were also analyzed to determine whether downregulation of TH phenotype contributed to the observed decreases in SNpc THir neurons. Using the optical fractionator principle, THir neurons or THir neurons and Nissl-positive/TH-negative neuronal nuclei in the 6-OHDA-injected and contralateral control nigral hemispheres were counted at × 60. A coefficient of error <0.10 was accepted. Data for comparisons between the selective TE quantification method and unbiased stereological assessment are reported as the percentage of remaining THir neurons. Data from Experiments 1 and 2 illustrating the impact of PTN transduction represent values obtained utilizing the selective TE counting method and are reported as the percentage of THir neurons remaining compared with the intact hemisphere. Data evaluating the impact of PTN transduction on TH phenotype were collected using unbiased stereological assessment of THir neurons and Nissl-positive neuronal nuclei and are reported as true neuronal estimates for each hemisphere. Glial nuclei were excluded from cresyl violet neuronal counts based on smaller size and homogeneous intense Nissl substance staining (Figures 4i–k).
Statistics
Selective TE quantification and stereology analyses were completed using separate two-way repeated-measures analyses of variance (ANOVAs) for each experiment. Comparisons were made between the numbers of surviving THir neurons in the intact versus lesioned SN (treatment factor) and between injection site groups (group factor). For Experiment 3, two-way repeated-measures ANOVAs were used to assess all behavioral effects between rats injected in the ST+SN or the ST only (group factor) and between multiple time points (time factor). To determine whether significant differences were present between ST+SN, striatal only or control-injected groups (group factor), a one-way ANOVA was performed using percentage of remaining striatal TH intensities. For Experiments 2 and 3, a two-way ANOVA was used to determine differences in PTN nigral or striatal expression between ST+SN-injected versus ST-only-injected rats (group factor) and between long- and short-term studies (time factor). In all cases, Holm–Sidak post hoc analyses were applied when appropriate.
Acknowledgments
This research was supported by NS058682 (to CES), NS076158 (to SEG) and the Morris K. Udall Center of Excellence for Parkinson’s Disease Research at Michigan State University NS058830 (to TJC).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 2.Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 3.Ceregene. Ceregene reports data from Parkinson’s disease Phase 2b Study [press release] 2013 Retrived from http://www.ceregene.com/press_041913.asp.
- 4.NINDS. AAV2-GDNF for Advanced Parkinson’s Disease. 2013 [Google Scholar]
- 5.Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Gasmi M, Herzog CD, Brandon EP, Cunningham JJ, Ramirez GA, Ketchum ET, et al. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Mol Ther. 2007;15:62–68. doi: 10.1038/sj.mt.6300010. [DOI] [PubMed] [Google Scholar]
- 7.Herzog CD, Dass B, Holden JE, Stansell J, 3rd, Gasmi M, Tuszynski MH, et al. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov Disord. 2007;22:1124–1132. doi: 10.1002/mds.21503. [DOI] [PubMed] [Google Scholar]
- 8.Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, et al. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirik D, Rosenblad C, Bjorklund A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci. 2000;12:3871–3882. doi: 10.1046/j.1460-9568.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenblad C, Kirik D, Bjorklund A. Sequential administration of GDNF into the substantia nigra and striatum promotes dopamine neuron survival and axonal sprouting but not striatal reinnervation or functional recovery in the partial 6-OHDA lesion model. Exp Neurol. 2000;161:503–516. doi: 10.1006/exnr.1999.7296. [DOI] [PubMed] [Google Scholar]
- 12.Bartus RT. Translating the therapeutic potential of neurotrophic factors to clinical ‘proof of concept’: a personal saga achieving a career-long quest. Neurobiol Dis. 2012;48:153–178. doi: 10.1016/j.nbd.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Herzog CD, Brown L, Kruegel BR, Wilson A, Tansey MG, Gage FH, et al. Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120) Neurobiol Dis. 2013;58:38–48. doi: 10.1016/j.nbd.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Hida H, Jung CG, Wu CZ, Kim HJ, Kodama Y, Masuda T, et al. Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro. Eur J Neurosci. 2003;17:2127–2134. doi: 10.1046/j.1460-9568.2003.02661.x. [DOI] [PubMed] [Google Scholar]
