Abstract
Background
The conventional practice of analyzing overall age-adjusted cancer mortality rates heavily emphasizes the experience of older, higher mortality age groups. This may conceal shifts in lifetime cancer mortality experience emerging first in younger age groups.
Methods
We examined age-specific cancer mortality rates and birth-cohort-specific cancer mortality rates in US mortality data recorded since 1955 to assess the effects of age, period and cohort in secular mortality trends. Cancer mortality and population data were obtained from the World Health Organization's Statistical Information System (WHOSIS).
Findings
Age-specific cancer mortality rates have been steadily declining in the U.S. since the early 1950's, beginning with children and young adults and now including all age groups. During the second half of the 20th century, each successive decade of births from 1925 - 1995 experienced a lower risk of cancer death than its predecessor at virtually every age for which such a comparison can be made.
Conclusions
A major decline in cancer mortality has been occurring in the U.S. for the past fifty years, affecting birth cohorts born as long as eighty years ago. Excepting lung cancer, much of this decline has occurred despite relatively stable cancer incidence. These findings suggest that improvements in cancer detection, treatment, and/or prevention have reduced the risk of cancer death across the lifespan for individuals born in the last three-quarters of the 20th century.
Keywords: Cohort analysis; Cancer mortality; Cancer surveillance and screening; Methodology, modeling, and biostatistics
INTRODUCTION
Cancer mortality and incidence statistics are nearly universally age adjusted—and on sound theoretical grounds. Without age adjustment the aging of the population as a whole will tend to upwardly bias trends in incidence and mortality; furthermore, age adjustment permits comparison of subgroups (e.g. gender or racial subgroups or geographic regions) that differ in age distribution (1). Secular trends in age-adjusted cancer mortality rates were central to decennial reviews of national cancer mortality rates in the US in the 20th century (2, 3) that raised concerns about progress in the war against cancer and the balance of investment in research into prevention, early detection and treatment. These analyses have inspired energetic discussion in both the medical and lay literature (4-7), with, on balance, more pessimism than optimism. More recent annual reports on the status of cancer from the American Cancer Society, the U.S. Centers for Disease Control and Prevention, the U.S. National Cancer Institute (NCI), and the North American Association of Central Cancer Registries have been more encouraging, documenting progress against cancer in noting that age-adjusted cancer mortality rates began to decline in the mid 1990's (8-12).
However, because the preponderance of cancer mortality occurs in older age groups, analysis of summary age-adjusted rates heavily emphasizes the experience of these groups— most of whom were born around the turn of the 20th century. Age-adjustment can thus easily obscure the emergence of trends among those born more recently. An alternative to age-adjustment is to examine age- and birth-cohort-specific cancer mortality.
We hypothesized that efforts in prevention, early diagnosis, and/or treatment have been having effects on cancer mortality risk in the U.S. that go back much farther than the 1990s, but that these benefits were attained first and foremost by younger individuals. As a result, this progress would not be reflected in overall age-adjusted mortality rates until long after it began. To test this hypothesis, we conducted age- and birth-cohort-specific analyses of all-site cancer rates. The utility of birth cohort analysis has long been recognized (13-17), and has been applied to cancer mortality rates in the past (18, 19), as has age stratification (19, 20). To our knowledge recent applications of birth cohort analysis in the context of cancer mortality have been limited to individual cancer sites both in the U.S. (e.g. 21) and in other countries (e.g. 22). We are aware of no age- and birth-cohort specific analyses of all-site cancer mortality in the U.S. published in the peer review literature within the past 20 years.
METHODS
Mortality data from 1955 to 2004, as reported by the U.S. National Center for Health Statistics (NCHS), was obtained from the World Health Organization's Statistical Information System (WHOSIS). Annual U.S. population estimates for this same period were also obtained from WHOSIS. Multiple coding systems were in place at different points during the time span studied (ICD7-ICD10). Table 1 lists the codes (both WHOSIS and their ICD equivalents) used from each system for calculating cancer mortality rates.
Table 1.
