Abstract
Objective
Goals of the study are to estimate the pharmacokinetic(PK) parameters of standard dose betamethasone in a large obstetrical population and evaluate the effect of maternal body size and multiple gestation on the PK parameters and their observed variability.
Study Design
Prospective PK study. Liquid chromatography mass spectrometry was used to measure betamethasone plasma concentrations. PK parameters and significant clinical covariates were estimated using mixed effect modeling. Bootstrap analysis confirmed validity of the model.
Results
Two hundred and seventy four blood samples from 77 patients were obtained. Greatest effect on PK variability was observed with maternal lean body weight(LBW). The relationship between the PK parameters and LBW remained linear over a wide range of maternal body sizes. Multiple gestations did not affect the PK parameters.
Conclusion
Individualization of betamethasone dosing by maternal LBWreduces variability in drug exposure. Mutiple gestations do not require betamethasone dosing adjustment, because PK are the same as singleton gestations.
Keywords: Antenatal steroids, Betamethasone, prematurity, pharmacokinetics
Background
Pregnancy is characterized by many physiological changes that may alter the disposition of drugs and the gravida's response.1,2 To date pharmacokinetics during pregnancy are known only for a few drugs.
Antenatal steroid therapy has been demonstrated to improve neonatal outcomes in premature neonates.3 This treatment decreases the risk of respiratory distress syndrome (RDS), reduces cerebroventricular hemorrhage, and improves overall neonatal survival.4,5 In multiple gestations there may be a less beneficial effect of antenatal corticosteroidin reducing RDS and mortality.6 Ballabh et al reported a more rapid elimination half-life of betamethasone in twin compared to singleton pregnancies and believed a decrease in betamethasone exposure may explain the reduced effectiveness in twins7. However, the lack of difference in this medication's clearance and volume of distribution conflicts with this interpretation. Little data exists on whether betamethasone given to obese women has the same beneficial neonatal effects as in normal weight women. Recently a retrospective investigation of more than 1000 gravidas receiving antenatal steroid treatment and stratified by Body Mass Index (BMI) found no difference among groups in composite neonatal morbidity and mortality.8 Similar results were noted by another investigator.9 Aims of this study are to 1) examine the pharmacokinetics of standard dose intramuscular (IM) betamethasone in women between 24 and 34 weeks of gestation; 2) determine whether body size indicators influence the variability in betamethasone volume of distribution and clearance and 3) determine whether multiple gestations affect these pharmacokinetic parameters.
Methods
Patients
A prospective population pharmacokinetic study was conducted from March, 2008 to November, 2008 at the University of Illinois at Chicago (UIC). All pregnant women, between 24 and 34 weeks gestation, greater than 18 years, and clinically eligible for an inpatient antenatal corticosteroid treatment course were approached for enrollment. Participating subjects received the standard regimen of betamethasone [equal amounts of betamethasone sodium phosphate and betamethasone acetate at a dose of 12 mg every 24 hours for a total of two injections intra-muscularly (IM)]. A blood sample of 8 mL was collected from participants within each of 5 sampling windows: 5 to 20 minutes, 1 to 3 hours, 5 to 8 hours and 22 to 24 hours after the first IM dose and 2 to 6 hours after the second IM dose. Sampling windows were constructed from the D-optimal sample times determined using the ADAPT II software.10,11 Enrollment occurred 7 days a week, 24 hours daily. Participation in the study was limited to inpatients population to ensure compliance with blood draws. Less than 5 patients refused to participate in the study and the main reason for refusal was discomfort with the research-related venous punctures. The study was approved by the UIC Institutional Review Board and a written informed consent was obtained prior to any study related interventions.
Demographic and clinical characteristics were recorded for each participant along with betamethasone dosing and sampling times. Blood samples were collected in evacuated tubes, centrifuged and plasma separated. To minimize hydrolysis of the esters, the plasma was immediately transferred to tubes containing 100mM sodium arsenate and potassium fluoride and stored at -20°C.12,13
Betamethasone Assay
Betamethasone plasma concentrations were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay, adapted from previously published procedures.12,14 Quality control data for the assay is described in Appendix II.
Pharmacokinetic analysis
The pharmacokinetic methods are summarized below and described in greater detail in Appendix II. Population pharmacokinetic analysis of betamethasone plasma concentrations was performed by nonlinear mixed effect modeling using the first order conditional estimation method of the NONMEM software, version VI.15 Several alternative pharmacokinetic models were evaluated to describe the disposition of IM betamethasone. Model selection was guided by visual inspection of diagnostic plots, standard error of the parameter estimates and minimum value of the objective function (OFV).
Covariate analysis
Bayesian estimates of the pharmacokinetic parameters for individual patients were obtained from the pharmacokinetic model without covariates (base model), then graphical methods were used to screen for potential relationships between covariates and pharmacokinetic parameters, using the software S-Plus version 6.1, Insightful Corporation, Seattle. Variables evaluated were: total body weight (TBW), lean body weight (LBW)16, body surface area (BSA)17, body mass index (BMI)18, gestational age (GA) calculated by last menstrual period, if available and confirmed by first or second trimester ultrasound, race, age, twin or multiple gestation, concurrent liver or kidney disease and presence of pre-eclampsia. The LBW was calculated as described by Janmahasatian et al16:
Covariates identified above were first added alone to the base model; those producing a significant decrease in OFV (p<0.01) were entered into the model using stepwise forward inclusion backward elimination approach. Continuous covariates identified were normalized to an accepted population standard (70 Kg for TBW, 45 Kg for LBW and 1.73m2 for BSA) or study median (30 weeks gestational age). Linear and power functions for continuous covariates and an indicator function for categorical covariates were evaluated to relate the covariates with the PK parameters.
Validation of the model
The validity of the final population pharmacokinetic model was evaluated by bootstrap analysis. Re-sampling with replacement from the original dataset was used to construct 1000 bootstrap data sets. Each data set was fit to the final population model.
