Abstract
Background
Bullous pemphigoid has been reported in association with neurologic disorders.
Objective
To analyze the association between bullous pemphigoid and neurologic disorders.
Methods
We retrospectively identified residents of Olmsted County, Minnesota, with a first lifetime diagnosis of bullous pemphigoid between January 1, 1960, and December 31, 2009. Three age- and sex-matched Olmsted County residents without bullous pemphigoid were selected as controls for each patient. We compared history of or development of neurologic disorders (dementia, Alzheimer disease, Parkinson disease, multiple sclerosis, cerebrovascular disease, and seizures) between groups using case-control and cohort designs.
Results
A total of 87 patients with bullous pemphigoid were identified and matched to 261 controls. The odds of a previous diagnosis of any neurologic disorder or a history of dementia were significantly increased among cases compared with controls (odds ratios: 6.85 (3.00–15.64); P<.001, and 6.75 (2.08–21.92); P=.002, respectively). Both Parkinson disease (hazard ratio, 8.56 (1.55–47.25); P=.01) and any type of neurologic disorder (hazard ratio, 2.02 (1.17–3.49); P=.01) were significantly more likely to develop during follow-up in patients with bullous pemphigoid than in those without bullous pemphigoid.
Limitations
Small geographic area; retrospective study design.
Conclusion
Our study confirmed an association of bullous pemphigoid with neurologic disorders, especially dementia and Parkinson disease.
Keywords: bullous diseases, bullous pemphigoid, epidemiology, immunobullous, immunodermatology, pemphigoid
Introduction
Bullous pemphigoid (BP) is an acquired cutaneous blistering disorder associated with circulating autoantibodies against basement membrane zone hemidesmosomal proteins BP180 (BP antigen 2 or type XVII collagen) and BP230 (BP antigen 1) (1). Although variable in presentation, BP is typically characterized clinically by pruritus and tense cutaneous bullae on an erythematous background. Diagnosis of BP can be confirmed by the linear deposition of antibodies (IgG and/or C3) using direct immunofluorescence or by the detection of circulating autoantibodies against basement membrane proteins BP180 and/or BP230 via indirect immunofluorescence or enzyme-linked immunoabsorbent assays (2).
Prior studies have confirmed an increased incidence of BP in elderly patients (3–5), but the prevalence of antibodies to BP180 and BP230 is low in the unaffected population (6). The reasons both for the development of BP180 and BP230 antibodies and for the presence of antibodies in the absence of BP are unclear. Numerous case reports have linked BP with various neurologic disorders, and there are multiple descriptions of localization of BP to sites of neurologic injury in patients with hemiparesis (7,8). The hypothesis of a potential association and etiologic link between neurologic disease and BP has sparked increasing interest, especially given that BP230 has similar isoforms in neuronal and cutaneous tissues (9,10). In addition, there is evidence of cross-recognition of patient sera containing cutaneous BP230 with neuronal tissue in animal models (11). There is also evidence of IgG antibodies against the epidermal basement membrane zone via indirect immunofluorescence in 33% of serum samples from patients with multiple sclerosis with subsequent development of BP (12). Several retrospective studies have continued to support a possible association between BP and various neurologic disorders, including dementia, stroke, Parkinson disease, and multiple sclerosis (13–17).
The prevalence and the incidence of neurodegenerative disease have been increasing in recent decades as a result of the aging population (18–20), and the recognition of autoimmune-related dementias is increasing; both of these factors are prompting further investigation of the association between autoimmunity and dementia (21). To better understand the association between BP and neurologic disorders, we conducted a 50-year population-based study on the co-occurrence of neurologic disorders in patients with BP in one Midwestern county.
Methods
This study was approved by the institutional review boards of Olmsted Medical Center and Mayo Clinic. We searched the databases of the Rochester Epidemiology Project for patients who were residents of Olmsted County, Minnesota, at their first lifetime diagnosis of BP (using the search terms “bullous pemphigoid” and “pemphigoid”) between January 1, 1960, and December 31, 2009 (22). For each case identified, we randomly selected 3 controls without BP on the basis of Olmsted County residency at index date (ie, the BP diagnosis date), age at index date within 2 years, and sex, also using the resources of the Rochester Epidemiology Project. Matching by age at index date is functionally equivalent to matching on year of birth. Olmsted County’s population is predominantly urban, with more than 70% of county residents living in the city of Rochester. The remainder of the population is suburban or rural. Extensive details about the Olmsted County population have been reported elsewhere (23,24).