WHOSIS codes, and corresponding ICD codes, used in tabulating all-site mortality rates for malignant neoplasms.
| WHOSIS | |||
|---|---|---|---|
| Years | Coding System | Codes | ICD Codes |
| 1955-1967 | ICD7-A List (condensed) | A044-A059 | 140-148, 150-165, 170-181, 190-205 |
| 1968-1978 | ICD8-A List (condensed) | A045-A060 | 140-163, 170-174, 180-209 |
| 1979-1998 | ICD9 Basic Tabulation List | B08-B14* | 140-165, 170-175, 179-208 |
| 1999-2004 | ICD10 Mortality Tabulation List 1 | 1027-1045 | C00-C95 |
All rates were reported in five year age intervals ranging from 0 to 85 years of age. We calculated mortality rates for each 5 year age group by year of death and by 10-year birth cohort. Year of birth was estimated by subtracting age at death from year of death. Since age at death is expressed in five year intervals in the databases used for this analysis, age at death was approximated as the midpoint of the interval. All rates were log-transformed prior to plotting to allow clear visualization of trends in all age groups despite the wide range of values from youngest to oldest individuals.
While important shifts in the mortality trends plotted by age and birth-cohort seemed subjectively obvious to us upon inspection, we desired to quantify the contributions of age, period, and cohort more systematically as well. It is well known that identification of the independent contributions of age, period, and birth-cohort to secular trends is refractory to common methods of analysis such as multivariate regression. The reason for this is that given any two of these factors (age, period, and cohort), the third may be calculated directly and therefore they are confounded (23, 24). A Bayesian solution has been proposed which uses the data itself together with a set of a priori assumptions to identify the most probably relative contributions of age, period, and cohort (25, 26). We chose to utilize this approach, previously employed by others (27-31) and implemented in the BAMP software package (26), to quantitatively assess age, period, and cohort effects in cancer mortality. The first a priori assumption this approach requires is that the risk ratios for each effect (e.g. “age”) sum to zero over the observed interval. The second assumption required by the model is that the effects tend to be constant, such that small deviations from constant rate are favored over large ones. As a result of these assumptions the absolute magnitude of each effect cannot be identified with certainty by the model. However, this approach does allow us to identify second order differences: the most probable direction of change in rate as well as the inflection points in rate. In addition, the analysis requires starting parameters (“hyperpriors”) to be estimated (for the gamma distribution used to model the probabilities) from which the model will then attempt to converge to the “true” values. We intentionally chose highly non-informative starting parameters for the gamma prior distribution (1 and 0.0005) to avoid imposing assumptions for which we had no prior knowledge. Furthermore, the software discarded the first 5000 iterations of the Monte Carol simulation as a “burn in” to minimize the influence of starting values. Finally, we repeated the analysis across a wide range of starting values for the gamma prior distribution (an order of magnitude or more) and found that the selection of starting values did not substantively alter the results of the analysis.
We made use of annual percent change (APC) estimates (3, 32) to compare cumulative age adjusted rates and age stratified rates. To estimate the APC for each successive five year interval, we first fit a linear regression model to the natural log-transformed mortality rates for the given interval to obtain the maximum likelihood estimate of the slope, β. Estimated APC was then calculated as (eβ - 1) * 100. The APC calculated in this manner for age adjusted cumulative mortality rates is denoted as APCw, since age adjusted cumulative mortality rates represent the average rate across age groups weighted by the number of at-risk persons in each age group. For comparison, we also calculated the APC for the unweighted average rate across age groups in each interval, which we denoted APCu. Where rates are changing homogenously across age groups, APCw and APCu will be the same. Where rates of change in mortality are biased with respect to age, these two rates will diverge.
