Abstract
Patients with the autoimmune disease multiple sclerosis (MS) typically present with the clinically isolated syndromes (CIS) transverse myelitis (TM) or optic neuritis (ON). B-cell disturbances have been well documented in patients with MS and CIS patients with ON, but not in CIS patients with TM, despite the fact that these patients have the worst clinical outcome of all CIS types. The goal of this study was to characterize the B-cell populations and immunoglobulin genetics in TM patients. We found a unique expansion of CD27high plasmablasts in both the cerebrospinal fluid and periphery of TM patients that is not present in ON patients. Additionally, plasmablasts from TM patients show evidence for positive selection with increased somatic hypermutation accumulation in VH4+ B cells and receptor editing that is not observed in ON patients. These characteristics unique to TM patients may impact disease severity and progression.
Keywords: multiple sclerosis, clinically isolated syndrome, transverse myelitis, optic neuritis, plasmablast, antibody repertoire
INTRODUCTION
Patients who present with a first demyelinating attack are diagnosed with clinically isolated syndrome (CIS), which places them at high risk to develop multiple sclerosis (MS), an autoimmune inflammatory disease of the central nervous system characterized by the demyelination of axons and the formation of lesions.1–5 The most common presentations are optic neuritis (ON) and transverse myelitis (TM).6 ON is characterized by visual impairments due to demyelination of the optic nerve.7 TM symptoms involve weakening of limbs or sensations of numbness due to demyelination occurring across short segments of the spinal cord.8 Patients who present with either ON or TM have a high risk of converting to MS, but current risk stratification approaches depend on brain MRI (magnetic resonance imaging) findings. Compounding the importance of early and accurate diagnosis are the data suggesting that earlier treatment delays the progression of disease and accruement of disability.9,10 The presence of lesions in the brain of TM patients also increases the risk of conversion to MS11 and these patients typically have a faster occurrence of a second attack than ON patients.6,12 Additionally, ON patients have better long-term prognosis than other presentations, including TM.7,12,13 Differences in progression to clinically definite MS and location of initial lesions between ON and TM patients may suggest different underlying biology. One possible difference could be variations in the composition of lymphocytes involved in the autoimmune pathology associated with these disease presentations.
B cells have been implicated in the pathogenesis of MS,5,14–25 highlighted by the finding that rituximab, a B-cell-depleting agent, can decrease brain inflammation in MS patients.26–28 Of particular interest are plasmablasts, a B-cell subset that is indentified by high CD27 expression.29 An abnormal expansion of CD27high plasmablasts has been documented in the afflicted compartments of several autoimmune diseases, such as rheumatoid arthritis,30 Sjogren’s,31 systemic lupus erythematosus,32 neuromyelitis optica (NMO),33 ankylosing spondylitis34 and pediatric ulcerative colitis.35 In addition, patients with active systemic lupus erythematosus have greater counts of CD27high plasmablasts in the periphery than in healthy controls (HCs) or patients with inactive disease,32,36–39 suggesting these expanding plasmablasts may be contributing to damage associated with these autoimmune diseases.
As patients presenting with TM have a worse prognosis than patients presenting with ON,6,7,13 we hypothesized that the plasmablast subset would be expanded in TM patients and, subsequently, that the antibody genetics of TM patients would demonstrate irregularities. To test this hypothesis, we characterized subpopulations and antibody genetics of B cells in the cerebrospinal fluid (CSF) and peripheral blood of patients experiencing their first TM event. Interestingly, TM patients could be segregated into two groups based on the percentage of CD19+CD27high B cells in either the blood or CSF, which was not seen in ON patients. This increase in CD27high plasmablasts in TM patients who have the worst clinical prognosis parallels with what is seen in other autoimmune disorders. In addition, we found abnormal antibody gene distribution and mutation patterns in the CD27high plasmablasts isolated from the periphery of TM patients that are absent from the CD27+ memory B cells from the same patients.
RESULTS
A subset of TM patients have an expanded plasmablast compartment
Initial characterization of lymphocyte (CD45+), CD4+ T cell, CD8+ T cell, CD19+ B cell and CD138+ plasma cell percentages and absolute numbers were similar in ON and TM patients in both the CSF and peripheral blood compartments (Supplementary Table S1). The percentages and absolute numbers of CD27−naive B cells and CD27+ memory B cells were also similar between ON and TM patients, regardless of compartment (Supplementary Table S1). Additionally, the ratios of CD4:CD8 T cells and naive: memory B cells were also similar between ON and TM patients in both the CSF and peripheral compartments (Supplementary Table S1).