Diagnostic Criteria for BP
Confirmation of BP was based on a combination of clinical and laboratory criteria including review of the clinical presentation (symptoms of pruritus, urticaria, erosions, and/or bullae) in conjunction with supporting laboratory evidence including any of the following: 1) characteristic histopathologic findings (subepidermal cleft with eosinophils), 2) direct immunofluorescence study showing linear deposition of antibody or complement (ie, IgG or C3, or both), 3) indirect immunofluorescence detecting circulating IgG antibodies against basement membrane proteins, or 4) positive BP180 or BP230 IgG antibody measured with enzyme-linked immunosorbent assay. Patients with only oral disease or predominantly oral disease were excluded to avoid including bullous diseases caused by different autoantibodies. The diagnosis was verified if the clinical symptoms of BP were associated with a positive laboratory confirmation with 1 or more of the above modalities (2). Date of diagnosis was recorded as the first date the diagnosis appeared in the medical record. Localized disease was characterized as single-site disease (eg, scalp, neck, limbs, or chest), and generalized disease was characterized as involvement at more than 1 site.
Definition of Neurologic Disorders
During the medical record review, presence of a neurologic disorder at any point in time was recorded. In particular, we collected data on the presence of a diagnosis of dementia of any type, parkinsonism, multiple sclerosis, peripheral neuropathies, cerebrovascular events, and seizures. All medical records were also reviewed by a neurologist (R.S.) to confirm the presence of a neurologic disorder and to adjudicate the final diagnosis, according to the information available in the records. Diagnostic groupings included dementia, Parkinson disease, multiple sclerosis, cerebrovascular disease, and seizures. The diagnosis date of each disorder was recorded to determine if the disorder was present before or after the index date. Date of diagnosis was recorded as the first date the diagnosis appeared in the medical record.
Statistical Analyses
This study incorporated a case-control design and a cohort design, anchored around the diagnosis of BP. A case-control design was used to evaluate associations between neurologic disorders diagnosed before the index date and BP. The associations between a history of neurologic disorders and BP were evaluated using conditional logistic regression models and summarized with odds ratios (ORs) and 95% CIs. A cohort design was then used to evaluate the risk of neurologic disorders developing during follow-up among a cohort of patients with BP and a referent cohort of patients without BP. The risks of neurologic disorders developing during follow-up were compared between patients with and without BP using Cox proportional hazards regression models and summarized with hazard ratios (HRs) and 95% CIs. The duration of follow-up was defined from the index date to the date of the neurologic disorder of interest or the date of death or last follow-up. Proportional hazards assumptions were checked by plotting scaled Schoenfeld residuals from the Cox models against time. Survival free of a neurologic disorder was estimated using the Kaplan-Meier method. For the analyses of the risk of a neurologic disorder developing during follow-up, patients with a history of a neurologic disorder (ie, a prevalent neurologic disorder) were excluded. More specifically, if a patient with BP had a prevalent neurologic disorder, this patient and the 3 matched patients without BP in the referent cohort were excluded. If a patient without BP had a prevalent neurologic disorder, only this patient was excluded; the patient with BP and the remaining 2 matched patients in the referent cohort were included. Statistical analyses were performed using the SAS software package (SAS Institute, Inc). All tests were 2-sided, and P values less than .05 were considered statistically significant.
Results
Case-Control Study
We identified 87 patients with BP during the study period (cases). These patients were matched with 261 controls without BP. At the index date, mean age (median, range) was 77.5 years (79, 41–100) for the cases and 77.4 years (79, 40–101) for the controls. Both groups had more women than men (57% vs 43%).