RESULTS
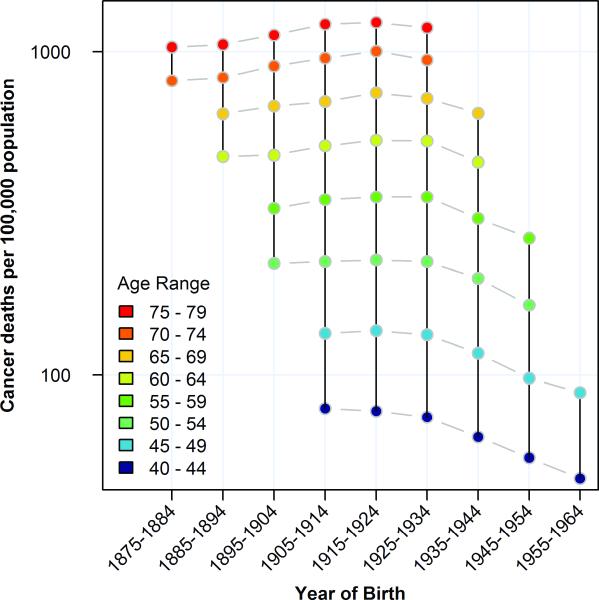
Age-specific cancer mortality rates were calculated by estimated year of birth to enable direct comparison of birth-cohorts. When these mortality rates are plotted with year of birth on the x-axis, the age-specific mortality rates for a given cohort align vertically (solid black vertical lines, figure 1). By comparing two columns of points, shifts in the lifetime mortality experience of two cohorts can be identified (gray dashes, figure 1). Thus we see that from 1875 to 1924, each birth cohort exhibited cancer mortality rates across the observed portions of the lifespan that were higher than or equivalent to (observed portions of) the preceding birth cohort. Then beginning with the cohort of individuals born after 1925, each subsequent birth cohort has experienced decreasing cancer mortality throughout the observed portion of the lifespan. In figure 1, we present only age groups for whom data were available spanning the two decadal birth cohorts (1915-1934) in which mortality began to decline. Mortality rates by year of birth for every available age group are shown in supplementary figure 1. Since our mortality dataset begins in 1955 and ends in 2004, not all age-specific rates are available for every birth cohort. Thus, for example, the 1925-34 birth cohort includes mortality data only for cohort members between the ages of thirty and eighty years of age.
Figure 1.
All-site cancer mortality rates at different ages by decade of birth. Mortality rates for 40-79 year olds are plotted stratified by age and plotted by year of birth.
We also analyzed age-specific mortality rates stratified by gender in light of the fact that some exposures, screening modalities, and cancers are gender specific. Mortality rates in both males (supplemental figure S2) and females (supplemental figure S3) exhibited a birth-cohort pattern similar to that of the combined data. Each cohort of women born since 1925 has experienced a progressive decrease in cancer mortality throughout the observed portion of the life span. The same is true for males.
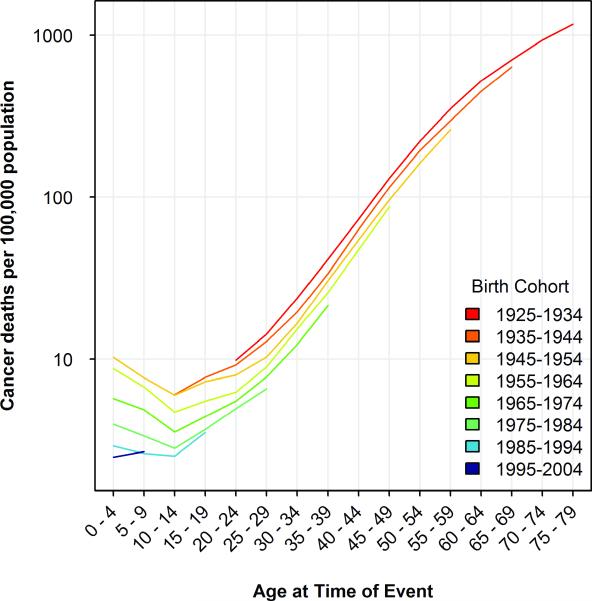
To more clearly visualize the cohort-dependent shifts in mortality, we plotted mortality rates by year of death for each cohort born between 1925 and 1994 (figure 2). Because of the time period for which we have mortality data (1955-2004), we have successively fewer cohorts to compare as age advances. Thus before age 30, we can compare six cohorts; for people in their thirties, five cohorts; and so on until age 70-79, for which data is available for only one cohort. However for nearly every comparison between cohorts that can be made, the later born cohort has lower mortality at the same age. The oldest cohort, born in 1925-1934, has a higher mortality rate than any other later born birth cohort at every age of comparison, from their early twenties until their late sixties. Subsequent birth cohorts have experienced a substantial decline in mortality. The total number of data points available for comparison between different cohorts at the same age is 59. Of these 59 adjacent age-cohort comparisons, the more recently born cohort had the lower mortality rate 57 times, with two ties (10-14 year olds in the 1935- and 1945- cohorts and the 5-9 year olds in the 1985- and 1995- cohorts—a comparison which is based on the smallest number of deaths of any comparison in the figure).
