Abstract
Objectives
To assess safety and efficiency of the dorsal slit and sleeve male circumcision (MC) procedures performed by physicians and clinical officers.
Methods
We evaluated the time required for surgery and moderate / severe adverse events (AEs), among circumcisions by trained physicians and clinical officers using sleeve and dorsal slit methods. Univariate and multivariate regression with robust variance was used to assess factors associated with time for surgery (linear regression) and adverse events (logistic regression).
Results
Six physicians and 8 clinical officers conducted 1934 and 3218 MCs, respectively. There were 2471 dorsal slit and 2681 sleeve procedures. The mean duration of surgery was 33 minutes for newly trained providers and decreased to ~20 minutes after ~100 circumcisions. The adjusted mean duration of surgery for dorsal slit was significantly shorter than that for sleeve method (Δ −2.8 minutes, p- <0.001). The duration of surgery was longer for clinical officers than physicians performing the sleeve procedure, but not the dorsal slit procedure. Crude AEs rates were 0.6% for dorsal slit and 1.4% with the sleeve method (p=0.006). However, there were no significant differences after multivariate adjustment. Use of cautery significantly reduced time needed for surgery (Δ − 4.0 minutes, p =0.008), but was associated with higher rates of AEs (adjusted odds ratio 2.13, 95%CI 1.26–3.61, p=0.005).
Conclusions
The dorsal slit resection method of male circumcision is faster and safer than sleeve resection, and can be safely performed by non-physicians. However, use of cautery may be inadvisable in this setting.
Keywords: Adult male circumcision, HIV, circumcision programs, task shifting, adverse events, safety, Uganda
Introduction
Male circumcision (MC) has been shown to reduce HIV, herpes simplex virus type 2 (HSV-2), human papilloma virus (HPV) infections, and genital ulcer disease (GUD) in men, 1,2,3,4 and circumcision is now recommended by WHO/UNAIDS for HIV prevention in men5. There are major efforts to scale up programs of MC, particularly in sub-Saharan Africa, but limited numbers of trained physicians present a constraint on provision of services6. Task shifting of procedures conventionally performed by physicians has been shown to be effective in overcoming human resource deficits7, 8. Therefore, there is a need to assess task shifting of male circumcision from physicians to other more numerous and lower cost cadres of health care personnel. Also, there are uncertainties as to which male circumcision procedure may be most appropriate for the African context in terms of safety and efficiency6. Following completion of a randomized trial of MC for HIV prevention, we provided circumcision as a service to the trial control arm participants and to the general male population in Rakai District, Uganda. The surgeries were performed by physicians and clinical officers, using two surgical resection methods; sleeve and dorsal slit. We report here the findings from an evaluation of this service program with respect to time required to perform circumcision and associated rates of moderate and severe adverse events.
Methods
Between May 2006 and May 2010 we provided circumcision services to men aged 15 years and older. For this program evaluation, we considered up to 700 male circumcision procedures performed by each surgeon during this period, with a total of 5152 surgeries. Adult men provided written informed consent for surgery and minors provided assent with parental consent. All men were provided with voluntary HIV counseling and testing (VCT), were instructed on postoperative wound care and the need to abstain from sexual intercourse until the wound was completely healed. All men were screened prior to surgery and if they had signs of penile pathology (e.g., balanitis or sexually transmitted infections (STIs), they were treated, and circumcision was delayed until the lesions resolved. The skin was prepared with 10% povidone-iodine. Circumcision was performed under local anesthesia using a dorsal penile nerve block with an equal volume of 2% lidocaine and 0.5% bupivacaine as a mixture. Circumcisions were performed using either sleeve or dorsal slit resection methods on alternate days of the week. Surgeries were performed by trained and certified physicians and clinical officers depending on the availability of personnel. Because of trial protocol requirements, trial control arm participants were circumcised only by physicians using the sleeve resection method. Non-trial participants who came for male circumcision services were circumcised by any provider available, using the resection method set for that day. For the sleeve resection method, the foreskin was retracted and a distal incision made 0.5–1 cm from the coronal sulcus, then a proximal incision was made following the coronal prominence of the unretracted foreskin. Bucks fascia was exposed and a sleeve of foreskin was freed and removed. For the dorsal slit method, the prepuce was secured by artery forceps at the 11 and 1 o’clock positions and an incision made at the 12 o’clock position between the two forceps. The foreskin was then removed using dissecting scissors. The sleeve procedure was used during the trial and during service provision, while the dorsal slit method was used only during the post-trial service provision. Hemostasis was secured by either bipolar electrocautery (mainly used by physicians), or by ligation (mainly used by clinical officers) For both procedures, the skin edges were apposed with four mattress sutures and additional simple sutures. The duration of surgery was recorded from the time of first skin incision to wound closure and dressing. A total of 2681 sleeve and 2,471 dorsal slit procedures were performed
Surgery during the trial was performed by trained general physicians, but to meet the increased demand for services, clinical officers who are equivalent to physicians’ assistants, were trained by a consultant urologist (SW) to perform male circumcision. Thus, 1934 service surgeries were performed by general physicians (1511 sleeve circumcisions and 423 dorsal slit circumcisions) and 3218 MCs were performed by clinical officers: (1170 sleeve circumcisions and 2048 dorsal slit circumcisions.)