Our case-control analysis indicated that the odds of a history of dementia were nearly 7 times higher among BP cases compared with controls (OR, 6.75 (2.08–21.92); P=.002) (Table 1). The odds of a history of any type of neurologic disorder were also significantly higher among BP cases than controls (OR, 6.85 (3.00–15.64); P<.001). Of note, 1 patient with BP had a history of both dementia and cerebrovascular disease.
Table 1.
History of Neurologic Disorders Among Cases With BP and Controls
| Neurologic Disorder | Controlsa (n=261) |
Casesa (n=87) |
OR (95% CI)b |
P Value |
|---|---|---|---|---|
| Dementia | 4 (2) | 9 (10) | 6.75 (2.08–21.92) | .002 |
| Parkinson disease | 1 (<1) | 3 (3) | 9.00 (0.94–86.52) | .06 |
| MS | 1 (<1) | 1 (1) | 3.00 (0.19–47.96) | .44 |
| Cerebrovascular disease | 5 (2) | 5 (6) | 3.00 (0.87–10.36) | .08 |
| Seizures | 0 | 3 (3) | 11.54 (1.24– infinity)c | .01 |
| Any of the above | 11 (4) | 20 (23) | 6.85 (3.00–15.64) | <.001 |
| Dementia or Parkinson disease | 5 (2) | 12 (14) | 8.55 (2.75–26.63) | <.001 |
| Dementia, Parkinson disease, or MS | 6 (2) | 13 (15) | 7.45 (2.65–20.97) | <.001 |
| Dementia, Parkinson disease, MS, or cerebrovascular disease | 11 (4) | 17 (20) | 5.74 (2.46–13.40) | <.001 |
Abbreviations: BP, bullous pemphigoid; MS, multiple sclerosis; OR, odds ratio.
Values are No. of patients (%).
Odds of a history of the neurologic disorder among BP cases compared with matched controls.
OR, 95% CI, and P value estimated using exact conditional logistic regression.
Eighty-five of the 87 BP cases had data regarding the extent of disease; of these, 12 (14%) had localized disease and 73 (86%) had generalized disease. When the subset of cases with generalized BP was evaluated further, along with their matched controls, the odds of a history of seizure were 11 times higher (OR, 11.54 (1.24-infinity); P=.03) and the odds of a history of dementia were 9 times higher (OR, 9.00 (2.44–33.24); P=.001) among BP cases than controls (Table 2). Odds of dementia combined with any other neurologic disorder were also significantly higher among BP cases (all P<.001).
Table 2.
History of Neurologic Disorders Among Cases With Generalized BP and Controls
| Neurologic Disorder | Controlsa (n=219) |
Casesa (n=73) |
OR (95% CI)b |
P Value |
|---|---|---|---|---|
| Dementia | 3 (1) | 9 (12) | 9.00 (2.44–33.24) | .001 |
| Parkinson disease | 0 | 2 (3) | 7.24 (0.56-infinity)c | .13 |
| MS | 1 (<1) | 1 (1) | 3.00 (0.19–47.96) | .44 |
| Cerebrovascular disease | 5 (2) | 3 (4) | 1.80 (0.43–7.53) | .42 |
| Seizures | 0 | 3 (4) | 11.54 (1.24-infinity)c | .03 |
| Any of the above | 9 (4) | 17 (23) | 6.79 (2.80–16.45) | <.001 |
| Dementia or Parkinson disease | 3 (1) | 11 (15) | 11.00 (3.07–39.43) | <.001 |
| Dementia, Parkinson disease, or MS | 4 (2) | 12 (16) | 9.00 (2.90–27.91) | <.001 |
| Dementia, Parkinson disease, MS, or cerebrovascular disease | 9 (4) | 14 (19) | 5.52 (2.21–13.76) | <.001 |
Abbreviations: BP, bullous pemphigoid; MS, multiple sclerosis; OR, odds ratio.
Values are No. of patients (%).
Odds of a history of neurologic disorder among generalized BP cases compared with matched controls.
OR, 95% CI, and P value estimated using exact conditional logistic regression.