Figure 2.
All-site cancer rates in successive birth cohorts by age of death. Mortality rates for decadal birth cohorts between 1925 and 2004 are plotted by age at death.
The cohorts of individuals born in the second quarter of the 20th century have aged to the point that now every age group exhibits declining rates of cancer mortality. However, mortality rates are decreasing more rapidly among younger individuals; the average rate of decrease in cancer mortality among the youngest individuals is nearly four times greater than that of the oldest individuals (25.9% per decade vs. 6.8%).
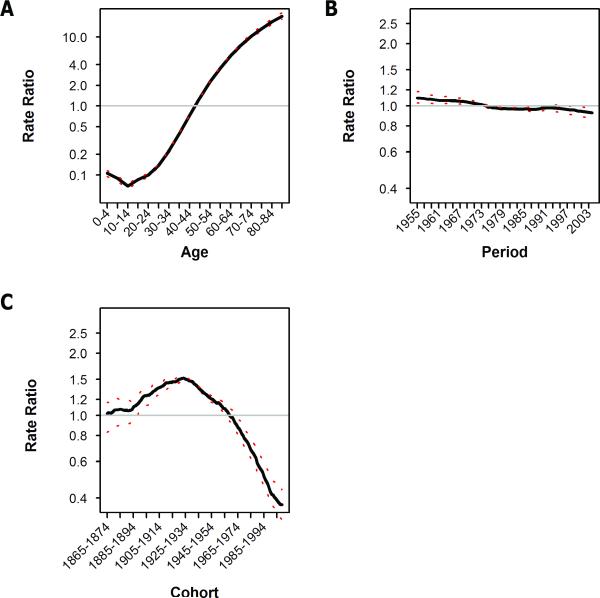
BAPC analysis was used to further characterize the relative contributions of age, period, and birth cohort effects. Cancer mortality risk falls during early childhood and then rises steadily with age (figure 2 and supplemental figure S1). The BAPC analysis identifies this pattern as well (figure 3A), and over the same order of magnitude—suggesting that the a priori assumptions required by the BAPC analysis are not unreasonable. Controlling for the effects of age and birth-cohort, the risk of dying of cancer has exhibited a consistent downward trend over the past 50 years (figure 3B). Finally, the BAPC model confirmed our impression—derived from the age-stratified mortality curves—that an inflection in birth cohort specific risk occurred among those born in the late 1920's and that cohorts born since that time period have experienced a progressive decline in cancer mortality risk (figure 3C). Results of BAPC analysis of mortality rates stratified by gender (supplemental figures S4 and S5) yielded results essentially identical to those described above for both genders combined.
Figure 3.
Bayesian age, period, and birth-cohort analysis of all-site cancer mortality data. The contributions of (A) age, (B) period and (C) birth-cohort to cancer mortality risk are plotted. Rate ratio refers to the ratio of the mortality rate at the specified interval compared to the average over the entire range plotted. The dotted red line indicates the 95% credible bounds of the estimate.