Men were followed up at 24–48 hours; 7–9 days and at 4 weeks postoperatively to assess adverse events (AEs) related to surgery and wound healing. At each visit, men were interviewed to ascertain symptoms of circumcision-related complications and the penis was examined. Surgery-related AEs were predefined and graded into mild (requiring no treatment), moderate (requiring treatment) and severe (requiring surgical intervention, hospitalization or referral for specialized care).
The characteristics of men were assessed according to provider and resection method to evaluate comparability of the patient populations. The frequencies of moderate and severe surgery-related AEs were estimated per 100 surgeries, by provider and resection method. Univariate and multivariate GEE regression with robust variance estimation to account for repeated surgeries by the same provider, were used to assess factors associated with operation time using linear regression. The odds ratio of adverse events was estimated by logistic regression with robust variance. Only variables with a univariate p-value<0.15 were included in the multivariate analyses.
Results
Table 1 shows the characteristics and behaviors reported by men prior to surgery, stratified by provider and procedure. Participants circumcised with the dorsal slit method were younger than those circumcised using the sleeve method (p<0.001). Of those circumcised with dorsal slit, 29% were below 20 years compared to 20% for the sleeve method. The clients receiving dorsal slit were predominantly unmarried (62%) compared to 52% for the sleeve method (p<0.001).
Table 1.
The characteristics of men prior to surgery, stratified by provider and procedure.