Cohort Study
We subsequently analyzed the risks of a neurologic disorder developing during follow-up in the cohort of patients with BP compared with the referent cohort of patients without BP (Table 3). For the analysis of dementia, the 9 patients with BP with a history of dementia, the corresponding 27 matched patients without BP in the referent cohort, and the 4 patients without BP with a history of dementia were excluded. The risk of dementia developing during follow-up was not significantly different between patients with and without BP (HR, 1.25 (0.61–2.55); P=.54). Patients with BP were significantly more likely to have development of Parkinson disease (HR, 8.56 (1.55–47.25); P=.01), as well as any type of neurologic disorder (HR, 2.02 (1.17-3.49); P=.01), during follow-up than were patients without BP. Survival free of the development of any neurologic disorder in patients with and without BP is shown in the Figure. An HR for multiple sclerosis could not be calculated because this disorder did not develop in any patients with BP during follow-up.
Table 3.
Risk of Neurologic Disorders Developing During Follow-Up Among Patients With and Without BP
| Neurologic Disorder | Patients Without BPa |
Patients With BPa |
HR (95% CI)b |
P Value |
|---|---|---|---|---|
| Dementia | 34/230 (15) | 10/78 (13) | 1.25 (0.61–2.55) | .54 |
| Parkinson disease | 2/251 (1) | 4/84 (5) | 8.56 (1.55–47.25) | .01 |
| MS | 1/257 (<1) | 0/86 (0) | NA | .64c |
| Cerebrovascular disease | 10/241 (4) | 6/82 (7) | 2.54 (0.92–7.05) | .07 |
| Seizures | 1/252 (<1) | 1/84 (1) | 5.56 (0.35–88.93) | .23 |
| Any of the above | 41/192 (21) | 19/67 (28) | 2.02 (1.17–3.49) | .01 |
| Dementia or Parkinson disease | 36/220 (16) | 14/75 (19) | 1.64 (0.88–3.06) | .12 |
| Dementia, Parkinson disease, or MS | 37/216 (17) | 14/74 (19) | 1.58 (0.85–2.95) | .15 |
| Dementia, Parkinson disease, MS, or cerebrovascular disease | 43/201 (21) | 19/70 (27) | 1.90 (1.10–3.26) | .02 |
Abbreviations: BP, bullous pemphigoid; HR, hazard ratio; MS, multiple sclerosis; NA, not applicable.
Values are No. of patients/No. of patients assessed (%).
Risk of neurologic disorder developing during follow-up for patients with BP compared with patients without BP.
Log-rank test.
Figure 1.
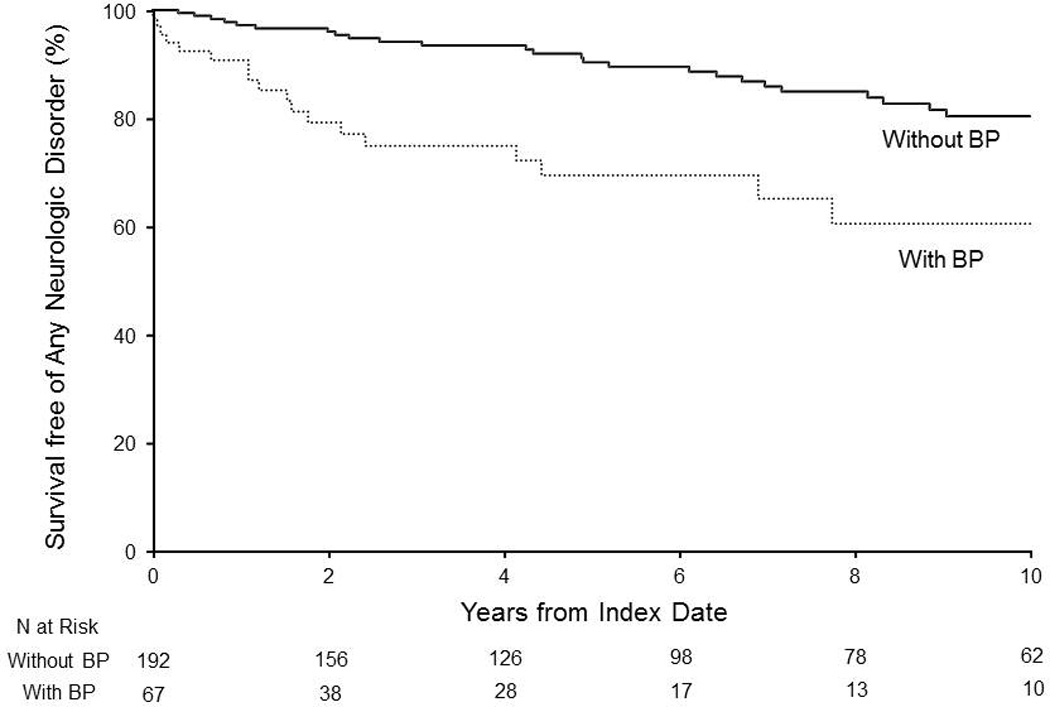
Kaplan-Meier curves illustrating survival free of the development of any neurologic disorder in patients with and without bullous pemphigoid (BP).