- 15.Marchionini D, Lehrmann E, Chu Y, He B, Sortwell C, Becker K, et al. Role of heparin binding growth factors in nigrostriatal dopamine system development and Parkinson’s disease. Brain Res. 2007;1147:77–88. doi: 10.1016/j.brainres.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 16.Gombash SE, Lipton JW, Collier TJ, Madhavan L, Steece-Collier K, Cole-Strauss A, et al. Striatal pleiotrophin overexpression provides functional and morphological neuroprotection in the 6-hydroxydopamine model. Mol Ther. 2012;20:544–554. doi: 10.1038/mt.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K, et al. Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss. Neurobiol Dis. 2010;39:105–115. doi: 10.1016/j.nbd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taravini IR, Chertoff M, Cafferata EG, Courty J, Murer MG, Pitossi FJ, et al. Pleiotrophin over-expression provides trophic support to dopaminergic neurons in parkinsonian rats. Mol Neurodegener. 2011;6:40. doi: 10.1186/1750-1326-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrario JE, Rojas-Mayorquin AE, Saldana-Ortega M, Salum C, Gomes MZ, Hunot S, et al. Pleiotrophin receptor RPTP-zeta/beta expression is up-regulated by L-DOPA in striatal medium spiny neurons of parkinsonian rats. J Neurochem. 2008;107:443–452. doi: 10.1111/j.1471-4159.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 20.Mi R, Chen W, Hoke A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. Proc Natl Acad Sci USA. 2007;104:4664–4669. doi: 10.1073/pnas.0603243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsueh YP, Sheng M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J Neurosci. 1999;19:7415–7425. doi: 10.1523/JNEUROSCI.19-17-07415.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rohrbough J, Broadie K. Anterograde Jelly belly ligand to Alk receptor signaling at developing synapses is regulated by Mind the gap. Development. 2010;137:3523–3533. doi: 10.1242/dev.047878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi N, Oohira A, Miyata S. Synaptic localization of receptor-type protein tyrosine phosphatase zeta/beta in the cerebral and hippocampal neurons of adult rats. Brain Res. 2005;1050:163–169. doi: 10.1016/j.brainres.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 24.Stoica GE, Kuo A, Aigner A, Sunitha I, Souttou B, Malerczyk C, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 25.Powers C, Aigner A, Stoica GE, McDonnell K, Wellstein A. Pleiotrophin signaling through anaplastic lymphoma kinase is rate-limiting for glioblastoma growth. J Biol Chem. 2002;277:14153–14158. doi: 10.1074/jbc.M112354200. [DOI] [PubMed] [Google Scholar]
- 26.Bowden ET, Stoica GE, Wellstein A. Anti-apoptotic signaling of pleiotrophin through its receptor, anaplastic lymphoma kinase. J Biol Chem. 2002;277:35862–35868. doi: 10.1074/jbc.M203963200. [DOI] [PubMed] [Google Scholar]
- 27.Kinnunen T, Raulo E, Nolo R, Maccarana M, Lindahl U, Rauvala H. Neurite outgrowth in brain neurons induced by heparin-binding growth-associated molecule (HB-GAM) depends on the specific interaction of HB-GAM with heparan sulfate at the cell surface. J Biol Chem. 1996;271:2243–2248. doi: 10.1074/jbc.271.4.2243. [DOI] [PubMed] [Google Scholar]
- 28.Rauvala H, Huttunen HJ, Fages C, Kaksonen M, Kinnunen T, Imai S, et al. Heparin-binding proteins HB-GAM (pleiotrophin) and amphoterin in the regulation of cell motility. Matrix Biol. 2000;19:377–387. doi: 10.1016/s0945-053x(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 29.Maeda N, He J, Yajima Y, Mikami T, Sugahara K, Yabe T. Heterogeneity of the chondroitin sulfate portion of phosphacan/6B4 proteoglycan regulates its binding affinity for pleiotrophin/heparin binding growth-associated molecule. J Biol Chem. 2003;278:35805–35811. doi: 10.1074/jbc.M305530200. [DOI] [PubMed] [Google Scholar]
- 30.Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/ phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase zeta/RPTPbeta, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) J Biol Chem. 1996;271:21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- 31.Tsui CC, Pierchala BA. The differential axonal degradation of Ret accounts for cell-type-specific function of glial cell line-derived neurotrophic factor as a retrograde survival factor. J Neurosci. 2010;30:5149–5158. doi: 10.1523/JNEUROSCI.5246-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leitner ML, Molliver DC, Osborne PA, Vejsada R, Golden JP, Lampe PA, et al. Analysis of the retrograde transport of glial cell line-derived neurotrophic factor (GDNF), neurturin, and persephin suggests that in vivo signaling for the GDNF family is GFRalpha coreceptor-specific. J Neurosci. 