| Physicians | Clinical Officer | |||||
|---|---|---|---|---|---|---|
| Sleeve | Dorsal Slit | All | Sleeve | Dorsal Slit | All | |
| Total | 1511(78.1) | 423(21.9) | 1934(100.0) | 1170(36.4) | 2048(63.6) | 3218 (100.0) |
| Age | ||||||
| Below 15 | 7(0.5) | 11(2.6) | 18(0.9) | 13(1.1) | 61(3.0) | 74(2.3) |
| 15–19 | 195(12.9) | 129(30.5) | 324(16.8) | 355(30.3) | 592(28.9) | 947(29.4) |
| 20–29 | 758(50.2) | 185(43.7) | 943 (48.8) | 516(44.1) | 906(44.2) | 1422(44.2) |
| 30–39 | 406(26.9) | 71(16.8) | 477(24.7) | 209(17.9) | 350(17.1) | 559(17.4) |
| 40+ | 145(9.6) | 27(6.4) | 172(8.9) | 77(6.6) | 139(6.8) | 216(6.7) |
| Marital Status | ||||||
| Married | 831 (55.0) | 159(37.6) | 990(51.2) | 467(39.9) | 770(37.6) | 1237(38.4) |
| Not Married | 680(45.0) | 264(62.4) | 994(48.8) | 703(60.1) | 1278(62.4) | 1981(61.6) |
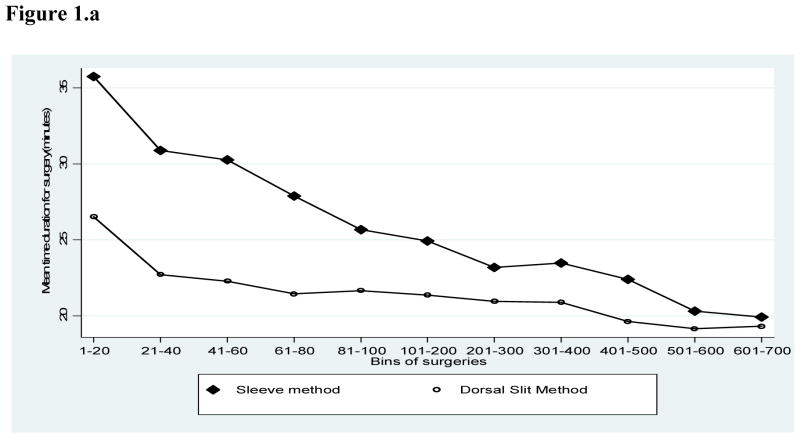
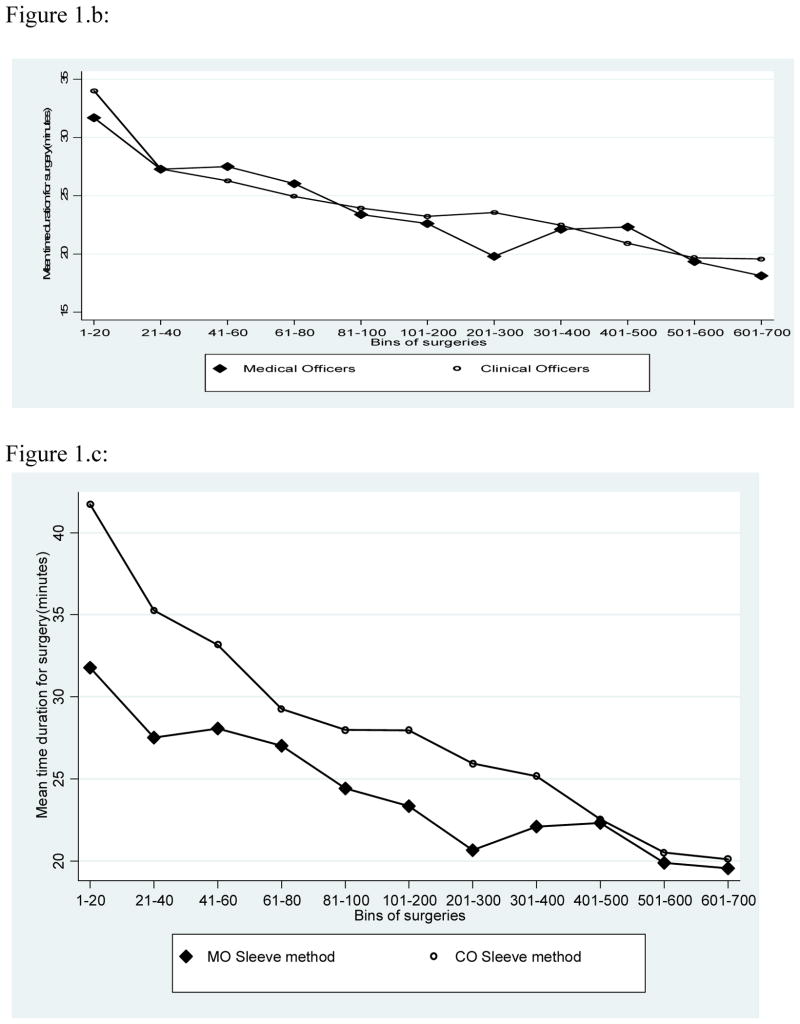
Figure 1.a shows the time required for surgery by the number of procedures performed by a surgeon. Irrespective of experience, the time required for sleeve resection was consistently longer than for dorsal slit and this differential in surgical time was particularly marked for the first 100 procedures performed. With both procedures, the duration of surgery declined with the number of procedures performed. There were no overall difference in the duration of surgery between physicians and clinical officers (Fig 1.b.), but medical officers required less time to perform the sleeve procedure (Fig. 1.c.). This shorter duration of surgery by physicians was less marked and inconstant for the Dorsal slit procedure (Fig 1.d.).
