Abstract
The transcription factor Sox9 was first discovered in patients with campomelic dysplasia, a haploinsufficiency disorder with skeletal deformities caused by dysregulation of Sox9 expression during chondrogenesis. Since then, its role as a cell fate determiner during embryonic development has been well characterized; Sox9 expression differentiates cells derived from all three germ layers into a large variety of specialized tissues and organs. However, recent data has shown that ectoderm- and endoderm-derived tissues continue to express Sox9 in mature organs and stem cell pools, suggesting its role in cell maintenance and specification during adult life. The versatility of Sox9 may be explained by a combination of post-transcriptional modifications, binding partners, and the tissue type in which it is expressed. Considering its importance during both development and adult life, it follows that dysregulation of Sox9 has been implicated in various congenital and acquired diseases, including fibrosis and cancer. This review provides a summary of the various roles of Sox9 in cell fate specification, stem cell biology, and related human diseases. Ultimately, understanding the mechanisms that regulate Sox9 will be crucial for developing effective therapies to treat disease caused by stem cell dysregulation or even reverse organ damage.
Keywords: Development, Sox9, Stem cells, Transcription factor
Introduction
Stem cells are undifferentiated biological cells that can self-renew or differentiate into one or more mature cellular lineages.1 In mammals, stem cells can differentiate into specialized cells of every germ layer—ectoderm, endoderm and mesoderm—in a developing embryo, but can also maintain the normal turnover of regenerative tissues, such as in skin and intestines.1 In adult organisms, stem and progenitor cells are kept in pools known as “niches,” which act as a repair system to replenish damaged tissues.2 A stem cell's decision between self-renewal and differentiation is a tightly regulated process that requires expression of cell type-specific transcription factors. Over the last few years, several such molecules have been implicated in stem cell biology, although their versatile functions in different tissues remain to be fully elucidated.
Sox family proteins are a group of transcriptional regulators containing a high mobility group (HMG) domain that is highly conserved.3 The HMG domain was first identified in Sry, a crucial factor involved in mammalian male sex determination.3, 4 In general, proteins containing an HMG domain with 50% or higher amino acid similarity to the HMG are referred to as Sox proteins (Sry-related HMG box). Around 20 Sox proteins to this date has been identified in mice and humans, and are grouped A through H based on the structural homology outside of their HMG boxes. Notably, Sox-like proteins are identified in invertebrate lineages and in the unicellular organisms, suggesting that it is evolutionarily conserved.5 Early insights into the function of Sox factors have involved cell fate determination during development, although recent findings reveal its crucial role in establishing and maintaining stem and progenitor cell pools.
In this review, we confine our discussion to one of the well-characterized SoxE proteins, Sox9. After discussing multiple levels of regulation and mechanisms, we review the versatile functions of Sox9 in germ layers and adult tissues as a stem cell regulator. We then discuss its function in disease pathogenesis while highlighting the Sox9-related pathology of fibrosis and cancer.
Molecular characteristics of Sox9
Structural domains of Sox9
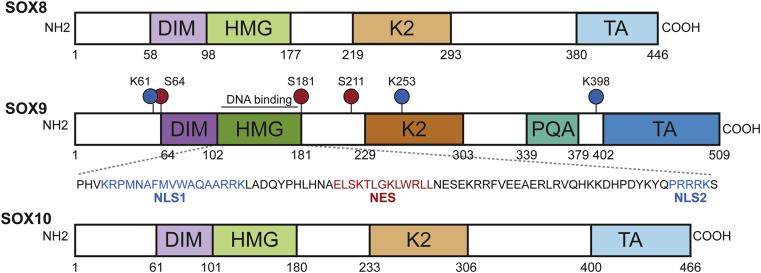
Research on Sox9 began with its seminal discovery as the gene underlying campomelic dysplasia (CD), a haploinsufficiency disorder characterized by defective chondrogenesis and a high proportion of male-to-female sex reversals in XY males.6 Along with Sox8 and 10, Sox9 belongs to the SoxE subgroup, and, characteristic of all Sox proteins, contains the HMG domain which induces significant bending at the consensus-binding motif (A/TA/TCAAA/TG) by forming an L-shaped complex in the minor groove of DNA.3 Members of the SoxE subgroup share regions of significant homology outside the HMG domain, and constitute two additional functional domains: a self-dimerization domain and a transactivation domain at the C-terminus (Fig. 1).3, 7
Figure 1.
Schematic structures of SoxE proteins. In all SoxE proteins, the dimerization domain (DIM) precedes the DNA-binding high mobility group (HMG) domain and two separate transactivation domains are located in a central position (K2) and at the C-terminus (TA). For Sox9, two independent nuclear localization sequences (NLS) and the nuclear export sequences (NES) in the HMG domain, phosphorylation sites (red), and ubiquination/sumolyation sites (blue) are highlighted.3, 7. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
A recurring theme among Sox proteins, Sox9 shares functions redundant within the SoxE subgroup. This is well demonstrated in knockout mutants, where individual Sox9 mutants often have a starkly less severe phenotype than double or triple SoxE mutants. For instance, separate deletions of either Sox9 or Sox10 retain normal formation of oligodendrocytes, whereas the deletion of both results in widespread apoptosis.8 However, depending on the tissue in question, the individual contribution of each member may differ temporally and in the amount of expression. While replacing Sox8 with Sox10 resumes normal development of glial cells and neurons in the sensory and sympathetic parts of the peripheral nervous system, only Sox10-deficient mice show defective melanocyte development.9 Another report showed that, despite functional redundancy of Sox8 and Sox10 in oligodendrocyte development, Sox8 expression levels are significantly lower than those for Sox10.10
Posttranscriptional regulation of Sox9
Sox9 is subject to context-dependent regulation at multiple levels. One type of regulation is posttranscriptional modification, which modulates the stability, intracellular localization, and the overall activity of Sox9.11, 12, 13 Phosphorylation by protein kinase A (PKA) enhances DNA-binding affinity of Sox9 and leads to its translocation into the nucleus in testis cells.14 Interestingly, this same event is also required in the neural crest (NC) cells for the Sox–Snail interaction during NC delamination, and is necessary for parathyroid hormone-related peptide (PTHrP)-mediated regulation of chondrocyte maturation.11, 12
SUMOlaytion, or post-transcriptional regulation by small ubiquitin-related modifier, has also been noted to influence Sox9-dependent transcription, although context determines whether it is activational or repressive. For example, co-transfection with a SUMO-expressing vector enhances the transcriptional activity of Sox9-dependent Col2a1 reporter.15 On the other hand, covalent attachment of SUMO-1 to Sox9 by gene fusion dramatically compromises its transcription activity on the reporter gene.16 In some situations, SUMOlyation of Sox9 acts as a switch to drive tissue differentiation one way or another. In Xenopus, non-SUMOlyated SoxE proteins promote NC development, whereas SUMOylated SoxE proteins promote inner ear development.13
MicroRNAs, small noncoding RNAs that control gene expression, inhibit Sox9 expression in lung development, during chondrogenesis and neurogenesis, and in developing mouse ovarian cells.17, 18, 19, 20 The ubiquitin-proteasome pathway represses Sox9 transcriptional activity by degrading Sox9 in hypertrophic chondrocytes.21 The regions of Sox9 subject to these posttranslational modifications are shown in Fig. 1.
The Sox9-partner complexes
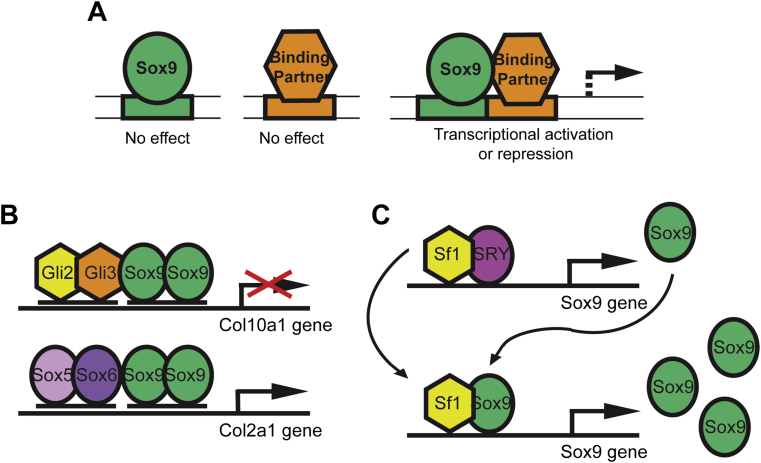
Sox proteins generally exhibit their gene regulatory functions by forming complexes with partner transcriptions factors, which can be transcription factors from another protein family, homologous Sox protein or heterologous Sox protein (Fig. 2A). Binding of either a single Sox protein or the partner protein alone to a DNA site does not elicit transcriptional activity.22 Target genes often have binding sites for a partner protein adjacent to a functional Sox-binding site, as in the case with the homodimer-binding sequences on the enhancer regions of chondrogenic genes.22, 23 It is inferred that a Sox-partner complex forms first, and then recognizes target DNA sites as a complex.23
Figure 2.