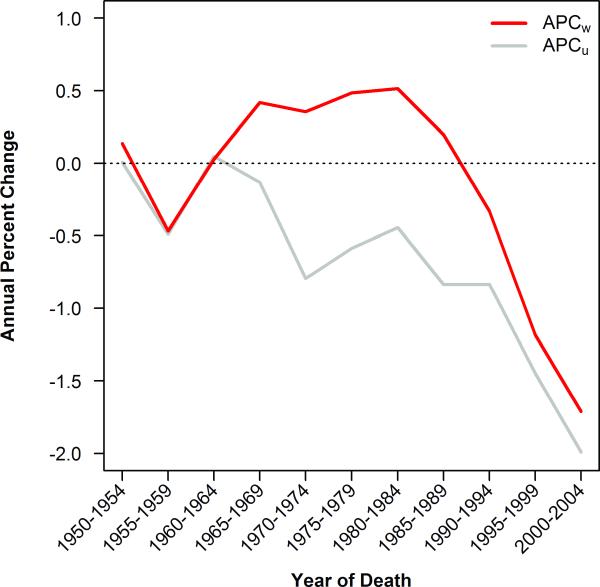
If the rate of change in mortality rates were either homogenous or stochastic with respect to age, analysis of cumulative age-adjusted mortality rates could be assumed to provide a reasonable summary estimate of trends in cancer mortality. But as we have shown above, rate of change in mortality trends are neither homogenous nor stochastic with respect to age. As a result of the birth-cohort effect we describe here, rates drop first among younger individuals. In order to quantify the difference between cumulative age-adjusted mortality rates and age-stratified rates, we compared the annual percent change (APC) in mortality experienced by the average person (ACPw) to the annual percent change in mortality in theaverage age group (APCu). As described in the methods section, where rates are changing homogenously across age groups APCw and APCu will be the same; where rates of change are biased with respect to age, APCw and APCu will diverge.
We calculated the APCw and APCu in cancer mortality rates across eleven 5-year intervals from 1950 to 2004 (figure 4). These two figures were essentially the same during the 1950s. Then, as those born after 1935 entered adulthood, the two lines begin to diverge, and remain divergent for thirty years. Finally, as those born prior to 1935 wash out of our population, the lines are again converging. At its most severe, the difference between the two curves was nearly 1.5 percentage points per year and, worse, the rate of change had opposite signs between the two measures for almost 30 years.
Figure 4.
Annual percent change in age-adjusted mortality rate (red line) and unweighted average mortality rate across all age groups from 20 to 85+ years of age (gray line).
CONCLUSIONS
It is estimated that about 41% of individuals born today in the U.S. will be diagnosed with cancer at some point in their lives, and 21% will succumb to the disease (33). The annual national investment in cancer research in the U.S. numbers in the tens of billions of dollars, in addition to which it is estimated we spend over $40 billion annually in cancer related health care. In light of the socioeconomic burden of cancer, it is not surprising that trends in cancer incidence and mortality receive a great deal of attention in the biomedical literature (1-3, 11, 12). These reports usually employ age-adjustment to remove bias related to the aging of the population as well as to allow comparison between populations with differing age distributions.
The canonical interpretation of age-adjusted all-site cancer mortality data is that mortality rates were rising through most of the 20th century and only began to decline slightly in the mid 1990's. But because age-adjustment and summarization emphasizes the trends in older groups with high mortality rates at the expense of trends in younger, lower mortality groups, this interpretation conceals the fact that mortality has been systematically decreasing among younger individuals for many decades. Moreover, birth cohort analysis shows a decline in lifetime risk of dying from cancer beginning with individuals born between 1925 and 1934. As an example of the magnitude of this decrease it is noteworthy that the cancer mortality rates for 30-59 year olds born between 1945 and 1954 was 29% lower than for people of the same age born three decades earlier. These observations too have been obscured by age-adjusted analyses that have largely reflected the experience of people born in the first quarter of the 20th century. The present analysis of unadjusted age- and birth-cohort specific rates demonstrates that substantial changes in cancer mortality risk across the lifespan have been developing over the past half century in the US.
An alternative to our approach would have been to analyze age-stratified incidence rates as a measure of the effects of prevention, and age-stratified survival rates as a measure of the effects of earlier diagnosis and improved treatment. Such analyses are routinely reported by the NCI in their valuable annual reports on cancer rates (8-12). Such analyses, however, have two limitations. First, incidence and survival data are only available on the portion of the population covered by the SEER registries in place since 1973. Second, neither set of analyses can describe the generation-specific pattern of cancer mortality seen in birth-cohort figures. Our analysis, by contrast, provides a composite picture of the net effects of changes in both incidence and survival upon cancer mortality rates.