Power analysis
To determine the power of the design implemented in this study for identifying important differences in the PK parameters between women with singleton and multiples pregnancies, a simulation approach was used. These simulations utilized a modified form of the final population model, which involved adding a covariate in the expression for the betamethasone apparent clearance (CL/F). This covariate represented a proportional increase, from 0% to 40% in increments of 5%, in the apparent clearance in women with multiple pregnancies. For each incremental increase, 200 replicate datasets were simulated and fit to the modified model. The percentage of the 200 replications at each incremental increase showing a significantly higher apparent clearance in multiple pregnancies represented the power of the study to detect such difference.
Simulation of betamethasone plasma concentrations
The effect on betamethasone systemic exposure of different body size adjusted dosing schemes was examined by simulation. Using the final population model, betamethasone plasma concentrations from time zero to 24 hours post dose were simulated for 77 patients for each of the following doses: 12 mg (standard), 12 mg per 45 Kg LBW (LBW adjusted dose) and 12 mg per 70Kg TBW (TBW adjusted dose). Covariates values from the pharmacokinetic dataset were reproduced in the simulation dataset. At each dose, 1000 replicates of dataset were simulated. The area under betamethasone plasma concentrations time curve from time zero to infinity (AUC) was calculated at each simulated plasma concentration profile. The findings were examined graphically by constructing box-plots of the AUCs categorized by dosage regimen and BMI (<25, between 25 and 30, more than 30 and 40, more than 40 Kg/m2).
Results
Eighty four pregnant women were enrolled into the study. However, 7 patients had no evaluable betamethasone plasma concentrations and were not included in the population analysis. Isolated plasma samples from other patients were excluded from the dataset for the following reasons: 13 were outside the quantifiable limit of the assay, 3 samples had incomplete labeling, and 2 samples were frozen prior to separating the red cells and plasma. Thus, the final pharmacokinetic dataset consisted of 77 pregnant women contributing 274 plasma samples for analysis. Of the 274 plasma samples, 233 (3.5 samples per patient) were from mother carrying singleton and 51 (3.9 per patient) carrying multiple pregnancies. The demographic and clinical characteristics of subjects are summarized in Table 1. Sixty-four women were carrying singleton pregnancies, 12 twin gestation and 1 set of triplets. Gestational age ranged from 21 and 34 weeks. One patient received steroids at 21 weeks gestation due to an error in determining her gestational age at time of admission.
Table 1. Demographic characteristics of subjects.
| Patients (N) | 77 |
|
| |
| Blood Samples (N) | 274 |
|
| |
| Age, median (range) | 27 (16-45) years |
|
| |
| Gestational Age, median (range) | 30 (21-34) weeks |
|
| |
| Total Body Weight, median (range) | 85 (36-159) kg |
|
| |
| Lean Body Weight, median (range) | 48 (26-68) kg |
|
| |
| Body Mass Index,, median (range) | 30 (16-53) kg/m2 |
| Distribution: <25 kg/m2 | 18 % |
| 25-30 kg/m2 | 32 % |
| 31-39 kg/m2 | 36 % |
| ≥40 kg/m2 | 13 % |
|
| |
| Race/Ethnicity Black | 64 % |
| Hispanic | 22 % |
| Caucasian | 14 % |
|
| |
| Fetuses | |
| Singleton (N) | 64 |
| Multiple (N) | 13 |
|
| |
| PPROM/PTL/CI | 65 % |
| Pre-eclampsia | 28 % |
| NRFWB | <1 % |
| Other | 6 % |
PPROM – Preterm premature rupture of membrane
PTL- Preterm labor
CI- Cervical insufficiency
NRFWB- non reassuring fetal well being
BMI- Body Mass Index (kg/m2)
A two compartment model with first order of absorption and no lag time fit the betamethasone plasma concentration profile well. The pharmacokinetic parameters estimated included absorption rate constant (ka), apparent distribution clearance (Q/F), CL/F, apparent volume of distribution of the central compartment (Vc/F) and volume of distribution at steady state (Vss/F). Data supporting the structural and covariate model selection is provided in Appendix III. Table 2 lists the pharmacokinetic parameters, covariate coefficients, inter-individual variability and residual variability for the base and final population pharmacokinetic models with their relative standard errors. The models included estimates for the inter-individual variability for CL/F, Q/F, Vss/F and Ka along with a covariance term between CL/F and Vss/F.
Table 2. Population pharmacokinetic parameter estimates of betamethasone from the base model (no covariates), final (covariate) model and bootstrap analysis.