Regulation by Sox9-partner complexes. A) Sox9 requires a binding partner to elicit either transcriptional activation or repression.22, 23 B) Sox9 can function to activate or repress transcription, depending on the partner factor and on the tissue in which it is expressed. During earlier chondrogenesis, Sox9-Gli2/3 complex represses Col10a1 while the “sox trio,” Sox9-Sox5/6 complex, activates Col2a1.24, 25 C) Sox-partner complexes form a feedforward, self-reinforcing pathway. During male gonad genesis, Sf1 and SRY cooperatively upregulate Sox9 and then, together with Sf1, Sox9 maintains its own expression.26.
Whether Sox9 elicits transcriptional activation or repression depends on the target site, partner factors, and the subsequent recruitment of either co-activators or repressors (Fig. 2B). During hypertrophic chondrocyte maturation, Sox9 recruits Gli protein as the partner factor, and the complex represses the gene transcription of Col10a1, the gene that is required for chondrocyte maturation.24 On the other hand, a Sox9 dimer recruits SoxD (Sox5/6) dimers to activate Col2a1, which is required for chondrogenic differentiation and extracellular matrix (ECM) deposition.25
One of the advantages of Sox-partner interactions is that they allow for stepwise progression of developmental processes (Fig. 2C). For instance, Sox-partner complexes can activate a second Sox gene that acts downstream, employing the same partner factor. In male gonad, Sry and steroidogenic factor-1 (Sf1, also known as AD4BP) form a complex to induce Sox9 expression, and this newly transcribed Sox9 partners with Sf1 to promote subsequent development processes. This self-pertetuating pathway helps maintain continued Sox9 expression, even after that of SRY has ceased.26 Knowing how such binding partners work is important for the ensuing discussion on diverse functions of Sox9 in different organs and tissue.
The roles of Sox9 in mesoderm development
Sox9 in chondrogenesis and skeletal development
During chondrogenesis and endochondral ossification, mesenchymal cells condense and differentiate into chondrocytes in a pattern that will define the eventual shape of the skeletal elements.27, 28 In this process, Sox9 is essential for mesenchymal condensation prior to chondrogenesis, and for inhibiting hypertrophy. Inactivation of Sox9 in chondrocytes at different stages of differentiation suggests that its expression is essential for the survival of chondrocytes to progress to hypertrophy.25 Upon hypertrophy, the chondrocytes down-regulate Sox9 expression to allow for vascular invasion and bone marrow formation.29
Sox9 activates many genes in proliferating chondrocytes, including the ECM genes Col2a1, Col9a1, Col11a2 and Acan (aggrecan).30 Sox9 directly trans-activates Col2a1, the collagen II gene that is expressed most strongly in proliferating chondrocytes, in vivo via a conserved enhancer sequence within the first intron.31 In addition to trans-activating genes expressed in non-hypertrophic chondrocytes, Sox9 directly represses expression of Col10a1 just prior to the onset of hypertrophy.24 Given the importance of Sox9 in chondrogenesis, it was reported that Sox9 may be explored as an important biofactor to treat or prevent intervertebral disc degeneration.32 The versatile functions of Sox9 in developmental and homeostatic processes are shown in Fig. 3, and the related signaling pathways are summarized in Table 1.
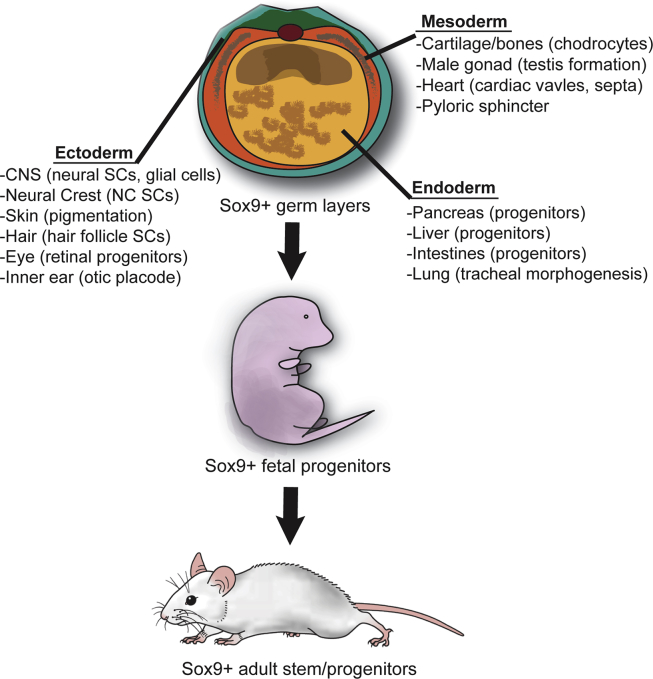
Figure 3.
Sox9 expression in pluripotent, fetal, and adult stem and progenitor cells. Sox9 is expressed throughout development, initially in pluripotent founder cells and subsequently in ectodermal, endodermal, and mesodermal derivatives. Sox9 expression is maintained in fetal and adult tissues derived from Sox9+ fetal progenitor cells and also in differentiated cells in some cases.26, 27, 28, 29, 30, 31, 32, 39, 40, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101.
Table 1.
Signaling pathways that regulate Sox9 during development and in human diseases.
| Key factors | Mesoderm | Ectoderm | Endoderm |
|---|---|---|---|
| Hh | Sonic hedgehog (Shh) upregulates Sox9 to generate chondrogenic precursors33; Indian hedgehog (Ihh) upregulates Sox9 for proliferation and maturation of chondrocytes34 | N/A | Upregulates Sox9 to modulate OPN in liver fibrosis35 |
| Wnt/β-catenin | Wnt5 upregulates Sox9 during early stages of chondrogenesis and inhibits it during chondrocyte maturation36, 37; Sox9 interacts with β-catenin to inhibit its transcription38 | Phosphorylates Sox9 for NC cell delamination along with BMP12 | Upregulates Sox9 for intestinal SC proliferation and Paneth cell differentiation41; upregulates Sox9 to inhibit villus maturation96 |
| Notch | Inhibits Sox9 expression in vivo and in vitro102; upregulates Hes1 and Hey1, which compete for Sox9 binding of the Col2a1 enhance to prevent Sox9-mediated activation103 | Induces Sox9 expression for stem cell maintenance and strogliogenesis55; regulates Sox9 and Hes1 in the developing and mature retina67 | Regulates Sox9 in the liver development81; regulates Sox9 in dose-dependent manner to induce Ngn3 for pancreatic endocrine and ductal cell differentiation75 |
| TGF-β | Upregulates Sox9 and Smad3,36 and activates Sox9 in vitro to mediate chondrogenic commitment104 | N/A | Induces Sox9 expression to inhibit hepatogenic differentiation potential of ADHLSCs105 |
| NFκB | Reduces Sox9 activity and cartilage gene expression by converging with RAR pathway106; RelA activates Sox9 for chondrogenic differentiation107 | N/A | Epigenetically regulates Sox9 in pancreatic cancer stem cells108 |
| BMP | BMP2 induces chromatin remodeling, and modifies the Sox9 promoter109; BMP4 upregulates Sox9 in semilunar valve cells110; upregulates Sox9 and Nkx2.5 to determine the pyloric sphincter epithelium54 | Phosphorylates Sox9 with Wnt for NC cell delamination along with Wnt12 | Induces Sox9 expression in endoderm and pancreatic lineage differentiation along with Activin and FGF pathway111 |
| Fgf | Fgf9 upregulates Sox9 to induce endochondral ossification112 | Activates Sox9-Sox10 pathway for branching morphogenesis of mouse ocular glands113; upregulates Sox9140 | Creates feed-forward loop to maintain pancreatic organ identity114 |
Sox9 in male gonad genesis
In mammals, Sry on the Y chromosome initiates the testis differentiation program, and Sox9 carries out the process by specifying the Sertoli cell lineage. The role of Sox9 in testis formation and subsequent sex determination was first recognized by genetic analysis of human campomelic dysplasia, in which about 75% of XY males with one mutant Sox gene exhibit male-to-female sex reversal.42 Similarly, duplicate Sox9 genes have been linked with male gonad genesis even in karyotypically XX subjects.43 In the male gonad, the combination of Sry and Sf1 initiates Sox9 expression, which is continued even after Sry expression disappears in positive auto-regulatory feedback loops.26 In the female gonad, on the other hand, Sox9 expression disappears due to the lack of Sry expression.42 Sox9-axis signaling induces ovary–testis transition in zebrafish, suggesting that its role in sex reversal is conserved.44
To complete gonad genesis, Sox9 recruits different binding partners to elicit two separate trans-activating functions.45, 46 In the former, Sox9 homodimerizes to activate prostaglandin D synthase (Ptdgs), the gene that encodes an enzyme responsible for producing prostaglandin D2 (Pgd2). Pgd2 then recruits cells of the supporting lineage to become Sertoli cells.45 In the latter, the Sox9-Sf1 complex upregulates anti-Mullerian hormone (AMH) in a cyclic AMP-dependent manner, which inhibits the development of the female Mullerian ducts.46 Sox8 is also important for testis cord differentiation. In mice, an experiment using Sox9 conditional knockout on a Sox8 mutant background showed that Sox8 expression follows that of Sox9, being required for the maintenance of testicular function at a later stage.47 However, the regulation of AMH by SoxE proteins is not conserved in mice and chickens. In the developing chicken, AMH is expressed one day before Sox9, suggesting that another AMH activating factor exists, and Sox8 is expressed at similar levels in both sexes during the sex-determining period.48, 49