In contrast, a subset of TM patients demonstrated an expansion of the plasmablast B-cell pool identified by high expression of CD27 (Figure 1e) in both the CSF (Figure 1a) and peripheral blood (Figure 1b) compared with ON patients. Therefore we stratified TM patients into TMA (Above) and TMB (Below) patient subsets. This analysis demonstrated that 41% (9/22) of TM patients had an expansion of plasmablasts in the CSF and 45% (10/22) of TM patients had an expansion of plasmablasts in the periphery (Supplementary Table S2). Additionally, the absolute plasmablast cell numbers per milliliter were significantly higher in TMA patients compared with TMB patients in both the compartments (CSF: 132.3 vs 2.4 cells ml−1, P = 0.004; Blood: 6580 vs 453.4 cells ml−1, P = 0.014; Figures 1c and d). In fact, the plasmablast pool was expanded 55-fold in the CSF and 14.5-fold in the periphery of TMA patients. Of the nine TMA patients with plasmablast expansion in their CSF, seven of them also had plasmablast expansion in the periphery (Supplementary Table S2). This suggests that abnormal plasmablast expansion can be detected in the periphery of some TM patients.
Figure 1.
Percentage of CD27high B cells in CSF (a) and peripheral blood (b) of patients initially presenting with ON or TM. The TM patients were segregated into two groups: TMA (Above) and TMB (Below) using the mean of the ON group plus 2 s.e.m. as cutoff criteria. This was done separately for both the CSF (c) and the peripheral blood (d) compartments. Bars shown in the plots are the means with s.e.ms. The mean, s.e.m. and N are shown below each respective group. Additionally, the average cells ml−1 in each group for (c) and (d) are also shown below. Representative flow plots for the gating of CD19+ CD27 naive B cells, CD19+ CD27+ memory B cells and CD19+ CD27high plasmablasts are shown in a TM and ON patient CSF (e).
We also analyzed the percentage of CD27high plasmablasts present in nine patients with paraneoplastic disease (PND), another neuroinflammatory disease group. PND patients also have characteristic high levels of various autoantibodies and expansion of CSF B cells,40–42 and thus could potentially have elevated levels of plasma cells or plasmablasts. Eight of the PND patients had a mean of 0.050% CD27high plasmablasts within the blood that falls below the TMA threshold (data not shown). One of the patients had a significant elevation of plasmablasts in the blood that may be due to the patient having a possible lymphoma. None of the nine PND patients had elevated CD27high plasmablast levels within the CSF compartment with the mean being 0.051% (data not shown). In both the blood and CSF compartments, there is no evidence of elevated plasmablast levels in PND patients.
Due to the increase in plasmablasts in TMA patients, we reasoned that the CSF immunoglobulin (Ig) synthesis rate (mg per 24 h) and the CSF Ig index could be affected. Despite the increased plasmablast frequency in the CSF, there was no correlation of these two clinical markers with plasmablast expansion (Supplementary Table S3). There were also no correlations with age at the time of sampling for any of the patient groups (Table 1). However, there was a positive correlation of peripheral plasmablast expansion with the length of time TMA patients remained untreated for their disease (R = 0.67, P = 0.034) (Table 1). ON and TMB patients did not have this correlation.
Table 1.
Pearson’s correlations between age and time of spinal tap from initial attack with CD27high plasmablast percentage in ON, TMA and TMB patient groups
| CD27high % CSFa |
CD27high % bloodb |
|||||
|---|---|---|---|---|---|---|
| Mean (s.d.) | R | P-valuec | Mean (s.d.) | R | P-valuec | |
| Age (years) | ||||||
| ON | 38.4 (13.4) | –0.20 | 0.63 | 38.5 (12.6) | –0.44 | 0.24 |
| TMA | 38.0 (14.2) | –0.27 | 0.52 | 44.1 (17.0) | –0.07 | 0.86 |
| TMB | 48.6 (13.6) | 0.06 | 0.86 | 44.7 (12.5) | –0.27 | 0.45 |
| Disease duration at the time of sampling (months)c | ||||||
| ON | 11.4 (16.7) | –0.07 | 0.87 | 10.1 (15.8) | 0.18 | 0.67 |
| TMA | 6.9 (11.4) | –0.21 | 0.61 | 16.9 (33.6) | 0.67 | 0.034d |
| TMB | 18.1 (32.4) | 0.50 | 0.10 | 10.3 (17.2) | –0.22 | 0.53 |
Abbreviations: CSF, cerebrospinal fluid; ON, optic neuritis; TMA, transverse myelitis (Above); TMB, transverse myelitis (Below).
The number of patients per CD27high percentage in CSF group is 8 ON, 8 TMA and 12 TMB.