| Parameters | Base Mode Estimate (RSE,%) | Final Model Estimate (RSE,%) | Bootstrap Median (2.5th – 97.5th Percentile) |
|---|---|---|---|
| Fixed Effects | |||
| ka (h-1) | 3.1 (12.8) | 3.0 (16.8) | 2.8 (1.4 – 4.2) |
| Q (L/h) | 2700 (65.2) | 2480 (63.7) | 2425 (473 – 3960) |
| CL (L/h) | 17.6 (4.3) | --- | --- |
| CL45kg woman (L/h/45kg) (LBW effect on CL/F) | --- | 17.2 (4.0) | 17.2 (15.8 – 18.6) |
| Vc (L) | 48.5 (17.4) | 43.7 (21.6) | 34.9 (1.2 – 59.3) |
| Vss (L) | 205 (7.4) | --- | --- |
| Vss45kg woman (L/45kg) (LBW effect on Vss/F) | --- | 166 (13.5) | 167 (102 – 215) |
| Θ for effect of gestational age on Vss/F (L/45kg) | --- | 121 (37.6) | 114 (37 – 196) |
| Interindividual Variability | |||
| Ka (h-1) | 1.3 (49.3) | 1.3 (55.3) | 1.2 (0.3 – 2.2) |
| Q/F (CV%) | 271 (43.0) | 221 (42.9) | 200 (110 – 330) |
| CL/F (CV%) | 35.1 (17.0) | 31.8 (20.0) | 30.9 (25.2 – 37.0) |
| Vss/F (CV%) | 27.0 (17.0) | 19.1 (37.1) | 16.8 (10.1 – 22.7) |
| Covariance CL/F, Vss/F (CV%) | 26.0 (20.6) | 19.3 (27.0) | 22.1 (7.2, 37.0) |
| Residual Variability (CV%) | 17.5 (20.7) | 17.6 (20.4) | 18.1 (14.6 – 21.8) |
ka typical value of the absorption rate constant (ka)
Q typical value of apparent distribution clearance (Q/F)
CL typical value of apparent clearance (CL/F)
CL45kg woman typical value of apparent clearance in a 45 kg LBW pregnant woman
Vc typical value of apparent volume of distribution of the central compartment (Vc/F)
Vss typical value of apparent volume of distribution at steady-state (Vss/F)
Vss45kg woman typical value of apparent volume of distribution at steady-state in a 45 kg LBW woman
Θ covariate
LBW lean body weight
CV coefficient of variation
RSE relative standard error
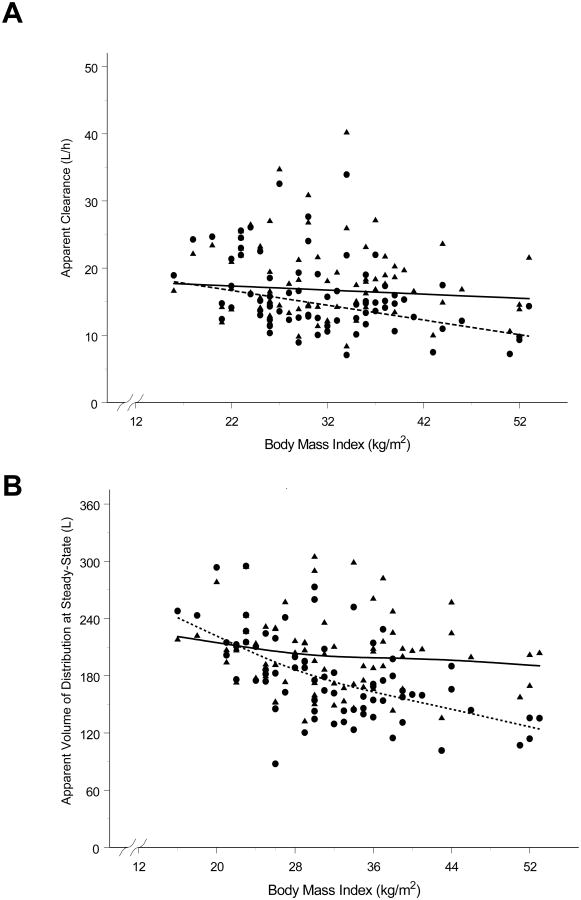
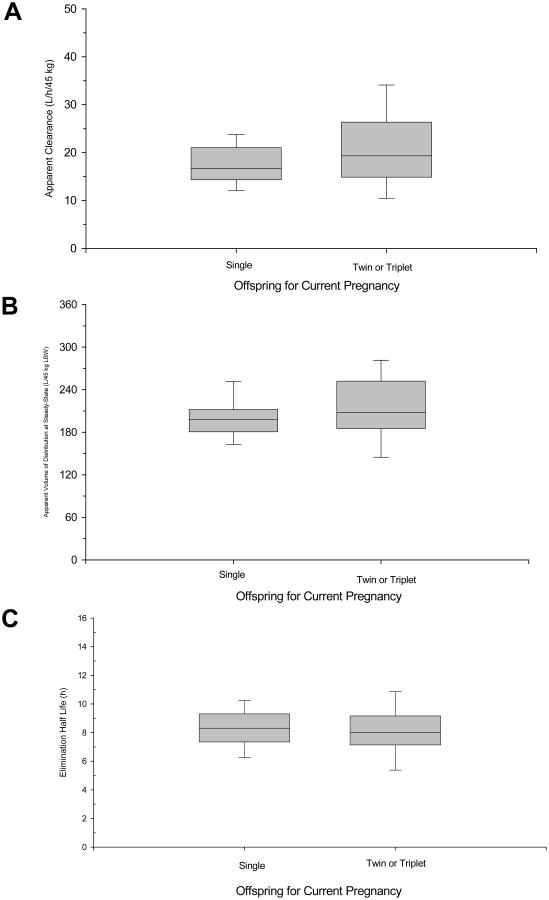
The covariate analysis identified LBW for CL/F and gestational age as well as LBW for Vss/F as factors significantly explaining their variability. The influence of LBW on the inter-individual variability of CL/F was small with variability reduced by 10.4%. On the other hand, LBW and gestational age explained nearly 40% of the inter-individual variability on Vss/F. No factors significantly influencing inter-individual variability in Q/F or ka were identified. The final covariate models are provided in Appendix III. Other descriptors of body size (TBW, BSA and BMI), when individually input into the covariate models for CL/F and Vss/F, produced significant decreases in the OFV. However, the decrease in OFV was less than with LBW, and their addition to covariate models containing LBW during the forward stepwise process produced no further improvement in the model fitting. They were, therefore, not retained in the final covariate models. Interestingly, in contrast to the linear relationship between LBW and CL/F or Vss/F, a power function better described the relationship of TBW with CL/F or Vss/F. The differences in the form of the relationships with TBW compared to LBW are apparent in Figures 1A and 1B. As can be seen in Figure 1, the individual Bayesian estimates of CL/F and Vss/F normalized by LBW remain constant over the range of observed body sizes, represented by BMI. On the other hand, TBW normalized CL/F and Vss decrease with increasing BMI. The final population pharmacokinetic model was validated from 1000 bootstrap replicates. The bootstrapped medians for the fixed and random effect parameters are provided in Table 2. The mean estimates for the parameters from the final model were within 15% of the bootstrapped medians, supporting the stability of the population model and accuracy of the parameter estimates. Additionally, the small relative standard errors and narrow bootstrapped 95% confidence intervals (Table 2) confirm the precision of the population parameters. Multiple gestation was not identified by the population analysis as significantly influencing the pharmacokinetics of betamethasone in pregnant women. Box plots of the individual CL/F, Vss/F and elimination half time grouped by number of offspring (single versus twin or triplet) are shown in Figure 2. No statistically significant differences (p>0.1, Student's t-test) were observed for parameters between single and multiple gestations. The power for detecting a statistically significant difference (p<0.01) in CL/F between singleton and multifetal pregnancies was 65% when CL/F was assigned a value 25% higher in women with twin or triplet pregnancies, 78% when CL/F was 30% higher in women with multiple pregnancies, and 86% when CL/F was 35% higher in women with multiple pregnancies.