Sox9 in other mesoderm tissues: cardiac valves/septa, and pyloric sphincter
In the heart, Sox9 is highly expressed in cardiac cushion cells, and is required for the normal development of valves and septa.50 Furthermore, Sox9 is required for precursor cell expansion and ECM organization during mouse heart development.51 In these instances, Sox9 seems to promote epithelial-mesenchymal transition (EMT) after delamination and initial migration of endocardial endothelial cells.50 Given the significance of EMT in fibrosis and cancer prognosis, there is much consideration about the relevance of Sox9 in these diseases.52
In the pyloric sphincter, a structure that demarcates the stomach from the duodenum, Sox9 is important in specifying its epithelium. Misexpression of Sox9 in the mesoderm of the stomach inhibits the differentiation of the gastric epithelium into pyloric sphincter-like epithelium.53 Similarly, another finding showed that Sox9 is regulated by BMP signaling in the pyloric sphincter, a pathway involved in epithelial-mesenchymal interactions for organ-specific morphogenesis of the gut tube.54
The roles of Sox9 in ectoderm development
Sox9 in neural stem cells (NSCs), gliogenesis, and neural crest (NC) stem cells
Sox9 regulates wide-ranging aspects of development in the central nervous system (CNS) and in neural crest (NC). Gain- and loss-of-function studies indicated that, during the CNS development, Sox9 is necessary and sufficient to initiate the induction of embryonic and adult neural stem cells.55, 56 Moreover, Sonic Hedgehog (SHH) induces Sox9 expression, which in return stimulates precocious generation of NSCs.56
In the CNS, Sox9 drives the differentiation program away from neurogenesis and towards gliogenesis.8, 55, 57, 58 Sox9 expression in NSCs continues in glial cells, but not in neurons. A study using Cre/loxP recombination system that ablates Sox9 expression showed that, in the developing spinal cord, Sox9 elicits the specification of myelin-forming oligodendrocytes and astrocytes, the two main types of glial cells in the CNS.59 For glial initiation, Sox9 recruits the transcription factor NFIA as a binding partner to co-regulate migratory and metabolic genes in astrogliogenesis, such as Apcdd1 and Mmd2.57 Importantly, Sox9 and Sox10 play redundant functions in survival and migration of oligodendrocyte precursors.8 Notch1 seems to be a part of the upstream pathway in astrogliaogenesis and stem cell maintenance, as demonstrated in the studies involving transient activation and knockdown of Notch1 during neuroectodermal differentiation.55
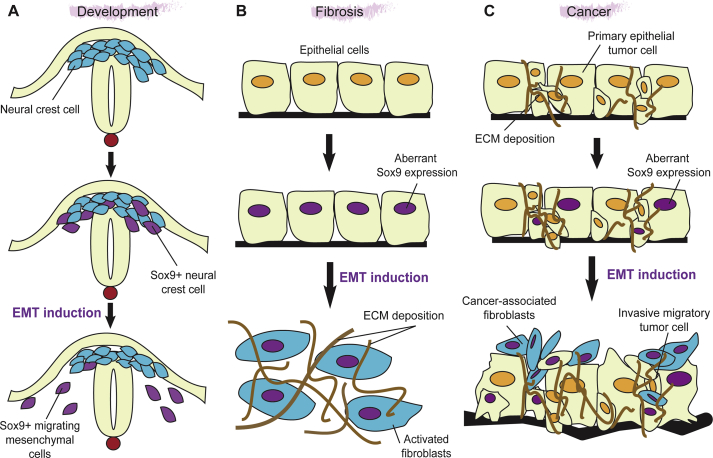
Neural crest is a population of multipotent stem cells derived from dorsal neural folds at the border between neural and non-neural ectoderm in the vertebrate embryo. Once induced, neural crest cells undergo epithelial-mesenchymal transition (EMT), delaminate from the neural tube, and migrate into the periphery to give rise to multiple differentiated cell types.60 Sox9 plays a crucial role in NC development, and is required for NC progenitor specification. Forced expression of Sox9 promotes neural crest-like properties in neural tube progenitors at the expense of CNS neuronal differentiation, and in migratory NC cells, SoxE expression guides NC stem cells towards a glial cell fate.61 As in the heart, Sox9 is important for EMT (Fig. 4A). In avian neural tube, Sox9 is essential for BMP signal-mediated induction of Snail2 and subsequent EMT, and cotransfection of Sox9 and Snail2 is sufficient to induce ectopic EMT.12, 39 In Xenopus, however, Sox9 is required only for neural crest specification but not migration,40 implying that the fates of NC progenitors are not conserved between species.
Figure 4.
EMT induction by Sox9 in acquired diseases. A) Sox9 is involved in epithelial-mesenchymal transition (EMT) for neural crest delamination during development.12, 39, 40 B) Sox9 plays a role in excess extracellular matrix (ECM) deposition and EMT,25 which may be related to fibrosis. C) Sox9 is important for ECM deposition and EMT,12, 25, 39, 40 which implies its role in tumor formation and invasive metastasis.
Sox9 in hair follicle stem cells (HF-SCs)
The function of Sox9 in HF-SCs was first noted in the HF bulge, an adult-specific stem cell niche that provides an appropriate microenvironment to preserve the proliferative potential of hair follicles.62 Although the study by Vidal et al in 2005, which will be discussed in the later section, established the importance of HF-SCs in adult life, whether SCs exist or function earlier during development was largely unknown.63 However, the findings by Nowak et al in 2008 demonstrated that HF-SCs are formed at earlier stages and that the niche formation dependent on Sox9. In this newer study, embryonic ablation of Sox9 using Sox9-Cre genetic marking and K14-Cre led to a reduced number of Sox9-expressing cells in all skin epithelial lineages. In the absence of early SCs, hair follicle and sebaceous gland morphogenesis is blocked and epidermal wound repair is compromised.64
Sox9 in other ectodermal tissues: retinal progenitor cells (RPCs) and otic placode
In the retina, Sox9 maintains a multipotent pool of retinal progenitor cells (RPCs), playing a role similar to that in the CNS. In multipotent murine RPCs, Sox9 is expressed throughout retinogenesis, and is continuously expressed in Muller glial cells into adulthood.65 Sox9 is induced by Notch signaling during retinal development, and once expressed, it recruits binding partners such as microphtahlmia-associated transcription factor (MITF) and OTX2 to maintain retinal pigment epithelium (RPE).66, 67 During this process, another SoxE protein, Sox8, and a SoxB protein, Sox2, play compensatory roles.68
In inner ear, Sox9 plays essential functions, although its roles vary among species. In Xenopus and zebrafish, Sox9 is required for initial specification of the otic placode. A morpholino antisense oligonucleotide-mediated depletion of Sox9 in Xenopus results in loss of early otic markers and failure of otic vesicle development, and overexpression of Sox9 leads to enlarged or ectopic otic vesicles.13, 69 Similarly in zebrafish, loss of Sox9a and Sox9b results in absence or severe reduction of the otic vesicle.12 On the other hand, in mice, Sox9 is not required for initial specification of the otic placode but instead controls adhesive properties and invagination of placodal cells.70 Interestingly, covalent attachment of SUMO to Sox9 by gene fusion inhibits expression of neural crest markers but increases expression of markers of inner ear development, suggesting that the posttranslational SUMOlyation may act as a switch.13
The roles of Sox9 in endoderm development
Sox9 in pancreas, liver, and intestine
During embryonic development in mammals, the upper digestive tract organs—the liver, pancreas, and duodenum—are derived from the primitive foregut endoderm, and share Sox9 expression in their progenitor populations. The pancreas has two different secretory structures—endocrine and exocrine—that originate from different sources of progenitors.71 Sox9 is necessary for normal pancreas development, as pancreas-specific Sox9 depletion result in severe pancreas hypoplasia.72 In line with abundant cases of pancreatic endocrine impairments in patients with campomelic dysplasia (CD), the endocrine lineage seems to be more sensitive to Sox9 than the exocrine lineage.73 Experimentally reducing Sox9 gene expression to 50% in mouse pancreatic progenitors led to a reduction in endocrine progenitors expressing neurogenin 3 (Ngn3), a gene necessary and sufficient for establishing an endocrine cell fate.74 During this process, Notch/Ngn3/Hes1 signaling regulates Sox9 in a dose-dependent manner. Too little or too much Notch results in tight regulation of Sox9: activated Ngn3 downregulates Sox9 expression in the endocrine cell compartment, and with high Notch activity, the transcription factor hairy and enhancer of split-2 (Hes1) represses Ngn3.75 In exocrine pancreatic development, a similar mechanism seems to be at work, with pancreas-specific transcription factor 1a (Ptf1a) substituting for Ngn3.76