The number of patients per CD27high percentage in blood group is 9 ON, 10 TMA and 10 TMB.
P–values were calculated using a Pearson’s correlation of the CD27high parameter and the clinical measure.
Statistically significant P–value. The bold-faced R and P–values highlight the statistical significance of this correlation within the table.
Increased VH4 usage and mutation accumulation in CSF repertoires
As TMA patients had elevated plasmablasts in the CSF, we reasoned that the B-cell repertoires of this subgroup would display skewing of the antibody repertoire. Previous data from our group and others demonstrates that CSF-isolated B cells from MS and ON patients often display enrichment of VH4 antibody genes that is not observed in peripheral memory B-cell populations of HC donors.43–49 Indeed, the TMA and TMB patient subgroups were enriched for VH4+ B cells in the CSF as observed in established MS patients (MS: 38.84%, TMA: 43.86%, TMB: 30.59%; Figure 2a). ON patients displayed a further expansion of VH4 usage compared with MS patients (ON: 49.32%, P = 0.032; Figure 2a). VH3 usage by MS and all CIS patient subgroups was similar to that observed in the periphery of HC subjects (data not shown).
Figure 2.
Gene and mutation characteristics of VH4+ B cells in the CSF. All data in (a–f) are shown for each of the five patient groups, indicated above (a) and (b). The panels are: percentage of VH4 family gene usage out of the entire VH repertoire, with the dotted line representing VH4% in the HC peripheral repertoire (a), MFs of VH4+ B cells compared with VH3+ B cells within the same group (b), ratio of JH4:JH6 gene segment usage with the dotted line representing JH4:JH6 in the HC peripheral repertoire (c), MF of VH4+JH4+ B cells compared with VH4+JH6+ B cells within the same group (d), mean CDR3 charge (e) and mean CDR3 amino-acid length (f). P-values were calculated using a Chi-square analysis for (a–d). P-values for (e) and (f) were calculated using a Student’s t-test. *P < 0.05, **P < 0.001, #P < 0.0001. The mean, ratios and s.e.m. are shown below each respective group as applicable. The number of patients per group is 11 MS, 17 CIS, 6 ON, 6 TMA and 5 TMB.
Accumulation of somatic hypermutations (SHMs) into antibody genes is a second method to determine whether particular B cells are being selected in a repertoire and driven by antigen. To determine whether B cells expressing VH4 genes were being selected at this level in ON, TMA and TMB patients, we calculated mutation frequencies (MFs) of VH3+ B cells and compared them with MFs of VH4+ B cells (Figure 2b). As expected, we found that the VH4+ B cells from the CSF of MS patients accumulate more mutations when compared with VH3+ B cells from the same patients (Figure 2b). VH4+ B cells from the CSF of CIS patients also accumulate more mutations when compared with VH3+ B cells from the same patients (Figure 2b). This positive selection of VH4+ B cells was also evident independently in each CIS patient subtype: ON, TMA and TMB (Figure 2b). Preferential accumulation of mutations in VH4+ B cells in the CSF compartment is a shared characteristic across all the disease groups.
Positive selection of CSF VH4+JH4+ B cells is not maintained in TMA
A characteristic of typical selection is the preferential usage of JH4 segments over JH6 segments in the memory B-cell antibody repertoire.50 All patient groups had a 1.5–3-fold increased usage of JH4 segments compared with JH6 in the VH4+ B-cell pool (Figure 2c). In addition, VH4+ B cells from MS, CIS, ON and TMB patients utilizing JH4 segments had higher MFs than VH4+ B cells utilizing JH6 segments (Figure 2d). However, VH4+ B cells from TMA patients using JH4 segments had equal MFs compared with those using JH6 segments (Figure 2d). TMA patients had more VH4+JH4+ B cells (Figure 2c), but they are not selected relative to the VH4+JH6+ B cells (Figure 2d).
CSF VH4+ B cells have similar complementarity determining region 3 (CDR3) charge and length
Self-reactive B cells from HC donors emerge from the bone marrow with an enrichment of positively charged CDR3 residues in their VH genes51 and VH receptor editing favors the addition of positively charged arginines in the CDR3.52 Following peripheral selection, enrichment of positively charged CDR3 residues is diminished. To address whether VH4+ B cells from these groups displayed selection at this level, we calculated the overall charge of the CDR3 segments of VH4+ B cells from MS patients and compared them with the CIS, ON, TMA and TMB patient subgroups. As expected, none of the patient groups were enriched for positively charged residues in the VH4+ B-cell pool (Figure 2e). The VH4+ B-cell pool from MS patients trended towards accumulating more negative charges in the CDR3, but this did not reach statistical significance.