Figure 1.
Graphical evaluation of relationship between body size and apparent clearance (CL/F) and volume of distribution at steady-state (Vss/F) normalized by LBW or TBW. (A) Individual Bayesian estimates of CL/F normalized to 45 kg LBW (▲) or to 70 kg TBW (●) versus body mass index. The solid line represents a loess smoother fit to the 45 kg LBW CL/F and the dashed line the fit of a loess smoother to the 70 kg TBW CL/F. (B) Individual Bayesian estimates of Vss/F normalized to 45 kg LBW (▲) or to 70 kg TBW (●) versus body mass index. The solid line represents a loess smoother fit to the 45 kg LBW Vss/F and the dashed line the fit of a loess smoother to the 70 kg TBW Vss/F.
Figure 2.
Effect of single and multiple gestation on Bayesian estimates of betamethasone pharmacokinetic parameters for individual patients. (A) Box plots of apparent betamethasone clearance for singleton and multiples pregnancies. (B) Box plots of apparent volume of distribution at steady-state for singleton and multiples pregnancies. (C) Box plots of betamethasone elimination half lies for singleton and multiples pregnancies. The limits of the box represent the 25th to 75th percentile of the distribution, the solid line in the box is the median value and the whiskers represent the 10th and 90th percentiles of the distribution.
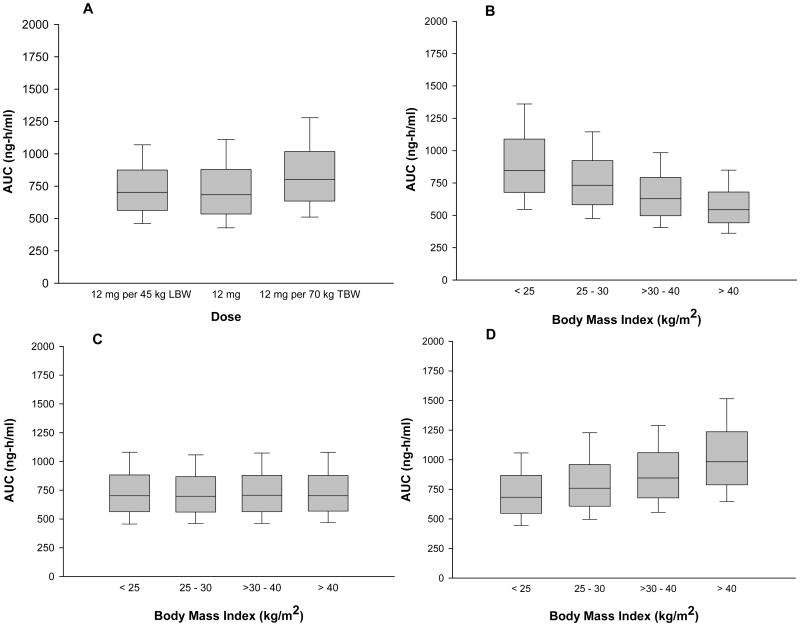
Figure 3 summarizes the betamethasone AUCs from the simulated datasets. The 12 mg (standard), 12 mg per 45 kg LBW (LBW-adjusted) and 12 mg per 70 kg TBW (TBW-adjusted) doses produced comparable median betamethasone AUCs (Figure 3A). This finding reflects the similarity between median LBW and TBW in this study and typical values in women of this age. Variability in betamethasone AUC is less with LBW-adjusted dose. The reason for the differing variability among dose groups is illustrated by the box plots in Figures 3B-3D. For LBW-adjusted dose, median betamethasone AUC is equivalent among the 4 BMI groups (Figure 3C). On the other hand, the median AUC for the other two dose groups varies with BMI (Figures 3B and 3D). For example, as BMI increases from <25 to > 40 mg/m2, median AUC with administration of the standard dose decreases by approximately 35% (Figure 3B) and with administration of TBW-adjusted dose increases by approximately 30% (Figure 3D).
Figure 3.
Graphical evaluation of the effects of dosage regimen and body size on betamethasone exposure, as represented by the area under the plasma concentration-time curve (AUC). AUCs were determined by simulating 1000 replicates of the final population model for each of the following doses: standard 12 mg, 12 mg per 45 kg LBW (LBW-adjusted dose) or 12 mg per 70 kg TBW (TBW-adjusted dose). (A) Box plot of simulated betamethasone AUCs for each of the 3 doses. (B) Box plot of simulated betamethasone AUCs following a 12 mg dose (not adjusted) grouped by the patients' body mass indices: < 25 kg/m2, 25-30 kg/m2, > 30-40 kg/m2 or > 40 kg/m2. (C) Box plot of simulated betamethasone AUCs following a 12 mg per 45 kg LBW dose (adjusted by LBW) grouped by body mass index: < 25 kg/m2, 25-30 kg/m2, > 30-40 kg/m2 or > 40 kg/m2. (D) Box plot of simulated betamethasone AUCs following a 12 mg per 70 kg TBW dose (adjusted by TWB) grouped by body mass index: < 25 kg/m2, 25-30 kg/m2, > 30-40 kg/m2 or > 40 kg/m2. The limits of the box represent the 25th to 75th percentile of the distribution, the solid line in the box is the median value and the whiskers represent the 10th and 90th percentiles of the distribution.