Bile ducts are structures within the liver that produce and secrete bile, and are divided into intrahepatic (within the liver) or extrahepatic (outside the liver).71 During liver development, Sox9 is expressed not in hepatocytes, the cells that secrete bile, but instead in cholangiocytes and mucin-producing cells that line the extrahepatic bile duct.77 Notably, studies using lineage tracing with Sox9-IRES-Cre knock-in mice and BAC Sox9-CreER transgenic mice demonstrated that embryonic Sox9+ cholangiocytes could differentiate into hepatocytes.78, 79 Evidence suggests that Sox9 determines the timing of bile duct morphogenesis: after a maturation step, the biliary tube is entirely composed of Sox9+ cholangiocytes, and embryonic liver-specific inactivation of Sox9 results in delayed duct maturation.80 In addition, Notch seems to regulate Sox9 in this process, as seen in the etiology of Alagille syndrome, a genetic disorder in the liver, heart, kidney, and other systems of the body caused by mutations in Notch pathway.81
In normal intestinal epithelium, Sox9 is localized to the nuclei of crypt cells, including terminally differentiated Paneth cells, stem cells, and a subset of transit-amplifying (TA) cells.82 Functionally, Sox9 suppresses proliferation in mouse intestinal epithelium in vivo, and inactivation of Sox9 results in increased proliferation.82, 83 A recent report demonstrated that Sox9 regulates insulin-like growth factor (IGF)-binding protein 4 (IGFBP-4), an inhibitor of the IGF/IGF-receptor pathway in cell proliferation.84
Sox9 in lung
The discovery of Sox9 expression in bronchial epithelium, and neonatal deaths of CD patients due to respiratory distress, first hinted at the significance of Sox9 in lung development.85 However, there are conflicting results regarding the role of Sox9 in the lung epithelium. Specific inactivation of Sox9 in respiratory epithelial cells of the mouse lung using a doxycycline-inducible Cre/loxP system leads to normal lung structure, postnatal survival, and repair following oxygen injury.86 However, other studies suggest that Sox9 is required for proper lung morphogenesis; loss of Sox9 leads to extracellular matrix defects, cytoskeletal disorganization and aberrant epithelial movement.87, 88 Another finding suggests that the role of Sox9 is crucial in tracheal development, as transgenic mice lacking Sox9 expression have morphological defects in the trachea, are unable to breathe, and die at birth.89 These contrasting reports on Sox9 regulation of lung epithelial lung branching may be due to the different genetic backgrounds of the mice.
The roles of Sox9 in adult tissues
To maintain homeostasis of an adult organ, either in the physiological state or a regenerative state after injury, an orchestrated mechanism ensures correct cell type and tissue architecture. Sox9 expression during development continues in adult stem and progenitor cells, and seems to be crucial in adult tissues. Here, we review recent data linking Sox9 with adult stem/progenitor cell maintenance and specification.
Sox9 in ectoderm-derived tissues: NPCs, retina, HF-SCs, and skin pigmentation
In the CNS, Sox9 continues to play a necessary role in the maintenance of multipotent NPCs throughout adult life, as shown by in vivo fate mapping experiments in the adult subependymal zone and olfactory bulbs.56 In the retina, Sox9 is crucial for retinogenesis, but continues to maintain these differentiated Muller glial cells postnatally as a result of Notch regulation, shown in a conditional knockout approach.65, 67 In addition, Sox9 expression in Muller glial cells persists in the adult tissues.65
In mature retinal pigment epithelium (RPE) cells, Sox9 acts synergistically with transcription factors orthodenticle homeobox 2 (OTX2) and the LIM homeobox family (LHX) to activate visual cycle genes by common miRNAs.90
Epithelial hair follicle stem cells (HF-SCs) reside in the “bulge” of the outer root sheath (ORS), and are essential for cyclic bouts of adult hair growth.91 Sox9 is crucial in maintenance and differentiation of adult skin by HF-SCs; postnatal conditional ablation of Sox9 results in mice born with fragile, atrophic hair shafts, suggesting that Sox9 is expressed by adult HF-SCs in the bulge and also may be required for their survival.63 Moreover, a recent study with conditional Sox9 targeting in adult HF-SCs demonstrated that Sox9 elicits an inhibitory function on epidermal differentiation in the SC bulge. While Sox9-defiecent HF-SCs transition from quiescence to proliferation and launch the subsequent hair cycle, they differentiate into epidermal cells rather than remaining as HF-SCs.92
Although many findings position Sox9 in stem cell homeostasis and regeneration during adult life, some evidence reveals its role in cell fate specification. One such case is in skin pigmentation. In Xenopus, along with Sox10, Sox9 induces NC stem cells to differentiate into melanocytes.93 Another finding showed that Sox9 is upregulated by ultraviolet B exposure in adult and neonatal melanocytes. This regulation results in activation of microphthalmia-associated transcription factor, dopachrome tautomerase, and tyrosinase promoters, all of which contribute to increases in key melanogenic proteins and subsequent pigmentation.94
Sox9 in endoderm-derived tissues: intestines, liver and pancreas
Developmentally derived Sox9+ progenitors in upper digestive tract organs from primitive foregut—the liver, pancreas, and duodenum—carry over as imprints into adult life.71 However, the extent of Sox9's effects within each organ varies considerably. While it is well established that Sox9+ progenitor zone serves as a continuous source of new tissues intestines, whether it has any physiological function in the adult liver and pancreas is still debated.
In intestinal epithelium, the most rapidly self-renewing tissue in adult mammals, a continuous supply of new cells and elimination of old cells preserves homeostasis at the top of the intestinal villi.95 In colon epithelium-derived cells, Sox9 transcriptionally represses the CDX2 and MUC2 genes, normally expressed in the mature villus cells, and may therefore contribute to the Wnt-dependent maintenance of a progenitor cell phenotype.96 In addition, Sox9 is required for differentiation of Paneth cells, which reside adjacent to the crypt's niche as post-mitotic, differentiated cells, and are important for maintaining epithelial cell renewal.83 Taken together, Sox9 maintains the homeostasis of the intestinal epithelium both directly and indirectly.
In adult liver, tamoxifen-related toxicity in lineage tracing studies has complicated the interpretation of Sox9 expression hepatocytes.79, 97 More recently, tamoxifen-independent tracing experiments argued against physiologically functioning progenitors in ducts. They revealed that hepatocytes labeled with this virus-mediated induction method were maintained solely through proliferation, and that Sox9+ duct cells do not participate in maintaining adult organ homeostasis.98 Interestingly, Sox9+ cells in the liver can be reprogrammed into insulin-secreting duct cells, implying that developmentally related cells can be modified to be used in a potential therapy for diabetes.99
In the adult pancreas, although Sox9 expression persists throughout the pancreatic ductal tree,79 it is not clear whether these Sox9+ cells are physiologically active. Lineage-tracing experiments using BAC Sox9-CreER transgenic mice show that Sox9+ duct cells lose their differentiation ability within a few days after birth,100 suggesting that adult Sox9+ duct cells do not function as stem/progenitor cells. A similar result was obtained from another lineage-tracing experiment, in which targeted adult ductal β cells failed to differentiate into functioning acinar/endocrine cells.101 Moreover, pulse and chase experiments support the notion that adult pancreatic β cells and acinar cells are maintained by the self-duplication of preexisting cells rather than by differentiation from progenitors.115, 116 Taken together, most of these results refute the existence of stem/precursor cells in the adult pancreatic duct.