Figure 1.
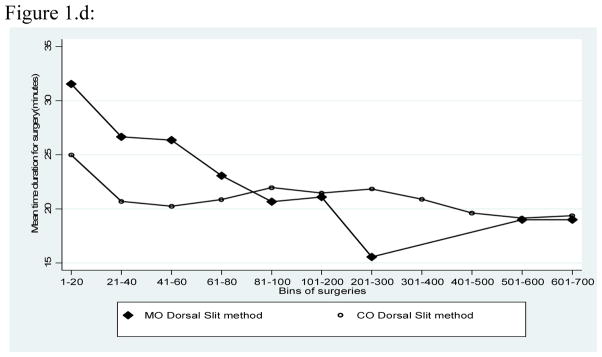
Figure 1.a Mean duration of surgery for Sleeve and Dorsal Slit Methods of Circumcision
Figure 1.b. Mean duration of surgery for Circumcisions Performed by Physicians and Clinical Officers
Figure 1.c. Mean duration of Surgery for the Sleeve Procedure by Physicians and Clinical Officers
Figure 1.d. Mean duration of surgery for the dorsal slit procedure by physcians and clinical officers
Table 2 shows factors associated with the duration of surgery. In multivariate analysis, there were no significant differences in adjusted surgical time between physicians and clinical officers. Dorsal slit required 2.7 minutes (p<0.001) less time than sleeve resection, use of cautery to control bleeding reduced surgical time by ~4 minutes (p-value=0.008), a unit increase in surgical experience reduced surgical time by 1.5 minutes (p-value<0.001). After performing 100 surgeries, dorsal slit took an average of 22.5 minutes compared to 25.3 minutes for the sleeve method.
Table 2.
Univariate and Multivariate Analyses of Factors associated with Surgical time (minutes)
| n/N | Unadjusted analysis
|
Adjusted analysis
|
|||
|---|---|---|---|---|---|
| time (95% CI) | P | time(95% CI) | P | ||
| All participants | 51/5152 | ||||
| Intercept | --- | 33.14(30.04 – 36.25) | <0.001 | ||
| Provider Cadre | |||||
| Medical Officer | 1934/5152 | 1.00 (referent) | 1.00 (referent) | ||
| Clinical Officer | 3218/5152 | −1.62(−4.35–1.11) | 0.246 | −0.62(−4.65 – 3.4) | 0.763 |
| Surgical Method | |||||
| Sleeve Method | 2681/5152 | 1.00 (referent) | 1.00 (referent) | ||
| Dorsal Slit | 2471/5152 | −4.63(−6.21 – −3.04) | <0.001 | −2.79(−3.59 – −1.98) | <0.001 |
| Blood control Method | |||||
| Non cautery | 3959/5152 | 1.00 (referent) | 1.00 (referent) | ||
| Cautery | 1193/5152 | 4.37(1.58 – 7.16) | 0.002 | −3.99(−6.95– −1.03) | 0.008 |
| Marital Status | |||||
| Not married | 3959/5152 | 1.00 (referent) | --- | --- | |
| Married | 1193/5152 | −0.11(−0.48 – 0.27) | 0.576 | --- | --- |
| Age of participant | 5152/5152 | −0.01(−0.02 – 0.00) | 0.083 | −0.01(−0.02 – −0.00) | 0.007 |
Table 3 shows the rates of moderate and severe AEs. Physicians experienced a higher rate of AEs (1.5%) than clinical officers (0.6%, p=0.007). AE rates were also higher with sleeve resection (1.34%) than dorsal slit (0.6%, p=0.01), and cautery was associated with higher AE rates (1.9%) than hemostasis by other methods (0.7%, p=0.0003). Both bleeding and dehiscence were more common with cautery (Table 3). In univariate analyses, provider, procedure and mode of hemosatasis were significantly associated with the risk of AEs (Table 4). However, in multivariate adjusted analyses, only use of cautery was significantly associated with and increased odds of a moderate/severe AEs (OR=2.13, 95%CI1.26–3.61, p=0.005).