Discussion
A two compartment model best described the disposition of betamethasone following IM administration in pregnant women between 21 and 34 weeks of gestation. The CL/F and Vss/F for the typical woman in the study of 30 weeks gestation and 48 kg LBW were 18.4 L/h and 202 L, respectively. These values are comparable to estimates derived from the study reported by Peterson et al following IM administration of the same formulation to pregnant women.19 Conversely, the betamethasone CL/F and distribution volume found by Ballabh et al in pregnant women following IM injection of the same formulation were lower.7 Potential factors explaining the discrepancy between Ballabh and our investigation as well as that of Peterson include their use of an immunoassay versus a chemical assay by Peterson and us for measuring betamethasone plasma concentrations, inability of their analysis to characterize the absorption and distribution phases of the betamethasone plasma concentration-time curves, and their failure to stabilize the betamethasone esters in the plasma samples prior to storage.7,19 Any of these factors may contribute to an artifactual elevation of AUC and, as a result, explain the lower CL/F and volume of distribution described by Ballabh.7
The betamethasone CL/F and Vss/F in our study are higher than observed in studies of non-pregnant women.13 Even after adjusting for a bioavailability of approximately 70% for the betamethasone phosphate/acetate suspension in pregnant women,19,20 our CL/F and Vss/F remain approximately 1.2 to 1.6-fold higher than values reported in non-pregnant women.13,19-20 Betamethasone is a low extraction ratio drug, and almost entirely eliminated by hepatic metabolism.13 Factors explaining a higher CL/F, therefore, include a decrease in extent of absorption, reduction in plasma protein binding, or enhanced hepatic metabolism. Observations of the same relative difference as we observed in CL/F and Vss/F between pregnant and non-pregnant women following intravenous administration argues against a change in extent of absorption.13,19-20 An alteration in plasma protein binding is also an unlikely explanation as the fraction of betamethasone bound to plasma proteins is equivalent in pregnant and non-pregnant women.13,19-20 Consequently, enhanced hepatic metabolism represents the most probable explanation for a pregnancy-related increase in CL/F. Although pathways mediating the heptic metabolism of betamethasone are not established, studies with other corticosteroids suggest a primary role for the cytochrome P450 isoenzyme 3A4.22 Pregnancy-related increases in clearances of other 3A4 substrates have been observed.1,23An increase in tissue binding of betamethasone provides the most apt explanation for the higher Vss/F in pregnancy19-20, 24. Among demographic and clinical variables evaluated, the covariates that affected betamethasone CL/F and Vss/F the most were descriptors of body size, including LBW, TBW, BSA, and BMI. Scaling of CL/F and Vss/F by LBW produced the greatest reduction in the OFV and inter-individual variability. After LBW was incorporated into the population pharmacokinetic models for CL/F and Vss/F, the addition of other body size measures yielded no further improvement in the model fitting. The form of the relationship with betamethasone CL/F or Vss/F also differed between LBW and TBW and other body size indicators. The CL/F and Vss/F increased linearly with increasing LBW, but nonlinearly with increasing TBW. Accordingly, betamethasone CL/F scaled to LBW remains constant and allows drug exposure to be satisfactorily estimated over a wide range of body compositions. On the other hand, CL/F scaled to TBW varies with changing body size and, when directly extrapolated from normal to obese individuals, underpredicts drug exposure in subjects with large BMIs. Figures 3B to 3D illustrate how these relationships impact the administration of betamethasone doses. Compared to use of a standard (unadjusted) or TBW-adjusted dose, a mg/kg LBW approach for dosing betamethasone offers the advantage of yielding consistent plasma concentrations across individuals of varying body compositions as represented by BMI in the figures.
Doubts about the clinical benefit of adjusting betamethasone doses for LBW are raised by recent retrospective analyses that failed to show any relationship between neonatal outcomes and maternal body size following standard courses of antenatal betamethasone.8,9 However, the assertion of Jobe and Soll25 that standard regimens deliver too high a dose suggests an alternative reason for the lack of differences in response between body composition groups, namely that everyone received a supra-therapeutic dose of betamethasone. The standard betamethasone regimen for treatment of prematurity derives from a 1995 NIH Consensus Panel,26 with the dose, timing and frequency selected from empiric rather than scientifically-derived evidence.27 Support for a lower dose is provided by a recent comparison of 4 different betamethasone regimens for inducing fetal lung maturation in sheep.28 Induction of fetal lung maturation was comparable among the 4 treatments, including a single dose regimen of IM betamethasone acetate. Notably, the single dose betamethasone acetate regimen produced no detectable betamethasone plasma concentrations in 2 of 3 fetuses and maternal betamethasone plasma concentrations approximately 1/10th of those seen following the standard regimen. The results of Jobe et al suggest the potential for unnecessary drug exposure during pregnancy with the current dosing regimen and emphasize the need to better understand the pharmacodynamic properties of betamethasone in the mother and fetus, particularly with respect to lung maturation and adverse effects on fetal growth. Whether individualizing doses by LBW offers any clinical advantages is uncertain. Once the range of effective betamethasone doses and plasma concentrations are identified, studies such as ours provide a framework for individualizing doses.
Studies involving other drugs substantiate the superiority of LBW compared to TBW for describing the effect of size on drug clearance across a broad range of body compositions.29-31 From a biological perspective, these findings suggest that LBW more directly mirrors the functional capacity of the liver than TBW. In contrast to our results, other studies have generally found TBW to be the best body size descriptor for volume of distribution of lipophilic drugs like betamethasone.29 We are uncertain of the reason for this discrepancy, but the lack of pregnant subjects in the other studies may offer an explanation. Finally, although we demonstrated that LBW was the best body size indicator among those evaluated in the current study, none of the body size indicators, including LBW, were specifically developed for pregnancy. Development and evaluation of pregnancy-specific measures of body composition are indicated.
Gestational age also significantly contributed to variability in Vss/F, with Vss/F increasing as a power function of gestational age. The time profile of this change and relatively minor impact on Vss/F, approximately 18% from 24 to 34 weeks, suggest pregnancy-induced increases in extracellular fluid as a possible factor.1
Higher order of pregnancy was not found to alter the maternal pharmacokinetics of betamethasone. The study had sufficient power to detect at least a 30% difference in CL/F. The only other study to compare pharmacokinetics in singleton and twin pregnancies found a significantly faster elimination half life time in women with twin pregnancies.7 Comparable to our findings, neither clearance nor volume of distribution significantly differed for the two groups. The more rapid elimination was interpreted by the investigators as having the potential to lower betamethasone plasma concentrations and, thus, to explain the poorer response to antenatal betamethasone in twin pregnancies. This interpretation ignores that clearance and volume of distribution, not elimination half life, are the primary determinants of the plasma concentration-time profile. Future investigations should focus on alternative explanations for decreased efficacy of betamethasone in twin pregnancies, including reduced fetal bioavailability due to enhanced activity of placental drug metabolizing enzymes, efflux transporter or altered pharmacological responsiveness to betamethasone in fetuses of twin or triplet pregnancies.2 Also, more pharmacodynamic studies of betamethasone given antenatally should focus on the identification of effective betamethasone maternal doses and relative therapeutic plasma concentrations in mothers and fetuses, before individualization of dosing by LBW can be clinically utilized.