Sox9 in developmental disorders
Campomelic dysplasia (CD)
Campomelic dysplasia (CD) refers to a rare autosomal dominant skeletal dysmorphology syndrome characterized by congenital bowing of the limb long bones, a small, bell-shaped thoracic cage, and hypoplastic scapulae.85 Other features not related to chondrogenesis include respiratory deficiencies with softening of the laryngo-tracheal cartilages, male-to-female sex reversal in XY patients, and a variety of congenital heart defects.117 CD is caused by haploinsufficiency of Sox9 due to deletions or mutations in or around the Sox9 gene.6, 42 Furthermore, disrupting the homodimerizing capacity of Sox9 has been linked to CD but not male-to-female sex reversal, indicating that homodimerization of Sox9 is required for proper cartilage formation but not for gonad formation.23
XY gonad dysgenesis
The role of Sox9 in gonad dysgenesis was first speculated due to a high proportion of male-to-female sex reversals in XY males with CD, as mentioned above.85 This is logical considering that Sox9 is downstream of Sry, the gene that encodes a crucial factor in triggering Sertoli cell development. Ectopic expression of Sox9 in the female gonad of XX mice causes complete female-to-male sex reversal, demonstrating that Sox9 is sufficient to trigger testis differentiation in the absence of Sry.118
Hypertrichosis and alopecia areata
Sox9 has been implicated in hereditary disorders of hair growth. Hypertrichosis is a rare syndrome defined as excessive hair growth in a particular body area that is not hormone dependent.119 Evidence suggests that Trps1, a gene associated with hypertrichosis in mice and humans, directly represses Sox9, and the absence of this gene activity results in premature proliferation of HF-SCs.120 In a family with a history of hypertrichosis, a copy number variation upstream of Sox9 showed decreased expression of HF genes.120 On the other end of the spectrum is alopecia areata, a condition that causes characteristic patches of hair loss.121 In mice, skin-specific knockout of Sox9 leads to the loss of hair shaft stem cells and causes similar bald patches.
Sox9 in acquired diseases
Sox9 in fibrosis, sclerosis and related disorders
One of the common characteristics among fibrosis, sclerosis, and related disorders is excessive, inappropriate extracellular matrix (ECM) deposition, and subsequent destruction of tissue architecture and function in response to injury.122 Considering its role in ECM deposition, evidenced in chondrogenesis, it seems logical that Sox9 has been implicated in the pathology of fibrotic diseases (Fig. 4B).
When damage occurs in the liver, a Sox9-dependent process causes hepatic stellate cells (HSCs) to proliferate into myofibroblasts, migrate to the surrounding parenchymal cells, and secrete ECM components for repair. In human fetal hepatocytes, aberrant induction of Sox9 causes ectopic expression of genes that encode the ECM components, Col2a1 and Comp1, which are normally expressed during chondrogenesis. Inducing transforming growth factor-β (TGF-β) signaling in activated HSCs leads to Sox9 expression, and causes type1 collagen production.123 Moreover, in vivo experiments using culture-activated HSCs posited Sox9 as a critical regulator of Osteopontin (OPN), an ECM component that is a biomarker for the severity of liver fibrosis. However, the same study also suggested that it is Hedgehog signaling, not TGF-β, that lies upstream of Sox9.35
In the kidney, high Sox9 expression is correlated with glomeruloscelerosis. A microarray gene expression profiling diseased glomeruli showed strongly upregulated expression of Sox9.124 In addition, highly elevated expressions of OPN and other TGF-β pathway-related genes were observed, suggesting that Sox9 activity is similar in both glomeruloscelerosis and liver fibrosis.124 In keeping with this finding, Sox9 appears to function downstream of TGF-β1 to activate Col4a2 transcription in mesangial cells, the specialized cells that surround blood vessels in the kidney.125
Sox9 in tumorigenesis and cancer
Dysregulation of tissue differentiation pathways and stem cell homeostasis can contribute to the development and progression of cancer. Sox9 has been implicated in the formation and growth of tumors in prostate, the CNS, skin, pancreas, ovary, and esophagus.126, 127, 128, 129, 130, 131, 132, 133, 134, 135 It seems logical that the role of Sox9 in controlling progenitor cells, to either proliferate or differentiate during development and adult life, could actually promote neoplasia if dysregulated (Fig. 4C).
Studies in human and mice place Sox9 as a key player in prognosis of prostate cancers. In phosphatase and tensin homolog (PTEN)+/− mice, overexpression of Sox9 in adult mouse prostate epithelia induces an early high-grade prostate intraepithelial neoplasia (PIN) lesion, indicating that Sox9 augments the loss of PTEN to promote disease.136 Furthermore, Sox9 levels are found to be increased in advanced lesions of human prostate cancer, and overexpression of Sox9 in LNCaP prostate cancer xenografts enhances growth, angiogenesis, and tumor invasion.134, 137 One possible mechanism by which Sox9 functions here is by trans-activating the androgen receptor, as some prostate cancers are androgen-dependent.135 However, one study showed that Sox9 suppresses growth and tumorigenesis in the prostate tumor cell line M12.127
Sox9 is also implicated in nervous system tumors. In glioma cell lines, siRNA knockdown of Sox9 reduced cell proliferation in vitro.132 In vivo, Sox9 production is increased in malignant nerve sheath tumors, and repressing this expression by small hairpin RNA causes cell death in culture.129
In skin, Sox9 is expressed in basal cell carcinomas, and is detected in over 80% of melanomas.130, 133 Sox9 is thought to lie downstream of Sonic hedgehog (Shh) and Gli2 transcription factor both of which have been implicated in skin tumors.63, 138, 139 However, another study demonstrated an inhibitory function of Sox9 in melanomas, as vector-derived Sox9 in both melanoma cell lines and xenografts decreased cell proliferation and tumor growth by direct upregulation of the cell cycle arrest gene, p21.130
In the pancreatic ductal system, clinical adenoma and carcinoma samples showed Sox9 overexpression localized to the bottom part of the crypts, suggesting that dysregulation of stem cell homeostasis may be responsible.131 In addition, Sox9 accelerates the formation of precursor lesions of pancreatic ductal adenocarcinoma (PDA) when co-expressed with a PDA-initiating Kras mutation.140 In adenocarcinomas, a potential NF-κB binding site was found in the Sox9 promoter with NF-κB subunits up-regulating Sox9 expression, indicating that Sox9 is epigenetically regulated by NF-κB signaling pathway.108
Taken together, these data present opposing roles for Sox9 in tumors, either inducing or potentially inhibiting cell proliferation. It should be kept in mind that the difference between these studies could be attributed, in part, to the differences in cell lines and levels of Sox9. These factors should be controlled for in future experiments.
Concluding remarks and future directions
Most insight into the biological properties of Sox9 has come from developmental studies, particularly involving chondrogenesis and male gonad genesis. Recent molecular and functional analyses of Sox9 have documented an additional role in stem cell biology of mesoderm-, ectoderm-, and endoderm-derived tissues and organs. While Sox9 maintains adult stem and progenitor cells with high turnover, as in intestine and hair follicles, it is also crucial for postnatal injury repair in endodermic and ectodermic organs. Identifying partner factors, signaling pathways, and posttranscriptional modifications have provided a better understanding of Sox9's versatility in different tissues and at different stages in mammalian life. The availability of appropriate mouse models and the ability to maintain rare stem cell populations in culture, combined with genome-wide technologies, should now enable researchers to further address fundamental questions at the mechanistic level.
In human diseases, mutations in Sox9 can cause birth defects in skeletal deformity, male-to-female sex reversals, and hair growth, and has been implicated in fibrosis and cancer. In addition, recent findings regarding fibrosis and cancer correlate Sox9 with developmental roles in cell proliferation, extracellular matrix (ECM) deposition, and epithelial-to-mesehchymal (ETM) transition. However, conflicting results position Sox9 with opposing roles in tumorigenesis, as evidenced in melanoma studies. These discrepancies may be due to the differences in individual cancer cell lines or mouse models, with further investigation being warranted.
Immunostaining for Sox9 carries prognostic value in a wide range of tumors, including neurofibromatosis, meduloblastoma, pancreatic cancer, and protstate cancer, and can aid in diagnosis. Moreover, Sox9 can be a potential target for novel therapeutic intervention that might compensate for the current lack of effective anti-fibrotic therapies and cancer treatments. However, Sox9 expression in many tissue types complicates cell-specific effects, which is why investigating this protein's diverse mechanisms and pathways is so important. Another challenge of using Sox9 therapeutically is modulating transcription factor levels. One potential way of reducing Sox9 could be to use small peptides or neutralizing antibodies to modify its function and expression. The manipulation of Sox9 levels might also be possible indirectly by modulating key molecules involved in upstream signaling pathways, such as TGF-β1, Wnt, and Hh signaling, all three of which have been linked to cancer and fibrosis.