Table 3.
Moderate/Severe Adverse Events by Provider and Procedure, and by Method of Hemostasis
| Surgery related moderate/severe AE | PROVIDER | PROCEDURE | Blood Control Method | |||
|---|---|---|---|---|---|---|
| PHYSICIAN | CLINICAL OFFICER | SLEEVE | DORSAL SLIT | Cautery | Non cautery | |
| Number of Surgeries | 1934 | 3218 | 2681 | 2471 | 1193 | 3959 |
| No. AEs(%) | No. AEs(%) | No. AEs(%) | No. AEs(%) | No. AEs(%) | No. AEs(%) | |
| Infections | 8(0.41) | 9(0.28) | 12(0.45) | 5(0.20) | 9(0.75) | 8(0.2) |
| Bleeding | 17(0.88) | 12(0.37) | 20(0.75) | 9(0.36) | 11(0.92) | 18(0.45) |
| Wound Dehiscence | 1(0.05) | 0(0.00) | 1(0.04) | 0(0.00) | 2(0.17) | 0(0.0) |
| Dehiscence and Infection | 1(0.05) | 0(0.00) | 1(0.04) | 0(0.00) | 1(0.08) | 0(0.0) |
| Other | 2(0.10) | 1(0.03) | 2(0.07) | 1(0.04) | 1(0.08) | 2(0.05) |
| TOTAL | 29(1.50) | 22(0.68) | 36(1.34) | 15(0.61) | 23(1.93) | 28(0.71) |
Table 4.
The odds ratio of adverse events associated with age, provider, procedure, hemostasis, and surgeon’s experience
| n/N | Unadjusted analysis
|
Adjusted analysis
|
|||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| All participants | 51/5152 | ||||
| Provider Cadre | |||||
| Medical Officer | 29/1934 | 1.00 (referent) | 1.00 (referent) | ||
| Clinical Officer | 22/3218 | 0.45(0.26 – 0.76) | 0.003 | 0.87(0.57 – 1.33) | 0.525 |
| Surgical Method | |||||
| Sleeve Method | 36/2681 | 1.00 (referent) | 1.00 (referent) | ||
| Dorsal Slit | 15/2471 | 0.46(0.27 – 0.78) | 0.004 | 0.72(0.44 – 1.18) | 0.189 |
| Blood control Method | |||||
| Non cautery | 28/3959 | 1.00 (referent) | 1.00 (referent) | ||
| Cautery | 23/1193 | 2.91(1.78 – 4.75) | <0.001 | 2.13(1.26 – 3.61) | 0.005 |
| Marital Status | |||||
| Not married | 26/2925 | 1.00 (referent) | --- | --- | |
| Married | 25/2227 | 1.17(0.70 – 1.99) | 0.539 | --- | --- |
| Age of participant | 51/5152 | 0.99(0.96 – 1.02) | 0.451 | --- | --- |
| Surgical Experience*** | 51/5152 | 0.92(0.85 – 0.99) | 0.018 | 0.94(0.85 - 1.03) | 0.177 |
Surgical experience is expressed in sequence of bins of surgeries done
Discussion
This evaluation of a circumcision service program suggests that the dorsal slit method of circumcision requires less time to perform than the sleeve resection method (Figure 1.a), and that clinical officers can perform dorsal slit as efficiently as physicians with respect to surgical time (Fig. 1.d). Additionally, crude AEs rates were lower with the dorsal slit than with the sleeve resection method (Tables 2 and 3). Furthermore, although cautery reduced surgical time, it was associated with a higher rate of AEs compared to conventional methods of hemostasis (Table 4). Although circumcision time was reduced with cautery, we found an increased risk of moderate or severe adverse events, possibly because of inadequate bleeding control or overzealous use of cautery leading to necrotic tissue and infection. We conclude that dorsal slit is the preferred procedure for circumcision in this setting and that it can be safely and efficiently provided by trained clinical officers. We conclude that bipolar electrocautery may be contraindicated in this setting. This program evaluation shows that task shifting from physicians to clinical officers is safe and efficient in this setting and could help overcome shortage of physicians and contribute to reduced costs of circumcision. Our findings support those of the Kenyan MC trial9 and suggestions that non-physicians should be considered for MC programs.10
There are limitations to this study. This was an evaluation of a service program rather than a randomized trial or a pre-planned operations research study, so there is a possibility of bias and confounding. For example, physicians were over represented among sleeve circumcision providers and were the predominant users of cautery. However, we used multivariate methods to adjust for these factors,. Although it might be desirable to conduct a randomized trial, we believe that this program evaluation is sufficient to provide guidance for planning circumcision services.