The risks of a neurologic disorder developing during follow-up for patients with generalized BP and the matched patients without BP in the referent cohort are summarized in Table 4.
Table 4.
Risk of Neurologic Disorders Developing During Follow-Up Among Patients With and Without Generalized BP
| Neurologic Disorder | Patients Without BPa |
Patients With BPa |
HR (95% CI)b |
P Value |
|---|---|---|---|---|
| Dementia | 28/189 (15) | 9/64 (14) | 1.29 (0.60–2.75) | .51 |
| Parkinson disease | 2/213 (1) | 4/71 (6) | 8.44 (1.53–46.56) | .01 |
| MS | 1/215 (<1) | 0/72 (0) | NA | .64c |
| Cerebrovascular disease | 9/205 (4) | 4/70 (6) | 1.78 (0.54–5.81) | .34 |
| Seizures | 1/210 (<1) | 1/70 (1) | 5.30 (0.33–84.84) | .24 |
| Any of the above | 35/161 (22) | 16/56 (29) | 1.89 (1.04–3.42) | .04 |
| Dementia or Parkinson disease | 30/183 (16) | 13/62 (21) | 1.76 (0.91–3.39) | .09 |
| Dementia, Parkinson disease, or MS | 31/179 (17) | 13/61 (21) | 1.69 (0.88–3.25) | .12 |
| Dementia, Parkinson disease, MS, or cerebrovascular disease | 37/170 (22) | 16/59 (27) | 1.78 (0.99–3.21) | .05 |
Abbreviations: BP, bullous pemphigoid; HR, hazard ratio; MS, multiple sclerosis; NA, not applicable.
Values are No. of patients/No. of patients assessed (%).
Risk of neurologic disorder developing during follow-up for patients with generalized BP compared with patients without BP.
Log-rank test.
Discussion
We observed that BP cases were significantly more likely to have dementia than were controls, which has been reported in previous studies (15,16,25,26). Although a prior diagnosis of Parkinson disease or cerebrovascular disease was not found to be more likely in either group, we observed a significant risk of Parkinson disease developing subsequent to the diagnosis of BP.
Several studies have shown an association between BP and various neurologic disorders, but the specific subset of these disorders has varied. In a 2005 retrospective review of hospitalized patients with BP, Stinco et al (14) observed significant associations of BP with multiple sclerosis and Parkinson disease. Cordel et al (15) found that 36% of patients with BP had a neurologic disorder, with dementia, cerebral stroke, Parkinson disease, or a combination of these, being most prevalent. One prior case-control study reviewed numerous medical diagnoses in patients with BP and found neurologic disease in 42.7% of the affected subjects (vs in 19.1% of controls), with the most frequent associations being cerebral stroke in men and dementia in women (25). Another population-based case-control study by Langan et al (16) demonstrated that pre-existing diagnoses of dementia, Parkinson disease, epilepsy, or multiple sclerosis were significantly more likely to be found in cases with BP than in controls. The French Study Group for Bullous Diseases also found an association of dementia and Parkinson disease with BP (26).