In summary, accumulating evidence implicates Sox9 in pluripotent and multipotent stem cell biology and tissue regeneration, in addition to its role in cell fate decisions. A better understanding of the mechanisms by which Sox9 induces and maintains these stem cell populations should provide important insights into how tissue stem cells are regenerated and maintained, and might lead to new strategies for treating degenerative diseases and cancer.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The investigators who reported the work were supported in part by the research grant from the National Institutes of Health (AR50142 to RCH). This work was also supported in part by The University of Chicago Core Facility Subsidy grant from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant UL1 TR000430. SD was a recipient of The University of Chicago Pritzker Fellowship and AOA Carolyn L. Kuckein Fellowship.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Simons B.D., Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res. Nov 15 2011;317:2719–2724. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Rezza A., Sennett R., Rendl M. Adult stem cell niches: cellular and molecular components. Curr Top Dev Biol. 2014;107:333–372. doi: 10.1016/B978-0-12-416022-4.00012-3. [DOI] [PubMed] [Google Scholar]
- 3.Gubbay J., Koopman P., Collignon J., Burgoyne P., Lovell-Badge R. Normal structure and expression of Zfy genes in XY female mice mutant in Tdy. Development. Jul 1990;109:647–653. doi: 10.1242/dev.109.3.647. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair A.H., Berta P., Palmer M.S. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. Jul 19 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 5.Phochanukul N., Russell S. No backbone but lots of Sox: Invertebrate Sox genes. Int J Biochem Cell Biol. Mar 2010;42:453–464. doi: 10.1016/j.biocel.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Wagner T., Wirth J., Meyer J. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. Dec 16 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 7.Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. Mar 15 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finzsch M., Stolt C.C., Lommes P., Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor alpha expression. Development. Feb 2008;135:637–646. doi: 10.1242/dev.010454. [DOI] [PubMed] [Google Scholar]
- 9.Kellerer S., Schreiner S., Stolt C.C., Scholz S., Bosl M.R., Wegner M. Replacement of the Sox10 transcription factor by Sox8 reveals incomplete functional equivalence. Development. Aug 2006;133:2875–2886. doi: 10.1242/dev.02477. [DOI] [PubMed] [Google Scholar]
- 10.Stolt C.C., Lommes P., Friedrich R.P., Wegner M. Transcription factors Sox8 and Sox10 perform non-equivalent roles during oligodendrocyte development despite functional redundancy. Development. May 2004;131:2349–2358. doi: 10.1242/dev.01114. [DOI] [PubMed] [Google Scholar]
- 11.Huang W., Zhou X., Lefebvre V., de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol Cell Biol. Jun 2000;20:4149–4158. doi: 10.1128/mcb.20.11.4149-4158.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J.A., Wu M.H., Yan C.H. Phosphorylation of Sox9 is required for neural crest delamination and is regulated downstream of BMP and canonical Wnt signaling. Proc Natl Acad Sci U S A. Feb 19 2013;110:2882–2887. doi: 10.1073/pnas.1211747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor K.M., Labonne C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev Cell. Nov 2005;9:593–603. doi: 10.1016/j.devcel.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Malki S., Boizet-Bonhoure B., Poulat F. Shuttling of SOX proteins. Int J Biochem Cell Biol. Mar 2010;42:411–416. doi: 10.1016/j.biocel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Hattori T., Eberspaecher H., Lu J. Interactions between PIAS proteins and SOX9 result in an increase in the cellular concentrations of SOX9. J Biol Chem. May 19 2006;281:14417–14428. doi: 10.1074/jbc.M511330200. [DOI] [PubMed] [Google Scholar]
- 16.Oh H.J., Kido T., Lau Y.F. PIAS1 interacts with and represses SOX9 transactivation activity. Mol Reprod Dev. Nov 2007;74:1446–1455. doi: 10.1002/mrd.20737. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L.C., Pastrana E., Tavazoie M., Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. Apr 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y., Thomson J.M., Wong H.Y., Hammond S.M., Hogan B.L. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. Oct 15 2007;310:442–453. doi: 10.1016/j.ydbio.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyaki S., Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. Sep 2012;8:543–552. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Real F.M., Sekido R., Lupianez D.G., Lovell-Badge R., Jimenez R., Burgos M. A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol Reprod. Oct 2013;89:78. doi: 10.1095/biolreprod.113.110957. [DOI] [PubMed] [Google Scholar]
- 21.Hattori T., Kishino T., Stephen S. E6-AP/UBE3A protein acts as a ubiquitin ligase toward SOX9 protein. J Biol Chem. Dec 6 2013;288:35138–35148. doi: 10.1074/jbc.M113.486795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kamachi Y., Uchikawa M., Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. Apr 2000;16:182–187. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- 23.Bernard P., Tang P., Liu S., Dewing P., Harley V.R., Vilain E. Dimerization of SOX9 is required for chondrogenesis, but not for sex determination. Hum Mol Genet. Jul 15 2003;12:1755–1765. doi: 10.1093/hmg/ddg182. [DOI] [PubMed] [Google Scholar]
- 24.Leung V.Y., Gao B., Leung K.K. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet. Nov 2011;7:e1002356. doi: 10.1371/journal.pgen.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda T., Kamekura S., Mabuchi A. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. Nov 2004;50:3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 26.Sekido R., Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. Jun 12 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- 27.Alman B.A. Skeletal dysplasias and the growth plate. Clin Genet. Jan 2008;73:24–30. doi: 10.1111/j.1399-0004.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson E.M., Matsiko A., Farrell E., Kelly D.J., O'Brien F.J. Recapitulating endochondral ossification: a promising route to in vivo bone regeneration. J Tissue Eng Regen Med. 2014 doi: 10.1002/term.1918. [DOI] [PubMed] [Google Scholar]
- 29.Hattori T., Muller C., Gebhard S. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. Mar 2010;137:901–911. doi: 10.1242/dev.045203. [DOI] [PubMed] [Google Scholar]
- 30.Bell D.M., Leung K.K., Wheatley S.C. SOX9 directly regulates the type-II collagen gene. Nat Genet. Jun 1997;16:174–178. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 31.Lefebvre V., Huang W., Harley V.R., Goodfellow P.N., de Crombrugghe B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol Cell Biol. Apr 1997;17:2336–2346. doi: 10.1128/mcb.17.4.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paul R., Haydon R.C., Cheng H. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. Apr 15 2003;28:755–763. [PMC free article] [PubMed] [Google Scholar]
- 33.Akiyama H., Stadler H.S., Martin J.F. Misexpression of Sox9 in mouse limb bud mesenchyme induces polydactyly and rescues hypodactyly mice. Matrix Biol. May 2007;26:224–233. doi: 10.1016/j.matbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 34.St-Jacques B., Hammerschmidt M., McMahon A.P. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. Aug 15 1999;13(16):2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pritchett J., Harvey E., Athwal V. Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology. Sep 2012;56:1108–1116. doi: 10.1002/hep.25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akiyama H. Control of chondrogenesis by the transcription factor Sox9. Mod Rheumatol. 2008;18:213–219. doi: 10.1007/s10165-008-0048-x. [DOI] [PubMed] [Google Scholar]
- 37.Topol L., Chen W., Song H., Day T.F., Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem. Jan 30 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akiyama H., Lyons J.P., Mori-Akiyama Y. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. May 1 2004;18:1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakai D., Suzuki T., Osumi N., Wakamatsu Y. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development. Apr 2006;133:1323–1333. doi: 10.1242/dev.02297. [DOI] [PubMed] [Google Scholar]
- 40.Lee Y.H., Aoki Y., Hong C.S., Saint-Germain N., Credidio C., Saint-Jeannet J.P. Early requirement of the transcriptional activator Sox9 for neural crest specification in Xenopus. Dev Biol. Nov 1 2004;275:93–103. doi: 10.1016/j.ydbio.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 41.van Es J.H., Jay P., Gregorieff A. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. Apr 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 42.Foster J.W., Dominguez-Steglich M.A., Guioli S. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. Dec 8 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 43.Huang B., Wang S., Ning Y., Lamb A.N., Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. Dec 3 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 44.Sun D., Zhang Y., Wang C., Hua X., Zhang X.A., Yan J. Sox9-related signaling controls zebrafish juvenile ovary-testis transformation. Cell Death Dis. 2013;4:e930. doi: 10.1038/cddis.2013.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelm D., Hiramatsu R., Mizusaki H. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J Biol Chem. Apr 6 2007;282:10553–10560. doi: 10.1074/jbc.M609578200. [DOI] [PubMed] [Google Scholar]
- 46.Lasala C., Schteingart H.F., Arouche N. SOX9 and SF1 are involved in cyclic AMP-mediated upregulation of anti-Mullerian gene expression in the testicular prepubertal Sertoli cell line SMAT1. Am J Physiol Endocrinol Metab. Sep 2011;301:E539–E547. doi: 10.1152/ajpendo.00187.2011. [DOI] [PubMed] [Google Scholar]