The rate of circumcision related moderate and severe adverse events was 1.0%, which is lower than previously reported in our randomized trial (3.5%).11 During the trial, the clinical officers monitoring participants in this rural setting tended to be highly conservative and may have over-diagnosed possible AEs, as well as over-estimated AE severity and over-prescribed antibiotics. For example, the “infection” rate was 2.2% in the trial11, compared to 0.99% in this service program. The definition of infection was unchanged, but provision of antibiotics automatically entailed a severity grade 2 or higher. Clinical officers were prone to prescribe prophylactic antibiotics for any suspected infection during the trial, but with increasing experience and training, this overuse of antibiotics diminished during the post-trial service provision.
In summary, this evaluation of a circumcision program suggests that the dorsal slit method is preferable to the sleeve method in terms of efficiency, that task shifting from physicians to clinical officers is safe and efficient, and that the use of cautery for bleeding control is inadvisable in this rural setting.
References
- 1.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, Kigozi G, Serwadda D, Makumbi F, Watya S, Nalugoda F, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.Helen A. Weiss Male circumcision as a preventive measure against HIV and other sexually transmitted infections. Curr Opin Infect Dis. 20:66–72. doi: 10.1097/QCO.0b013e328011ab73. [DOI] [PubMed] [Google Scholar]
- 5.WHO and UNAIDS press release on recommendations for male circumcision on prevention of HIV infection. Mar 28, 2007. [Google Scholar]
- 6.WHO/UNAIDS. Operational guidance for scaling up male circumcision services for HIV prevention. [Google Scholar]
- 7.Chu K, et al. Surgical Task Shifting in Sub-Saharan Africa. PLoS Med. 6(5):e1000078. doi: 10.1371/journal.pmed.1000078.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shumbusho1 Fabienne, vanGriensven Johan, Lowrance David, Turate Innocent, Weaver Mark A, Price Jessica, Binagwaho Agnes. Task Shifting for Scale-up of HIV Care: Evaluation of Nurse-Centered Antiretroviral Treatment at Rural Health Centers in Rwanda. PLoS Medicine. 2009 Oct;6(10):e1000163. doi: 10.1371/journal.pmed.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krieger John N, Bailey Robert C, Opeya John C, Ayieko Benard O, Opiyo Felix A, Omondi Dickens, Agot Kawango, Parker Corette, Ndinya-Achola JO, Moses Stephen. Adult Male Circumcision Outcomes: Experience in a Developing Country Setting. Urol Int. 2007;78:235–240. doi: 10.1159/000099344. [DOI] [PubMed] [Google Scholar]
- 10.Shumbusho1 Fabienne, vanGriensven Johan, Lowrance David, Turate Innocent, Weaver Mark A, Price Jessica, Binagwaho Agnes. Task Shifting for Scale-up of HIV Care: Evaluation of Nurse-Centered Antiretroviral Treatment at Rural Health Centers in Rwanda. PLoS Medicine. 2009 Oct;6(10):e1000163. doi: 10.1371/journal.pmed.1000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kigozi Godfrey, et al. The Safety of Adult Male Circumcision in HIV Infected and Uninfected Men in Rakai. Uganda PLoS Medicine. 2008 Jun;5(6):e116. doi: 10.1371/journal.pmed.0050116. www.plosmedicine.org0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valerian Kiggundu, et al. Number of procedures required to achieve optimal competency with male circumcision: Findings from a randomized trial in Rakai. Uganda BJU int. 2009 Aug;104(4):529–532. doi: 10.1111/j.1464-410X.2009.08420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]