We reasoned that if VH4+ B cells from the patient subgroups were undergoing selection typical of what occurs in the germinal center, bias for productive sequences with short CDR3 lengths53,54 would also be intact, as they are in MS and CIS patients. CDR3 length analysis was also important because receptor editing can elongate the VH CDR3 region.52 To address this, we calculated the CDR3 length of VH4+ B cells from the CSF of MS patients and compared it with the CDR3 length of VH4+ B cells from CIS, ON, TMA and TMB patients. In all the cases, the CIS as a whole and the CIS patient subgroups had similar CDR3 lengths in comparison to VH4+ B cells from established MS patients (Figure 2f).
Decreased VH1 usage and mutation accumulation in TMA and ON
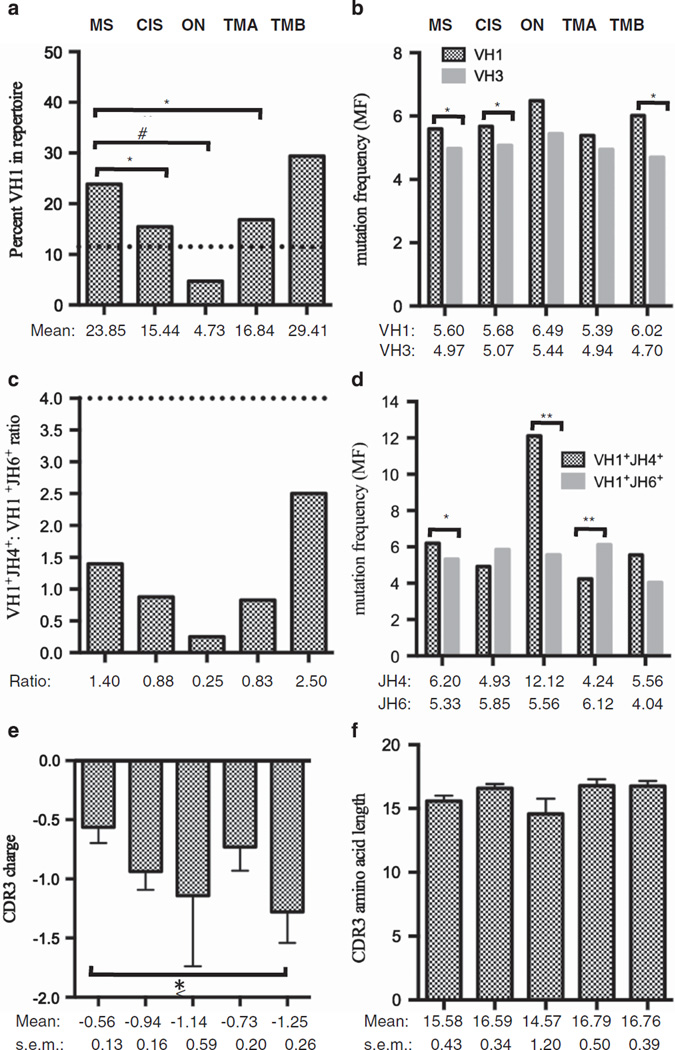
CIS patients and the ON, TMA and TMB patient subgroups had enrichment of VH4+ B cells similar to MS patients, as described above. The VH1 family also displayed variation in usage across the patient repertoires. The ON and TMA patient subgroups displayed a decrease in VH1+ B cells as compared with MS patients (MS: 23.85%, ON: 4.73% P < 0.0001, TMA: 16.84% P = 0.032; Figure 3a). TMB patients had a similar VH1+ B-cell frequency compared with MS patients (TMB: 29.41%) (Figure 3a).
Figure 3.
Gene and mutation characteristics of VH1+ B cells in the CSF. All data in (a–f) are shown for each of the five patient groups, indicated above (a) and (b). The panels are: percentage of VH1 family gene usage out of the entire VH repertoire, with the dotted line representing VH1% in the HC peripheral repertoire (a), MFs of VH1+ B cells compared with VH3+ B cells within the same group (b), ratio of JH4:JH6 gene segment usage with the dotted line representing JH4:JH6 in the HC peripheral repertoire (c), MF of VH1+JH4+ B cells compared with VH1+JH6+ B cells within the same group (d), mean CDR3 charge (e) and mean CDR3 amino-acid length (f). P-values were calculated using a Chi-square analysis for (a–d). P-values for (e) and (f) were calculated using a Student’s t-test. *P < 0.05, **P < 0.001, #P < 0.0001. The mean, ratios and s.e.m. are shown below each respective group as applicable. The number of patients per group is 11 MS, 17 CIS, 6 ON, 6 TMA and 5 TMB.