In summary, we estimated pharmacokinetic parameters for betamethasone for women between 21 and 34 weeks gestation at standard dosages. We demonstrated that across a wide range of maternal body size, individualization of betamethasone dosage by LBW may be preferable in order to limit the risk of overdosing slim mothers and underdosing mothers with larger body size. However, therapeutics levels must be identified as well as a better understanding of its fetal and maternal pharmacodynamics. Because we could not demonstrate any difference in maternal pharmacokinetics with multiple pregnancies, further work should focus on alternative reasons for why betamethasone does not provide the same beneficial effects in babies born of multiple gestations compared to their singleton counterparts.
Supplementary Material
Apppendix III Supplementary Figure: Goodness of fit plots for betamethasone plasma concentrations using one- and two compartment models with first order absorption and elimination. (A) Observed versus model predicted betamethasone plasma concentrations from the one-compartment model. (B) Weighted residuals versus model predicted betamethasone plasma concentrations from the one-compartment model. (C) Observed versus model predicted betamethasone plasma concentrations from the two-compartment model. (D) Weighted residuals versus model predicted betamethasone plasma concentrations from the two-compartment model. The solid line in (A) and (C) represents the line of identity. The solid line in (B) and (D) represents the zero-intercept line.
Acknowledgments
We would like to thank all the individuals who contributed to the data collection presented in alphabetical order; without them the project could not have been completed: Maria Colon, MD, Cecilia Gambala, MD, Dennie Rogers, MD and all the Obstetrics and Gynecology residents and Labor and Delivery/Mother Baby staff.
Appendix 1- Common Definitions
| Parmacokinetics (PK) | Time course of drug absorption, distribution, metabolism and excretion in the body. |
| Clinical Pharmacokinetics | Application of PK principles to safe and effective therapeutic management of drugs in an individual. |
| Pharmacodynamics | Relationship between drug concentration at the site of action and resulting effect, including time course and intensity of therapeutic and adverse effects. |
| One Compartment model | All body tissue and fluid are considered a unit; based on the assumption that after one dose of a drug is administered, it distributes instantaneously to all body areas. |
| Two Compartments model | At least two different units: a central compartment, with rapid drug distribution, usually the bloodstream and highly perfuse organs and a peripheral compartment, with slow distribution. The model implies the drug moves back and forth between the two compartments to remain in equilibrium |
| First Order elimination | Amount of drug eliminated over a period of time is directly proportional to the amount of drug in the body; the total amount of drug eliminated over a set time period changes, but the fraction of the drug eliminated over the given time remains constant. |
| Bioavailability (F) | Fraction of a given drug dose that reaches the systemic circulation. |
| Body Size Indicators |
Total Body Weight (Kg) [TBW] Body Surface Area (m2) [BSA] →calculated surface of a human body (for many clinical purposes BSA is a better indicator of metabolic mass than body weight because it is less affected by abnormal adipose mass) Body Mass Index (Kg/m2) [BMI]→ Weight (kg)/Height (m)2 Lean Body Weight (Kg) [LBW] → Lean weight includes the muscles, bones, tendons, ligaments, and water in the body; everything except fat tissue – Weight (women) = (1.07 × Weight(kg)) - 148 (Weight2/(100 × Height(m))2) |
| Clearance (CL/F) | Rate of drug removal from the plasma – expressed as volume of plasma per given unit of time. |
| Volume of Distribution (Vc/F) | Extent of drug distribution into body fluids and tissues – volume required to account for all of the drug in the body, if the concentration in all tissues is the same as plasma concentration. |
| Volume of Distribution at steady state (Vss/F) | Relates total amount of drug in the body to a particular plasma concentration under steady state conditions. |
| Inter Compartmental Clearance Q/F | Rate of drug moving back and forth between the central compartment and the tissues and fluid of the peripheral compartment, to maintain a status of pseudoequilibrium. |
| Area Under the plasma Concentration versus time curve (AUC) | Area formed under the curve when plasma concentration is plotted versus time; expressed also as total drug exposure. CL=Dose/AUC |
Appendix II
Betamethasone Assay
Betamethasone plasma concentrations were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay adapted from previously published procedures.12,13 The lower limit of quantitation was 1.05 ng/ml. Samples with concentrations above the upper limit of quantitation,105 ng/ml, were analyzed after diluting 1 to 4 with blank plasma. The between-run precision, based on the relative standard deviation of replicate (n=7) quality controls, was 1.3% at 402 ng/ml, 4.7% at 80.5 ng/ml, 4.2% at 40.2 n/ml, 5.5% at 4.02 ng/ml and 2.9% at 1.05 ng/ml. The assay was specific for betamethasone, with resolution of betamethasone base from the acetate and phosphate esters.
Explanation of Equations Utilized for Pharmacokinetic(PK) Modeling
Betamethasone plasma concentrations were analyzed with NONMEM version VI level 1.0 (Globomax LLC, Hanover, MD, USA). Model building first focused on identifying an appropriate structural (base) model, consisting of a PK compartmental model and expressions for describing the inter-individual and intra-individual (residual) variabilities. The best model was selected based on: 1). goodness of fit plots such as observed versus the model-predicted population betamethasone plasma concentrations and weighted residuals versus predicted plasma concentrations, 2). precision of the parameter estimates as indicated by the relative standard error from the model fitting, 3). minimum value of the objective function (OFV), i.e., the minimization criteria in NONMEM, and 4). physiological relevance of the parameter estimates. The difference in the OFV between competing models is approximately χ2-distributed with the degrees of freedom equal to the difference in number of parameters between the models.