All of the evidence to date points toward a relationship between neurologic disorders and BP, but the specific linking factors are yet to be determined. To investigate a possible physiologic basis for this link, Foureur et al (27) specifically compared the presence of antibodies to BP antigen 2 (BP180) in elderly subjects with and without BP. They found that, compared with age- and sex-matched controls, the presence of BP180 antibodies in patients with BP was associated with the diagnosis of dementia. Neurologic disorders have even been postulated as a cause of increasing mortality secondary to BP, although the mechanism underlying this proposition is unclear (28,29). One hypothesis is that those with BP and concomitant or subsequent neurologic disease have more severe disease on either front, leading to increased mortality while also favoring increased autoantibody cross-reactivity between the skin and brain. Indeed, Fichel et al (30) recently found that patients with more extensive disease at initial presentation and those with dementia had a greater risk of relapse.
Cross-reaction of autoantibodies toward similar antigens in the skin and brain has been proposed as an explanation for the association between BP and neurologic disease. The skin and neurons are both derived from the ectoderm, and the same hemidesmosomal protein antigens involved in the pathogenesis of BP are also expressed in human neurons (9,10). With regard to BP antigen 1 (BP230), a majority (54.5%) of serum samples from patients with BP and neurologic disease recognized a 230-kDa protein of human brain extract (31). Another study also showed that both human skin and brain contain immunogenic BP230 in patients with neurologic disease and BP (32).
There are several limitations to our study. Notably, the diagnoses of dementia were unable to be further subclassified (eg, as Alzheimer disease vs dementia with Lewy bodies vs frontotemporal dementia vs other) given the retrospective nature of our study. The date of diagnosis was dependent on the initial date of diagnosis as listed in the medical record. This may not accurately represent the date of onset of disease because the time from the first presenting symptoms to a clinical diagnosis may vary. Additionally, this is a study population from a small geographic area that may not be generalizable to all populations.
Despite these limitations, our results further support the evidence for a link between BP and certain neurologic disorders, particularly dementia and Parkinson disease. Further studies detailing the direct or indirect etiologic associations between neurologic disorders and BP may influence future disease diagnosis, prevention, and treatment.
Capsule Summary.
Bullous pemphigoid has been described in association with neurologic disorders.
Parkinson disease and dementia are significantly associated with bullous pemphigoid.
Recognition of these associations may improve our diagnostic abilities and understanding of disease etiology.
Acknowledgments
Funding: The Rochester Epidemiology Project supported this project (R01-AG034676; Principal Investigators: Walter A. Rocca, MD, MPH, and Barbara P. Yawn, MD, MSc).
Abbreviations
- BP
bullous pemphigoid
- HR
hazard ratio
- OR
odds ratio
Footnotes
Conflict of interest: The authors state no conflict of interest.
References
- 1.Jordon RE, Beutner EH, Witebsky E, Blumental G, Hale WL, Lever WF. Basement zone antibodies in bullous pemphigoid. JAMA. 1967 May 29;200(9):751–756. [PubMed] [Google Scholar]
- 2.Vaillant L, Bernard P, Joly P, Prost C, Labeille B, Bedane C, et al. French Bullous Study Group. Evaluation of clinical criteria for diagnosis of bullous pemphigoid. Arch Dermatol. 1998 Sep;134(9):1075–1080. doi: 10.1001/archderm.134.9.1075. [DOI] [PubMed] [Google Scholar]