1999;19:9322–9331. doi: 10.1523/JNEUROSCI.19-21-09322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomac A, Widenfalk J, Lin LF, Kohno T, Ebendal T, Hoffer BJ, et al. Retrograde axonal transport of glial cell line-derived neurotrophic factor in the adult nigrostriatal system suggests a trophic role in the adult. Proc Natl Acad Sci USA. 1995;92:8274–8278. doi: 10.1073/pnas.92.18.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartus RT, Brown L, Wilson A, Kruegel B, Siffert J, Johnson EM, Jr, et al. Properly scaled and targeted AAV2-NRTN (neurturin) to the substantia nigra is safe, effective and causes no weight loss: support for nigral targeting in Parkinson’s disease. Neurobiol Dis. 2011;44:38–52. doi: 10.1016/j.nbd.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 35.Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis. 2007;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson’s disease. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ, et al. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature. 1995;373:335–339. doi: 10.1038/373335a0. [DOI] [PubMed] [Google Scholar]
- 38.Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc Natl Acad Sci USA. 1997;94:14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oiwa Y, Yoshimura R, Nakai K, Itakura T. Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and photochemical measurements in a model of progressive Parkinson’s disease. Brain Res. 2002;947:271–283. doi: 10.1016/s0006-8993(02)02934-7. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, et al. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson’s disease. Gene Therapy. 2002;9:381–389. doi: 10.1038/sj.gt.3301682. [DOI] [PubMed] [Google Scholar]
- 41.Kozlowski DA, Connor B, Tillerson JL, Schallert T, Bohn MC. Delivery of a GDNF gene into the substantia nigra after a progressive 6-OHDA lesion maintains functional nigrostriatal connections. Exp Neurol. 2000;166:1–15. doi: 10.1006/exnr.2000.7463. [DOI] [PubMed] [Google Scholar]
- 42.McGrath J, Lintz E, Hoffer BJ, Gerhardt GA, Quintero EM, Granholm AC. Adeno-associated viral delivery of GDNF promotes recovery of dopaminergic phenotype following a unilateral 6-hydroxydopamine lesion. Cell Transplant. 2002;11:215–227. [PubMed] [Google Scholar]
- 43.Rosenblad C, Martinez-Serrano A, Bjorklund A. Intrastriatal glial cell line-derived neurotrophic factor promotes sprouting of spared nigrostriatal dopaminergic afferents and induces recovery of function in a rat model of Parkinson’s disease. Neuroscience. 1998;82:129–137. doi: 10.1016/s0306-4522(97)00269-8. [DOI] [PubMed] [Google Scholar]
- 44.Gorbatyuk OS, Li S, Sullivan LF, Chen W, Kondrikova G, Manfredsson FP, et al. The phosphorylation state of Ser-129 in human alpha-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc Natl Acad Sci USA. 2008;105:763–768. doi: 10.1073/pnas.0711053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson’s disease. PLoS One. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gombash SE, Manfredsson FP, Kemp CJ, Kuhn NC, Fleming SM, Egan AE, et al. Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS One. 2013;8:e81426. doi: 10.1371/journal.pone.0081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burger C, Nguyen FN, Deng J, Mandel RJ. Systemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol Ther. 2005;11:327–331. doi: 10.1016/j.ymthe.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 48.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15(Pt 2):3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fleming SM, Schallert T, Ciucci MR. Cranial and related sensorimotor impairments in rodent models of Parkinson’s disease. Behav Brain Res. 2012;231:317–322. doi: 10.1016/j.bbr.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 51.Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T. Enhanced function in the good forelimb of hemi-parkinson rats: compensatory adaptation for contralateral postural instability? Exp Neurol. 2008;211:511–517. doi: 10.1016/j.expneurol.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schallert T, Upchurch M, Wilcox RE, Vaughn DM. Posture-independent sensorimotor analysis of inter-hemispheric receptor asymmetries in neostriatum. Pharmacol Biochem Behav. 1983;18:753–759. doi: 10.1016/0091-3057(83)90019-9. [DOI] [PubMed] [Google Scholar]
- 53.Schallert T, Upchurch M, Lobaugh N, Farrar SB, Spirduso WW, Gilliam P, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- 54.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 55.Robinson TE, Becker JB. The rotational behavior model: asymmetry in the effects of unilateral 6-OHDA lesions of the substantia nigra in rats. Brain Res. 1983;264:127–131. doi: 10.1016/0006-8993(83)91129-0. [DOI] [PubMed] [Google Scholar]
- 56.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; Sydney, Australia, Orlando, FL, USA: 1986. [Google Scholar]