- 47.Barrionuevo F., Georg I., Scherthan H. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. Mar 15 2009;327:301–312. doi: 10.1016/j.ydbio.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 48.Oreal E., Pieau C., Mattei M.G. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev Dyn. Aug 1998;212:522–532. doi: 10.1002/(SICI)1097-0177(199808)212:4<522::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 49.Takada S., Mano H., Koopman P. Regulation of Amh during sex determination in chickens: Sox gene expression in male and female gonads. Cell Mol Life Sci. Sep 2005;62:2140–2146. doi: 10.1007/s00018-005-5270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiyama H., Chaboissier M.C., Behringer R.R. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci U S A. Apr 27 2004;101:6502–6507. doi: 10.1073/pnas.0401711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lincoln J., Kist R., Scherer G., Yutzey K.E. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. May 1 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pritchett J., Athwal V., Roberts N., Hanley N.A., Hanley K.P. Understanding the role of SOX9 in acquired diseases: lessons from development. Trends Mol Med. Mar 2011;17:166–174. doi: 10.1016/j.molmed.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Moniot B., Biau S., Faure S. SOX9 specifies the pyloric sphincter epithelium through mesenchymal-epithelial signals. Development. Aug 2004;131:3795–3804. doi: 10.1242/dev.01259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Theodosiou N.A., Tabin C.J. Sox9 and Nkx2.5 determine the pyloric sphincter epithelium under the control of BMP signaling. Dev Biol. Mar 15 2005;279:481–490. doi: 10.1016/j.ydbio.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 55.Martini S., Bernoth K., Main H. A critical role for Sox9 in notch-induced astrogliogenesis and stem cell maintenance. Stem Cells. Apr 2013;31:741–751. doi: 10.1002/stem.1320. [DOI] [PubMed] [Google Scholar]
- 56.Scott C.E., Wynn S.L., Sesay A. SOX9 induces and maintains neural stem cells. Nat Neurosci. Oct 2010;13:1181–1189. doi: 10.1038/nn.2646. [DOI] [PubMed] [Google Scholar]
- 57.Kang P., Lee H.K., Glasgow S.M. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. Apr 12 2012;74:79–94. doi: 10.1016/j.neuron.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stolt C.C., Wegner M. SoxE function in vertebrate nervous system development. Int J Biochem Cell Biol. Mar 2010;42:437–440. doi: 10.1016/j.biocel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Stolt C.C., Lommes P., Sock E., Chaboissier M.C., Schedl A., Wegner M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev. Jul 1 2003;17:1677–1689. doi: 10.1101/gad.259003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakai D., Wakamatsu Y. Regulatory mechanisms for neural crest formation. Cells Tissues Organs. 2005;179:24–35. doi: 10.1159/000084506. [DOI] [PubMed] [Google Scholar]
- 61.Cheung M., Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. Dec 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 62.Blanpain C., Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vidal V.P., Chaboissier M.C., Lutzkendorf S. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. Aug 9 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 64.Nowak J.A., Polak L., Pasolli H.A., Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. Jul 3 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poche R.A., Furuta Y., Chaboissier M.C., Schedl A., Behringer R.R. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in Muller glial cell development. J Comp Neurol. Sep 20 2008;510:237–250. doi: 10.1002/cne.21746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masuda T., Esumi N. SOX9, through interaction with microphthalmia-associated transcription factor (MITF) and OTX2, regulates BEST1 expression in the retinal pigment epithelium. J Biol Chem. Aug 27 2010;285:26933–26944. doi: 10.1074/jbc.M110.130294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu M.Y., Gasperowicz M., Chow R.L. The expression of NOTCH2, HES1 and SOX9 during mouse retinal development. Gene Expr Patterns. Mar–Apr 2013;13:78–83. doi: 10.1016/j.gep.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Muto A., Iida A., Satoh S., Watanabe S. The group E Sox genes Sox8 and Sox9 are regulated by Notch signaling and are required for Muller glial cell development in mouse retina. Exp Eye Res. Oct 2009;89:549–558. doi: 10.1016/j.exer.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Saint-Germain N., Lee Y.H., Zhang Y., Sargent T.D., Saint-Jeannet J.P. Specification of the otic placode depends on Sox9 function in Xenopus. Development. Apr 2004;131:1755–1763. doi: 10.1242/dev.01066. [DOI] [PubMed] [Google Scholar]
- 70.Barrionuevo F., Naumann A., Bagheri-Fam S. Sox9 is required for invagination of the otic placode in mice. Dev Biol. May 1 2008;317:213–224. doi: 10.1016/j.ydbio.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Belo J., Krishnamurthy M., Oakie A., Wang R. The role of SOX9 transcription factor in pancreatic and duodenal development. Stem Cells Dev. Nov 15 2013;22:2935–2943. doi: 10.1089/scd.2013.0106. [DOI] [PubMed] [Google Scholar]
- 72.Seymour P.A., Freude K.K., Tran M.N. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. Feb 6 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piper K., Ball S.G., Keeling J.W., Mansoor S., Wilson D.I., Hanley N.A. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. Aug 2002;116:223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 74.Seymour P.A., Freude K.K., Dubois C.L., Shih H.P., Patel N.A., Sander M. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. Nov 1 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shih H.P., Kopp J.L., Sandhu M. A Notch-dependent molecular circuitry initiates pancreatic endocrine and ductal cell differentiation. Development. Jul 2012;139:2488–2499. doi: 10.1242/dev.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawaguchi Y. Sox9 and programming of liver and pancreatic progenitors. J Clin Invest. May 1 2013;123:1881–1886. doi: 10.1172/JCI66022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carpino G., Cardinale V., Onori P. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. Feb 2012;220:186–199. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carpentier R., Suner R.E., van Hul N. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. Oct 2011;141(4) doi: 10.1053/j.gastro.2011.06.049. 1432–1438, 1438 e1431–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Furuyama K., Kawaguchi Y., Akiyama H. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. Jan 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 80.Antoniou A., Raynaud P., Cordi S. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. Jun 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kodama Y., Hijikata M., Kageyama R., Shimotohno K., Chiba T. The role of notch signaling in the development of intrahepatic bile ducts. Gastroenterology. Dec 2004;127:1775–1786. doi: 10.1053/j.gastro.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Mori-Akiyama Y., van den Born M., van Es J.H. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. Aug 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 83.Bastide P., Darido C., Pannequin J. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. Aug 13 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shi Z., Chiang C.I., Mistretta T.A., Major A., Mori-Akiyama Y. SOX9 directly regulates IGFBP-4 in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. Jul 1 2013;305:G74–G83. doi: 10.1152/ajpgi.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mansour S., Hall C.M., Pembrey M.E., Young I.D. A clinical and genetic study of campomelic dysplasia. J Med Genet. Jun 1995;32:415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perl A.K., Kist R., Shan Z., Scherer G., Whitsett J.A. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. Jan 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- 87.Chang D.R., Martinez Alanis D., Miller R.K. Lung epithelial branching program antagonizes alveolar differentiation. Proc Natl Acad Sci U S A. Nov 5 2013;110:18042–18051. doi: 10.1073/pnas.1311760110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rockich B.E., Hrycaj S.M., Shih H.P. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci U S A. Nov 19 2013;110:E4456–E4464. doi: 10.1073/pnas.1311847110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turcatel G., Rubin N., Menke D.B., Martin G., Shi W., Warburton D. Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol. 2013;11:117. doi: 10.1186/1741-7007-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Masuda T., Wahlin K., Wan J. Transcription factor SOX9 plays a key role in the regulation of visual cycle gene expression in the retinal pigment epithelium. J Biol Chem. May 2 2014;289:12908–12921. doi: 10.1074/jbc.M114.556738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cotsarelis G. Gene expression profiling gets to the root of human hair follicle stem cells. J Clin Invest. Jan 2006;116:19–22. doi: 10.1172/JCI27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kadaja M., Keyes B.E., Lin M. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. Feb 15 2014;28:328–341. doi: 10.1101/gad.233247.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aoki Y., Saint-Germain N., Gyda M. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev Biol. Jul 1 2003;259:19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 94.Passeron T., Valencia J.C., Bertolotto C. SOX9 is a key player in ultraviolet B-induced melanocyte differentiation and pigmentation. Proc Natl Acad Sci U S A. Aug 28 2007;104:13984–13989. doi: 10.1073/pnas.0705117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barker N., Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. Dec 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 96.Blache P., van de Wetering M., Duluc I. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. Jul 5 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dorrell C., Erker L., Schug J. Prospective isolation of a bipotential clonogenic liver progenitor cell in adult mice. Genes Dev. Jun 1 2011;25:1193–1203. doi: 10.1101/gad.2029411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Malato Y., Naqvi S., Schurmann N. Fate tracing of mature hepatocytes in mouse liver homeostasis and regeneration. J Clin Invest. Dec 2011;121:4850–4860. doi: 10.1172/JCI59261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banga A., Akinci E., Greder L.V., Dutton J.R., Slack J.M. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A. Sep 18 2012;109:15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kopp J.L., Dubois C.L., Hao E., Thorel F., Herrera P.L., Sander M. Progenitor cell domains in the developing and adult pancreas. Cell Cycle. Jun 15 2011;10:1921–1927. doi: 10.4161/cc.10.12.16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solar M., Cardalda C., Houbracken I. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. Dec 2009;17:849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 102.Mead T.J., Yutzey K.E. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc Natl Acad Sci U S A. Aug 25 2009;106:14420–14425. doi: 10.1073/pnas.0902306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grogan S.P., Olee T., Hiraoka K., Lotz M.K. Repression of chondrogenesis through binding of notch signaling proteins HES-1 and HEY-1 to N-box domains in the COL2A1 enhancer site. Arthritis Rheum. Sep 2008;58:2754–2763. doi: 10.1002/art.23730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deschaseaux F., Pontikoglou C., Sensebe L. Bone regeneration: the stem/progenitor cells point of view. J Cell Mol Med. Jan 2010;14:103–115. doi: 10.1111/j.1582-4934.2009.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paganelli M., Nyabi O., Sid B. Downregulation of Sox9 expression associates with hepatogenic differentiation of human liver mesenchymal stem/progenitor cells. Stem Cells Dev. Jun 15 2014;23:1377–1391. doi: 10.1089/scd.2013.0169. [DOI] [PubMed] [Google Scholar]
- 106.Rockel J.S., Kudirka J.C., Guzi A.J., Bernier S.M. Regulation of Sox9 activity by crosstalk with nuclear factor-kappaB and retinoic acid receptors. Arthritis Res Ther. 2008;10:R3. doi: 10.1186/ar2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ushita M., Saito T., Ikeda T. Transcriptional induction of SOX9 by NF-kappaB family member RelA in chondrogenic cells. Osteoarthritis Cartilage. Aug 2009;17:1065–1075. doi: 10.1016/j.joca.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 108.Sun L., Mathews L.A., Cabarcas S.M. Epigenetic regulation of SOX9 by the NF-kappaB signaling pathway in pancreatic cancer stem cells. Stem Cells. Aug 2013;31:1454–1466. doi: 10.1002/stem.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pan Q., Yu Y., Chen Q. Sox9, a key transcription factor of bone morphogenetic protein-2-induced chondrogenesis, is activated through BMP pathway and a CCAAT box in the proximal promoter. J Cell Physiol. Oct 2008;217:228–241. doi: 10.1002/jcp.21496. [DOI] [PubMed] [Google Scholar]
- 110.Zhao B., Etter L., Hinton R.B., Jr., Benson D.W. BMP and FGF regulatory pathways in semilunar valve precursor cells. Dev Dyn. Apr 2007;236:971–980. doi: 10.1002/dvdy.21097. [DOI] [PubMed] [Google Scholar]
- 111.Xu X., Browning V.L., Odorico J.S. Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev. Sep-Dec 2011;128:412–427. doi: 10.1016/j.mod.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Govindarajan V., Overbeek P.A. FGF9 can induce endochondral ossification in cranial mesenchyme. BMC Dev Biol. 2006;6:7. doi: 10.1186/1471-213X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Z., Huang J., Liu Y. FGF signaling activates a Sox9-Sox10 pathway for the formation and branching morphogenesis of mouse ocular glands. Development. Jul 2014;141:2691–2701. doi: 10.1242/dev.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seymour P.A., Shih H.P., Patel N.A. A Sox9/Fgf feed-forward loop maintains pancreatic organ identity. Development. Sep 2012;139:3363–3372. doi: 10.1242/dev.078733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Desai B.M., Oliver-Krasinski J., De Leon D.D. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. Apr 2007;117:971–977. doi: 10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dor Y., Brown J., Martinez O.I., Melton D.A. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. May 6 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 117.Houston C.S., Opitz J.M., Spranger J.W. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux, et al in 1971. Am J Med Genet. May 1983;15:3–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- 118.Vidal V.P., Chaboissier M.C., de Rooij D.G., Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. Jul 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- 119.Garcia-Cruz D., Figuera L.E., Cantu J.M. Inherited hypertrichoses. Clin Genet. May 2002;61:321–329. doi: 10.1034/j.1399-0004.2002.610501.x. [DOI] [PubMed] [Google Scholar]
- 120.Fantauzzo K.A., Kurban M., Levy B., Christiano A.M. Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis. PLoS Genet. 2012;8:e1003002. doi: 10.1371/journal.pgen.1003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Calvieri S., Rossi A. Alopecia in genetic diseases. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. Feb 2014;149:1–13. [PubMed] [Google Scholar]
- 122.Diehl A.M., Chute J. Underlying potential: cellular and molecular determinants of adult liver repair. J Clin Invest. May 1 2013;123:1858–1860. doi: 10.1172/JCI69966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hanley K.P., Oakley F., Sugden S., Wilson D.I., Mann D.A., Hanley N.A. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. J Biol Chem. May 16 2008;283:14063–14071. doi: 10.1074/jbc.M707390200. [DOI] [PubMed] [Google Scholar]
- 124.Bennett M.R., Czech K.A., Arend L.J., Witte D.P., Devarajan P., Potter S.S. Laser capture microdissection-microarray analysis of focal segmental glomerulosclerosis glomeruli. Nephron Exp Nephrol. 2007;107:e30–e40. doi: 10.1159/000106775. [DOI] [PubMed] [Google Scholar]
- 125.Sumi E., Iehara N., Akiyama H. SRY-related HMG box 9 regulates the expression of Col4a2 through transactivating its enhancer element in mesangial cells. Am J Pathol. Jun 2007;170:1854–1864. doi: 10.2353/ajpath.2007.060899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clemons N.J., Wang D.H., Croagh D. Sox9 drives columnar differentiation of esophageal squamous epithelium: a possible role in the pathogenesis of Barrett's esophagus. Am J Physiol Gastrointest Liver Physiol. Dec 15 2012;303:G1335–G1346. doi: 10.1152/ajpgi.00291.2012. [DOI] [PubMed] [Google Scholar]
- 127.Drivdahl R., Haugk K.H., Sprenger C.C., Nelson P.S., Tennant M.K., Plymate S.R. Suppression of growth and tumorigenicity in the prostate tumor cell line M12 by overexpression of the transcription factor SOX9. Oncogene. Jun 3 2004;23:4584–4593. doi: 10.1038/sj.onc.1207603. [DOI] [PubMed] [Google Scholar]
- 128.Kato N., Fukase M., Motoyama T. Expression of a transcription factor, SOX9, in Sertoli-stromal cell tumors of the ovary. Int J Gynecol Pathol. Apr 2004;23:180–181. doi: 10.1097/00004347-200404000-00014. [DOI] [PubMed] [Google Scholar]
- 129.Miller S.J., Jessen W.J., Mehta T. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med. Jul 2009;1:236–248. doi: 10.1002/emmm.200900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Passeron T., Valencia J.C., Namiki T. Upregulation of SOX9 inhibits the growth of human and mouse melanomas and restores their sensitivity to retinoic acid. J Clin Invest. Apr 2009;119:954–963. doi: 10.1172/JCI34015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sakamoto H., Mutoh H., Miura Y., Sashikawa M., Yamamoto H., Sugano K. SOX9 is highly expressed in nonampullary duodenal adenoma and adenocarcinoma in humans. Gut Liver. Sep 2013;7:513–518. doi: 10.5009/gnl.2013.7.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Swartling F.J., Ferletta M., Kastemar M., Weiss W.A., Westermark B. Cyclic GMP-dependent protein kinase II inhibits cell proliferation, Sox9 expression and Akt phosphorylation in human glioma cell lines. Oncogene. Sep 3 2009;28:3121–3131. doi: 10.1038/onc.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vidal V.P., Ortonne N., Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. Apr 2008;35:373–379. doi: 10.1111/j.1600-0560.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 134.Wang H., Leav I., Ibaragi S. SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. Mar 15 2008;68:1625–1630. doi: 10.1158/0008-5472.CAN-07-5915. [DOI] [PubMed] [Google Scholar]
- 135.Wang H., McKnight N.C., Zhang T., Lu M.L., Balk S.P., Yuan X. SOX9 is expressed in normal prostate basal cells and regulates androgen receptor expression in prostate cancer cells. Cancer Res. Jan 15 2007;67:528–536. doi: 10.1158/0008-5472.CAN-06-1672. [DOI] [PubMed] [Google Scholar]
- 136.Thomsen M.K., Ambroisine L., Wynn S. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Res. Feb 1 2010;70:979–987. doi: 10.1158/0008-5472.CAN-09-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schaeffer E.M., Marchionni L., Huang Z. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. Dec 4 2008;27:7180–7191. doi: 10.1038/onc.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alexaki V.I., Javelaud D., Van Kempen L.C. GLI2-mediated melanoma invasion and metastasis. J Natl Cancer Inst. Aug 4 2010;102:1148–1159. doi: 10.1093/jnci/djq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gomez-Ospina N., Chang A.L., Qu K., Oro A.E. Translocation affecting sonic hedgehog genes in basal-cell carcinoma. N. Engl J Med. Jun 7 2012;366:2233–2234. doi: 10.1056/NEJMc1115123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kopp J.L., von Figura G., Mayes E. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. Dec 11 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]