- 3.Bernard P, Vaillant L, Labeille B, Bedane C, Arbeille B, Denoeux JP, et al. Bullous Diseases French Study Group. Incidence and distribution of subepidermal autoimmune bullous skin diseases in three French regions. Arch Dermatol. 1995 Jan;131(1):48–52. [PubMed] [Google Scholar]
- 4.Serwin AB, Bokiniec E, Piascik M, Masny D, Chodynicka B. Epidemiological and clinical analysis of pemphigoid patients in northeastern Poland in 2000–2005. Med Sci Monit. 2007 Aug;13(8):CR360–CR364. [PubMed] [Google Scholar]
- 5.Jung M, Kippes W, Messer G, Zillikens D, Rzany B. Increased risk of bullous pemphigoid in male and very old patients: a population-based study on incidence. J Am Acad Dermatol. 1999 Aug;41(2 Pt 1):266–268. doi: 10.1016/s0190-9622(99)70061-7. [DOI] [PubMed] [Google Scholar]
- 6.Wieland CN, Comfere NI, Gibson LE, Weaver AL, Krause PK, Murray JA. Anti-bullous pemphigoid 180 and 230 antibodies in a sample of unaffected subjects. Arch Dermatol. 2010 Jan;146(1):21–25. doi: 10.1001/archdermatol.2009.331. [DOI] [PubMed] [Google Scholar]
- 7.Foureur N, Descamps V, Lebrun-Vignes B, Picard-Dahan C, Grossin M, Belaich S, et al. Bullous pemphigoid in a leg affected with hemiparesia: a possible relation of neurological diseases with bullous pemphigoid? Eur J Dermatol. 2001 May-Jun;11(3):230–233. [PubMed] [Google Scholar]
- 8.Tsuruta D, Nishikawa T, Yamagami J, Hashimoto T. Unilateral bullous pemphigoid without erythema and eosinophil infiltration in a hemiplegic patient. J Dermatol. 2012 Sep;39(9):787–789. doi: 10.1111/j.1346-8138.2012.01562.x. Epub 2012 Apr 16. [DOI] [PubMed] [Google Scholar]
- 9.Seppanen A, Autio-Harmainen H, Alafuzoff I, Sarkioja T, Veijola J, Hurskainen T, et al. Collagen XVII is expressed in human CNS neurons. Matrix Biol. 2006 Apr;25(3):185–138. doi: 10.1016/j.matbio.2005.11.004. Epub 2006 Jan 4. [DOI] [PubMed] [Google Scholar]
- 10.Claudepierre T, Manglapus MK, Marengi N, Radner S, Champliaud MF, Tasanen K, et al. Collagen XVII and BPAG1 expression in the retina: evidence for an anchoring complex in the central nervous system. J Comp Neurol. 2005 Jun 27;487(2):190–203. doi: 10.1002/cne.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown A, Bernier G, Mathieu M, Rossant J, Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat Genet. 1995 Jul;10(3):301–306. doi: 10.1038/ng0795-301. [DOI] [PubMed] [Google Scholar]
- 12.Peramiquel L, Barnadas MA, Pimentel CL, Garcia Muret MP, Puig LL, Gelpi C, et al. Bullous pemphigoid and multiple sclerosis: a report of two cases with ELISA test. Eur J Dermatol. 2007 Jan-Feb;17(1):62–66. doi: 10.1684/ejd.2007.0189. Epub 2007 Feb 27. [DOI] [PubMed] [Google Scholar]
- 13.Taghipour K, Chi CC, Bhogal B, Groves RW, Venning V, Wojnarowska F. Immunopathological characteristics of patients with bullous pemphigoid and neurological disease. J Eur Acad Dermatol Venereol. 2013 Mar 26; doi: 10.1111/jdv.12136. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Stinco G, Codutti R, Scarbolo M, Valent F, Patrone P. A retrospective epidemiological study on the association of bullous pemphigoid and neurological diseases. Acta Derm Venereol. 2005;85(2):136–139. doi: 10.1080/00015550410024481. [DOI] [PubMed] [Google Scholar]
- 15.Cordel N, Chosidow O, Hellot MF, Delaporte E, Lok C, Vaillant L, et al. French Study Group of Bullous Diseases. Neurological disorders in patients with bullous pemphigoid. Dermatology. 2007;215(3):187–191. doi: 10.1159/000106574. [DOI] [PubMed] [Google Scholar]
- 16.Langan SM, Groves RW, West J. The relationship between neurological disease and bullous pemphigoid: a population-based case-control study. J Invest Dermatol. 2011 Mar;131(3):631–636. doi: 10.1038/jid.2010.357. Epub 2010 Nov 18. [DOI] [PubMed] [Google Scholar]