One- and two-compartment models with first order absorption and elimination were evaluated to describe the disposition of betamethasone and provide estimates of the fixed effect PK parameters. Models were tested with and without an absorption lag time. The first-order conditional estimation (FOCE) method was selected for fitting the PK data. A log normal distribution was assumed for the pharmacokinetic parameters, and variability among individuals (i.e., interindividual variability) for the PK parameters was described using exponential random effects. This relationship is illustrated for clearance (CL) by the following equation:
| (1) |
Where: CLi is the estimated CL for individual i, CLTV is the typical population value for CL and ηi is the random effect representing the deviation of CLi from CLTV. The ηs are assumed to be normally distributed with a mean of zero and variance ω2. The ω2 is an estimate of the interindividual variance for the PK parameter. An additive model (equation 2) was evaluated when problems occurred with the exponential model.
| (2) |
A full variance-covariance matrix (i.e., inclusion of the off-diagonal elements, which represent the covariance between random effects and indicate their degree of correlation) was implemented for modeling the inter-individual random effects.
Residual (error) variability was described as a proportional error (Equation 3).
| (3) |
where Yij is the jth observed betamethasone plasma concentration in individual i, Ŷij is the jth predicted betamethasone plasma concentration in individual i, and εij denotes the random error between the measured and predicted jth observation in individual i. The ε's are assumed to be normally distributed with a mean of zero and variance of σ2. The σ2 is an estimate of the intraindividual variance. A diagonal variance-covariance matrix (i.e., covariance between random effects set to 0) was used for modeling the residual variability
After the base model was determined, the next step in the modeling process involved identifying clinically meaningful covariates for explaining the inter-individual variability in the PK parameters. This was performed by obtaining Bayesian estimates of the pharmacokinetic parameters for individual patients from the base model. Graphical and generalized additive modeling methods (S-Plus version 6.1, Insightful Corporation, Seattle) were used to screen for relationships between covariates and pharmacokinetic parameters. Covariates evaluated were: total body weight (TBW), lean body weight (LBW),15 body surface area (BSA),16 body mass index (BMI),17 gestational age (GA) (calculated by last menstrual period, if available, and confirmed by first or second trimester ultrasound), race, age, multifetal (twin or triplet) gestation, concurrent liver or kidney disease and presence of pre-eclampsia. Covariates identified in the screening analysis were first added alone to expressions for the pharmacokinetic parameters in the base model using NONMEM. Covariates producing a decrease in OFV > 3.84 (p<0.05, df=1) in the univariate analysis were entered in a stepwise fashion into an intermediate multivariate model and retained if their addition decreased the OFV by > 3.84. A backward elimination step followed with covariates entered into the model during the forward addition step individually eliminated and retained in the final population pharmacokinetic model if their removal increased the OFV by > 6.6 (p<0.01, df=1). Continuous covariates were normalized to an accepted population standard (70 kg for TBW, 45 kg for LBW, 1.73 m2 for BSA) or study median (30 weeks gestational age), and linear (equation 4) or power (equation 5) functions employed to assess their influence.
| (4) |
| (5) |
Where: θ represents the effect of the covariate on the TVCL.
Categorical covariates were input as indicator variables.
| (6) |
Where: I is an indicator variable with a value of 1 if the trait is present and 0 otherwise.
The simulations to determine the power of the design for identifying important differences in apparent clearance (CL/F) between women with single and multifetal (twin or triplet) gestations were performed using a modified form of the final population model. The modification is described in the following equation:
| (7) |
Where: CL/Fi is the estimated CL/F for individual i, CL/Ffinal model is the CL/F based on the final population PK and covariate model, θtwin pregnancy is an additional covariate for the simulation representing a proportional increase in CL/F in women with a twin or higher order pregnancy. θtwin pregnancy was varied from 0% to 40% in increments of 5%. The percentage of the 200 replications at each incremental increase showing a significantly higher CL/F in multiple pregnancies represented the power of the study to detect this difference. The increase in CL/F was considered significant if the decrease in the OFV was > 6.6 and the lower 95% asymptotic confidence limit for the coefficient representing the effect of multiple pregnancies on clearance, i.e., θtwin pregnancy, was > 0. For the simulations performed to examined the effect of different body size adjusted dosing schemes on betamethasone exposure, the area under the betamethasone plasma concentration-time curve from time zero to infinity (AUC) was calculated for each simulated plasma concentration profile by the trapezoidal rule from time zero to 24 hours postdose, with area from 24 hours to infinity extrapolated by dividing the betamethasone plasma concentration at 24 hours by the negative of the terminal slope.
Appendix III
Data Supporting Selection of the Structural Pharmacokinetic (PK) Model
A two-compartment model with first order absorption and no lag time fit the betamethasone plasma concentration profile well (Δ OFV= -183, p<0.001, df=2 compared to a one-compartment model). Pharmacokinetic parameters of the model included absorption rate constant (ka), CL/F, apparent distribution clearance (Q/F), apparent volume of distribution of the central compartment (Vc/F), and Vss/F. The less varied and more symmetric distribution of data around the line of identity in Appendix III Supplementary Figure 1C compared to 1A and zero intercept line in Supplementary Figure 1D compared to 1B support the suitability of the two-compartment model. The model included estimates for the IIV for CL/F, Q/F, Vss/F and Ka along with a covariance term between CL/F and Vss/F. The IIV in ka was modeled with an additive error, whereas an exponential model best described IIV for the other parameters. The data did not support allocation of IIV to Vc/F. Residual variability was expressed using a proportional error model. A linear relationship was selected for describing the relationship between LBW and CL/F or Vss/F. The use of a power equation did not improve the fit and yielded power coefficients that included 1 within the 95% confidence limits, strongly suggesting a linear relationship. After hypothesis testing was completed, the effect of LBW on CL/F and Vss/F was simplified from a linear expression to a direct proportion for the final model. This transformation provided a more clinically applicable expression and did not affect either the fit or amount of variability explained by LBW.
Covariate Models for CL/F and Vss/F
The final covariate models for CL/F and Vss/F were:
TVCL45kg woman and TVVss45kg woman represent typical values of CL/F and Vss/F for a pregnant woman of 45 kg LBW.