Our analysis demonstrates the fact that age-dependant heterogeneity in trends is revealed by divergence between weighted and unweighted average annual percent change in mortality rates (figure 4). In the case of cancer mortality, a divergence between APCu and APCw has been evident since the mid 1960's. This divergence may be taken as a signal that further age-stratified analysis is required to fully describe the developing trends. Where efforts at improved prevention and treatment benefit younger individuals first, analysis of aggregate age-adjusted rates will inevitably fail to detect the results of such efforts until long after they take effect. In such cases, age-stratified analysis and age-period-cohort analysis will provide important additional perspectives.
This analysis and interpretation of cancer mortality trends over the past 50 years is not without limitations. Any interpretation of vital records data must acknowledge the fact that the recording of death events is subject to changes in coding (e.g. ICD-9 vs. ICD-10), legislative requirements, and recording habits (20, 34). Secondly, the BAPC analysis presented requires significant a priori assumptions to be made, though this is no less true of frequentist approaches.
It is not our intention to suggest that the observed birth-cohort effect in cancer mortality must result entirely from direct biological effects of very early life experience. Rather, the birth-cohort effect is likely the net result of diverse behavioral, medical, and technological factors operating throughout the lifespan. The value of birth-cohort analysis in identifying shifts in the overall environmental milieu—including, but not limited to, early life experience—has long been recognized (13, 35).
Age-stratified cancer incidence data (see supplementary figure S6) does not demonstrate the same pattern of decreases documented for mortality. The mortality decline we describe in this paper cannot therefore be attributed to an overall decline in cancer incidence. Rather, the net improvement in cancer mortality in birth cohorts born since 1925 seems to reflect a succession of public health and medical care efforts. For one site—lung cancer—the decline in mortality does parallel a decline in incidence. The first wave of anti-smoking efforts in the 1950's produced a steady decline in smoking that began to be reflected in cancer mortality in younger males as early as the 1950's and 1960's. Harris has shown that smoking in men declined in every successive birth cohort born since 1920, paralleling the decline in cancer mortality (36). However, in women the decline in smoking was not evident until the cohort born in the 1940s. Further evidence that the birth-cohort dependent decline cancer mortality is not purely a result of changes in smoking behavior is the fact that breast cancer mortality, for which a relationship to cigarette smoking has not been consistently shown (37), follows essentially the same birth-cohort-dependent pattern as the all site trends discussed in this report (38).
At about the same time as the hazards of cigarette smoking began to impact smokingw behaviors, the first chemotherapeutic successes in childhood leukemias began to be noted (39) followed very closely by improvement in treatment of the lymphomas (40) and testicular cancers (41) of young adulthood. The final quarter of the century saw mortality reductions in older adults as a result of increasingly successful screening programs for breast (42), prostate (43), and colon cancer (44).
While there are likely other contributory factors, this analysis suggests that efforts in prevention, early detection, and/or treatment have significantly impacted our society's experience of cancer risk. We are optimistic that ongoing efforts in very early cancer prevention (such as use of HB and HPV vaccines) as well as ongoing clinical trials of targeted therapies will preserve the downward trend of cancer mortality. For the public and the healthcare community to be fully informed about trends in cancer mortality, we recommend supplementing time trends in age-adjusted cancer mortality with age-specific and cohort-specific analyses that provide more insight into the complex societal influences on the cancer death rate.
Supplementary Material
ACKNOWLEDGMENTS
EJK performed the data analysis and drafted the manuscript. NP guided the analysis and edited the manuscript. GVW conceived of the project, supervised and guided the analysis, and edited the manuscript. This work was supported by the generosity of Jay and Betty Van Andel, and by the National Institute of Child Health and Human Development (T32 program grant #HD046377-01A1). The authors would like to thank Dr. Joe Fraumeni and colleagues, as well as anonymous reviewers, for their critical review and helpful comments on the manuscript. We also thank Dr. Volker Schmid for assistance with interpretation of the BAPC analysis.
Grant Support: EJK was supported by the National Institute of Child Health and Human Development (T32 program grant #HD046377-01A1).
Footnotes
Potential Conflicts of Interest: None
The authors have no conflicts of interest to report.