- 17.Chen YJ, Wu CY, Lin MW, Chen TJ, Liao KK, Chen YC, et al. Comorbidity profiles among patients with bullous pemphigoid: a nationwide population-based study. Br J Dermatol. 2011 Sep;165(3):593–599. doi: 10.1111/j.1365-2133.2011.10386.x. Epub 2011 Jul 28. [DOI] [PubMed] [Google Scholar]
- 18.Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013 Nov;70(11):1396–1402. doi: 10.1001/jamaneurol.2013.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology. 2010 Sep 7;75(10):889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong EL, Goldacre R, Taghipour K. The relationship between motor neuron disease and bullous pemphigoid: an English cohort study. J Am Acad Dermatol. 2013 Nov;69(5):836–837. doi: 10.1016/j.jaad.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan EP, McKeon A, Lennon VA, Boeve BF, Trenerry MR, Tan KM, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010 Oct;85(10):881–897. doi: 10.4065/mcp.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brick KE, Weaver CH, Lohse CM, Pittelkow MR, Lehman JS, Camilleri MJ, et al. Incidence of bullous pemphigoid and mortality of patients with bullous pemphigoid in Olmsted County, Minnesota, 1960 through 2009. J Am Acad Dermatol. 2014 Apr 3; doi: 10.1016/j.jaad.2014.02.030. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011 May 1;173(9):1059–1068. doi: 10.1093/aje/kwq482. Epub 2011 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012 Feb;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jedlickova H, Hlubinka M, Pavlik T, Semradova V, Budinska E, Vlasin Z. Bullous pemphigoid and internal diseases: a case-control study. Eur J Dermatol. 2010 Jan-Feb;20(1):96–101. doi: 10.1684/ejd.2010.0805. Epub 2009 Oct 2. [DOI] [PubMed] [Google Scholar]
- 26.Bastuji-Garin S, Joly P, Lemordant P, Sparsa A, Bedane C, Delaporte E, et al. French Study Group for Bullous Diseases. Risk factors for bullous pemphigoid in the elderly: a prospective case-control study. J Invest Dermatol. 2011 Mar;131(3):637–643. doi: 10.1038/jid.2010.301. Epub 2010 Oct 14. [DOI] [PubMed] [Google Scholar]
- 27.Foureur N, Mignot S, Senet P, Verpillat P, Picard-Dahan C, Crickx B, et al. [Correlation between the presence of type-2 anti-pemphigoid antibodies and dementia in elderly subjects with no clinical signs of pemphigoid] Ann Dermatol Venereol. 2006 May;133(5 Pt 1):439–443. doi: 10.1016/s0151-9638(06)70935-8. French. [DOI] [PubMed] [Google Scholar]
- 28.Cortes B, Marazza G, Naldi L, Combescure C, Borradori L Autoimmune Bullous Disease Swiss Study Group. Mortality of bullous pemphigoid in Switzerland: a prospective study. Br J Dermatol. 2011 Aug;165(2):368–374. doi: 10.1111/j.1365-2133.2011.10413.x. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Zuo YG, Zheng HY. Mortality of bullous pemphigoid in China. JAMA Dermatol. 2013 Jan;149(1):106–108. doi: 10.1001/archdermatol.2012.2994. [DOI] [PubMed] [Google Scholar]
- 30.Fichel F, Barbe C, Joly P, Bedane C, Vabres P, Truchetet F, et al. Clinical and immunologic factors associated with bullous pemphigoid relapse during the first year of treatment: a multicenter, prospective study. JAMA Dermatol. 2014 Jan;150(1):25–33. doi: 10.1001/jamadermatol.2013.5757. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Li L, Chen J, Zeng Y, Xu H, Song Y, Wang B. Sera of elderly bullous pemphigoid patients with associated neurological diseases recognize bullous pemphigoid antigens in the human brain. Gerontology. 2011;57(3):211–216. doi: 10.1159/000315393. [DOI] [PubMed] [Google Scholar]
- 32.Seppanen A, Suuronen T, Hofmann SC, Majamaa K, Alafuzoff I. Distribution of collagen XVII in the human brain. Brain Res. 2007 Jul 16;1158:50–56. doi: 10.1016/j.brainres.2007.04.073. Epub 2007 May 6. [DOI] [PubMed] [Google Scholar]