Footnotes
This work was presented as oral presentation at the 30thAnnual Meeting of the Society of Maternal Fetal Medicine, Chicago, Illinois on February 4, 2010.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 2.Hodge LS, Tracy TS. Alterations in drug disposition during pregnancy: implications for drug therapy. Expert Opin Drug Metab Toxicol. 2007;3:557–571. doi: 10.1517/17425225.3.4.557. [DOI] [PubMed] [Google Scholar]
- 3.Antenatal corticosteroids revisited: repeated courses. NIH Consensus Development Conference Statement; August 17-18, 2000; pp. 1–10. [Google Scholar]
- 4.The effect of corticosteroids for fetal lung maturity on perinatal outcomes. NIH Consensus Development Conference Statement; February 28-March 2, 1994. [Google Scholar]
- 5.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm labor. Cochrane Database of Systematic Review. 2006;(3) doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- Burkett G, Baver CR, Morrison JC, Curet L. Effect of prenatal dexamethasone administration on prevention of respiratory distress syndrome in twin pregnancies. J Perinatol. 1986;6:304–8. [Google Scholar]
- 7.Ballabh P, Lo ES, Kumari J, Cooper TB, Zervoudakis I, Auld PA, Krauss AN. Pharmacokinetics of betamethasone in twin and singleton pregnancy. Clin Pharmacol Ther. 2002 Jan;71(1):39–45. doi: 10.1067/mcp.2002.120250. [DOI] [PubMed] [Google Scholar]
- 8.Della Torre M, Hibbard J. Antenatal steroids for prematurity and maternal obesity: does obesity decrease the beneficial effects?. Poster number 748, 29th Annual Meeting – The pregnancy meeting SMFM; San Diego CA. January 31st 2009. [Google Scholar]
- 9.Hashima J. The effect of maternal obesity on neonatal outcome in women receiving a single course of antenatal corticosteroids. Poster number 812, 29th Annual Meeting – The pregnancy meeting SMFM; San Diego CA. January 31st 2009. [Google Scholar]
- 10.D'Argenio D. Optimal sampling times for pharmacokinetic experiments. J Pharmacokinet Biopharm. 1981;9(6):739–56. doi: 10.1007/BF01070904. [DOI] [PubMed] [Google Scholar]
- 11.D'Argenio D, Schumitzky A. ADAPT II User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource; Los Angeles: 1997. [Google Scholar]
- 12.Samtani MN, Lohle M, Grant A, Nathanielsz PW, Jusko WJ. Betamethasone pharmacokinetics after two prodrug formulations in sheep: implications for antenatal corticosteroid use. Drug Metab Dispos. 2005 Aug;33(8):1124–30. doi: 10.1124/dmd.105.004309. Epub 2005 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Nation RL, Mc Bride WG, Ashley JJ, Moore RG. Pharmacokinetics of Betamethasone in Healthy Adults after intravenous administration. Eur J Clin Pharmacol. 1983;25:643–650. doi: 10.1007/BF00542353. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Uboh CE, Soma LR, Guan F, Rudy JA, Tsang DS. Resolution, quantification and confirmation of betamethasone and dexamethasone in equine plasma by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(6):825–32. doi: 10.1002/rcm.1851. [DOI] [PubMed] [Google Scholar]
- 15.Beal SL, Sheiner LB. NONMEM Users Guide. Icon Development Solutions; Elliott City, MD: 1989-2006. [Google Scholar]
- Janmhasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44(10):1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 17.DuBois D, DuBois EF. Clinical calorimetry. Tenth paper. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863. [Google Scholar]
- 18.Keys A, Fidanza F, Karvonen M, Kimura N, Taylor H. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Petersen MC, Ashley JJ, Mc Bride WG, Nation RL. Disposition of betamethasone in parturient women after intramuscular administration. By J Clin Pharmac. 1984;18:383–392. doi: 10.1111/j.1365-2125.1984.tb02480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MC, Collier CB, Ashley JJ, Mc Bride WG, Nation RL. Disposition of betamethasone in parturient women after intravenous administration. Eur J Clin Pharmacol. 1983;25:803–810. doi: 10.1007/BF00542524. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen BB, Larsen LS, Senderovitz T. Pharmacokinetic interaction studies of atosiban with labetalol or betamethasone in healthy female volunteers. BJOG. 2005;112:1492–9. doi: 10.1111/j.1471-0528.2005.00735.x. [DOI] [PubMed] [Google Scholar]
- 22.McCrea JB, Majumdar AK, Goldberg MR, Iwamoto M, Gargano C, Panebianco DL, Hesney M, Lines CR, Petty KJ, Deutsch PJ, Murphy MG, Gottesdiener KM, Goldwater DR, Blum RA. Effects of the neurokinin1 receptor antagonist aprepitant on the pharmacokinetics of dexamethasone and methylprednisolone. Clin Pharmacol Ther. 2003;74:17–24. doi: 10.1016/S0009-9236(03)00066-3. [DOI] [PubMed] [Google Scholar]
- 23.Tracy TS, et al. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–9. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Gibaldi M, McNamara PJ. Apparent volumes of distribution and drug binding to plasma proteins and tissues. Europ J Clin Pharmacol. 1978;13:373–8. doi: 10.1007/BF00644611. [DOI] [PubMed] [Google Scholar]
- 25.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190:878–81. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273(5):413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 27.Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD006764.pub2. Art. No.: CD006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jobe AH, Nitsos I, Pillow J, Polglase GR, Kallapur SG, Newnham JP. Betamethasone dose and formulation for induced lung maturation in fetal sheep. Am J Obstet Gynecol. 2009;201:611.e1–7. doi: 10.1016/j.ajog.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han PY, Duffull SB, Kirkpatrick CMJ, Green B. Dosing in obesity: a simple solution to a big problem. Clinical Pharmacology & therapeutics. 2007 Nov;82(Number 5):505–508. doi: 10.1038/sj.clpt.6100381. [DOI] [PubMed] [Google Scholar]
- 31.Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Apppendix III Supplementary Figure: Goodness of fit plots for betamethasone plasma concentrations using one- and two compartment models with first order absorption and elimination. (A) Observed versus model predicted betamethasone plasma concentrations from the one-compartment model. (B) Weighted residuals versus model predicted betamethasone plasma concentrations from the one-compartment model. (C) Observed versus model predicted betamethasone plasma concentrations from the two-compartment model. (D) Weighted residuals versus model predicted betamethasone plasma concentrations from the two-compartment model. The solid line in (A) and (C) represents the line of identity. The solid line in (B) and (D) represents the zero-intercept line.