REFERENCES
- 1.Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. Journal of the National Cancer Institute. 2005;97:1407–27. doi: 10.1093/jnci/dji289. [DOI] [PubMed] [Google Scholar]
- 2.Bailar JC, 3rd, Smith EM. Progress against cancer? The New England journal of medicine. 1986;314:1226–32. doi: 10.1056/NEJM198605083141905. [DOI] [PubMed] [Google Scholar]
- 3.Bailar JC, 3rd, Gornik HL. Cancer undefeated. The New England journal of medicine. 1997;336:1569–74. doi: 10.1056/NEJM199705293362206. [DOI] [PubMed] [Google Scholar]
- 4.Bazell R. More profit than progress in cancer research. Health / Second Opinion [Web page] 2008. [cited 2008 6/9/2008]; Available from: http://www.msnbc.msn.com/id/24930000/
- 5.Begley S. Newsweek. Sep 15, 2008. We Fought Cancer... And Cancer Won. [PubMed] [Google Scholar]
- 6.Kramer BS, Klausner RD. Grappling with cancer--defeatism versus the reality of progress. The New England journal of medicine. 1997;337:931–4. doi: 10.1056/NEJM199709253371312. discussion 7-8. [DOI] [PubMed] [Google Scholar]
- 7.Mayer RJ, Schnipper LE. Winning the war on cancer. The New England journal of medicine. 1997;337:935. doi: 10.1056/NEJM199709253371313. [DOI] [PubMed] [Google Scholar]
- 8.Howe HL, Wingo PA, Thun MJ, et al. Annual report to the nation on the status of cancer (1973 through 1998), featuring cancers with recent increasing trends. Journal of the National Cancer Institute. 2001;93:824–42. doi: 10.1093/jnci/93.11.824. [DOI] [PubMed] [Google Scholar]
- 9.Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. Journal of the National Cancer Institute. 1999;91:675–90. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 10.Ries LA, Wingo PA, Miller DS, et al. The annual report to the nation on the status of cancer, 1973-1997, with a special section on colorectal cancer. Cancer. 2000;88:2398–424. doi: 10.1002/(sici)1097-0142(20000515)88:10<2398::aid-cncr26>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 11.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–92. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 12.Weir HK, Thun MJ, Hankey BF, et al. Annual report to the nation on the status of cancer, 1975-2000, featuring the uses of surveillance data for cancer prevention and control. Journal of the National Cancer Institute. 2003;95:1276–99. doi: 10.1093/jnci/djg040. [DOI] [PubMed] [Google Scholar]
- 13.Susser M, Stein Z. Civilisation and peptic ulcer. Lancet. 1962;1:115–9. [PubMed] [Google Scholar]
- 14.Andvord KF, Wijsmuller G, Blomberg B. What can we learn by following the development of tuberculosis from one generation to another? 1930. Int J Tuberc Lung Dis. 2002;6:562–8. [PubMed] [Google Scholar]
- 15.Dorn HF. Morbidity and mortality from cancer of the lung in the United States. Acta Unio Int Contra Cancrum. 1953;9:552–61. [PubMed] [Google Scholar]
- 16.Korteweg R. The age curve in lung cancer. Br J Cancer. 1951;5:21–7. doi: 10.1038/bjc.1951.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuller LH. Age-adjusted death rates: a hazard to epidemiology? Annals of epidemiology. 1999;9:91–2. doi: 10.1016/s1047-2797(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 18.Breslow L, Cumberland WG. Progress and objectives in cancer control. JAMA. 1988;259:1690–4. [PubMed] [Google Scholar]
- 19.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; Betheshda, MD: 2008. [Google Scholar]
- 20.Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer. 2003;97:3133–275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93:277–83. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 22.Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975-2004: an ecological study. The lancet oncology. 2008;9:445–52. doi: 10.1016/S1470-2045(08)70104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glenn ND. Distinguishing Age, Period, and Cohort Effects. In: Mortimer JT, Shanahan MJ, editors. Handbook of the Life Course. Kluwer Academic/Plenum; New York: 2003. pp. 465–76. [Google Scholar]
- 24.Heuer C. Modeling of time trends and interactions in vital rates using restricted regression splines. Biometrics. 1997;53:161–77. [PubMed] [Google Scholar]
- 25.Berzuini C, Clayton D. Bayesian analysis of survival on multiple time scales. Stat Med. 1994;13:823–38. doi: 10.1002/sim.4780130804. [DOI] [PubMed] [Google Scholar]
- 26.Schmid VJ, Held L. Bayesian Age-Period-Cohort Modeling and Prediction -- BAMP. Journal of Statistical Software. 2007;21:1. [Google Scholar]
- 27.Bray I, Brennan P, Boffetta P. Projections of alcohol- and tobacco-related cancer mortality in Central Europe. Int J Cancer. 2000;87:122–8. doi: 10.1002/1097-0215(20000701)87:1<122::aid-ijc18>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Bray I, Brennan P, Boffetta P. Recent trends and future projections of lymphoid neoplasms--a Bayesian age-period-cohort analysis. Cancer Causes Control. 2001;12:813–20. doi: 10.1023/a:1012240117335. [DOI] [PubMed] [Google Scholar]
- 29.Knorr-Held L, Rainer E. Projections of lung cancer mortality in West Germany: a case study in Bayesian prediction. Biostatistics. 2001;2:109–29. doi: 10.1093/biostatistics/2.1.109. [DOI] [PubMed] [Google Scholar]
- 30.Eilstein D, Uhry Z, Lim TA, Bloch J. Lung cancer mortality in France Trend analysis and projection between 1975 and 2012, using a Bayesian age-period-cohort model. Lung Cancer. 2008;59:282–90. doi: 10.1016/j.lungcan.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Brennan P, Bray I. Recent trends and future directions for lung cancer mortality in Europe. Br J Cancer. 2002;87:43–8. doi: 10.1038/sj.bjc.6600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National CancerInstitute, Statistical Research and Applications Branch Average Annual Percent Change (AAPC) [cited 2009 05/18/2009]; Available from: http://srab.cancer.gov/joinpoint/aapc.html.
- 33.Ries L, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; Bethesda, MD: 2008. [Google Scholar]
- 34.Muir C, Fraumeni JF, Jr., Doll R. The intepretation of time trends. In: Doll R, Fraumeni JF Jr., Muir C, editors. Trends in cancer incidence and mortality. Cold Spring Harbor Laboratory Press; Plainview, NY: 1994. [Google Scholar]
- 35.Case RA. Cohort analysis of mortality rates as an historical or narrative technique. Br J Prev Soc Med. 1956;10:159–71. doi: 10.1136/jech.10.4.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900-80. J Natl Cancer Inst. 1983;71:473–9. [PubMed] [Google Scholar]
- 37.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 38.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–41. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 39.Burchenal JH, Murphy ML, Ellison RR, et al. Clinical evaluation of a new antimetabolite, 6-mercaptopurine, in the treatment of leukemia and allied diseases. Blood. 1953;8:965–99. [PubMed] [Google Scholar]
- 40.DeVita VT, Jr., Lewis BJ, Rozencweig M, Muggia FM. The chemotherapy of Hodgkin's disease: past experiences and future directions. Cancer. 1978;42:979–90. doi: 10.1002/1097-0142(197808)42:2+<979::aid-cncr2820420721>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Higby DJ, Wallace HJ, Jr., Albert DJ, Holland JF. Diaminodichloroplatinum: a phase I study showing responses in testicular and other tumors. Cancer. 1974;33:1219–5. doi: 10.1002/1097-0142(197405)33:5<1219::aid-cncr2820330505>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Cronin KA, Yu B, Krapcho M, et al. Modeling the dissemination of mammography in the United States. Cancer Causes Control. 2005;16:701–12. doi: 10.1007/s10552-005-0693-8. [DOI] [PubMed] [Google Scholar]
- 43.Mettlin C, Jones G, Averette H, Gusberg SB, Murphy GP. Defining and updating the American Cancer Society guidelines for the cancer-related checkup: prostate and endometrial cancers. CA Cancer J Clin. 1993;43:42–6. doi: 10.3322/canjclin.43.1.42. [DOI] [PubMed] [Google Scholar]
- 44.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.