MS patients had an increase of SHM accumulation in the VH1+ B cells when compared with the VH3+ B cells (Figure 3b), indicating that, in addition to selection of VH4+ B cells, VH1+ B cells were also selected over VH3+ B cells. TMB patients did not differ from MS patients in VH1 gene usage (Figure 3a) but displayed positive selection of VH1+ B cells over VH3+ B cells as measured by SHM accumulation (Figure 3b). ON and TMA patients had the lowest VH1 usage (Figure 3a) and similar SHM accumulation in the VH3+ and VH1+ B cells (Figure 3b), indicating a lack of positive selection for VH1+ B cells in these patient groups.
Positive selection of CSF VH1+JH6+ B cells in TMA
JH4 usage was similar to MS patients in the VH1+ B cells of the CIS, ON, TMA and TMB patient subtypes (Figure 3c). SHM accumulation in VH1+ B cells from MS, CIS, ON and TMB patients using JH4 segments was either greater or equal to SHM accumulation in VH1+ B cells using JH6 segments (Figure 3d), similar to what was found in the VH4+ B-cell pool. In contrast, SHM accumulation in VH1+ B cells from TMA patients using JH6 segments was higher than VH1+ B cells using JH4 segments (P = 0.001; Figure 3d).
CSF VH1+ B cells have similar CDR3 charge and length except TMB
The VH1+ B-cell pools were not enriched for positively charged residues in MS and CIS patients (Figure 3e), as was observed in the VH4+ B-cell pool (Figure 2e). However, there was a significant accumulation of negative charges in the CDR3s of the VH1+ B cells in TMB patients when compared with MS patients (Figure 3e). As with the VH4+ B cells, there were no statistically significant differences in CDR3 lengths for VH1+ B cells in the TMA and ON patient groups (Figure 3f).
Increased VH4 mutation accumulation in peripheral B-cell repertoires of TMA
As 41% of TM patients had an enrichment of peripheral CD27high plasmablast B cells, we next focused on determining whether the antibody repertoires from peripheral plasmablast B cells of these patients also demonstrated skewing of their antibody gene repertoire characteristics. To do this, we sorted memory (TMA-CD27+) and plasmablast (TMA-CD27high) B cells from the peripheral blood of four TMA patients. A MS peripheral blood database was used to determine antibody repertoire differences in established disease compared with the initial clinical stage. As expected, VH4+ CD27+ memory B cells were expanded in the periphery of MS patients compared with the HC (HC: 19.35%; MS: 36.21%, P = 0.007; Figure 4a). The frequency of peripheral VH4+ plasmablasts in TMA patients (23.77%) was also similar to HC subjects (19.35%; Figure 4a), but the frequency of peripheral memory B cells in TMA patients demonstrated an increased frequency of VH4+ B cells (28.99%, P = 0.027) but not to the extent observed in MS patients.
Figure 4.
Gene and mutation characteristics of peripheral blood VH4+ B cells. All data in (a–f) are shown for each of the four patient groups, indicated above (a) and (b). The panels are: percentage of VH4 family gene usage out of the entire VH repertoire (a), MFs of VH4+ B cells compared with VH3+ B cells within the same group (b), ratio of JH4:JH6 gene segment (c), MF of VH4+JH4+ B cells compared with VH4+JH6+ B cells within the same group (d), mean CDR3 charge (e) and mean CDR3 amino-acid length (f). P-values were calculated using a Chi-square analysis for (a–d). P-values for (e) and (f) were calculated using a Student’s t-test. *P < 0.05, **P < 0.001, #P < 0.0001. The mean, ratios and s.e.m. are shown below each respective group as applicable. The number of patients per group is 6 HC, 4 MS, 4 TMA-CD27high and 4 TMA-CD27+.
Next, we assessed the selection of VH4+ B cells by measuring the accumulation of SHMs. As expected, there was no SHM accumulation in VH4+ B cells from HCs, as their MF was similar to the MF of VH3+ B cells (Figure 4b). Positive selection for VH4+ B cells was observed in the periphery of MS patients (Figure 4b) similar to VH4+ B cells in the CSF of MS patients. Interestingly, the non-expanded VH4+ plasmablasts from the same patients (Figure 4a) had an increased MF compared with VH3+ plasmablasts from the same patients (Figure 4b). The expanded peripheral VH4+ memory B cells (Figure 4a) also had increased MF compared with VH3+ memory B cells from the same patients (Figure 4b). The peripheral memory B cells and CD27high plasmablasts from early TM disease shared the VH4+ dysregulation observed in CSF memory B cells from established MS patients.
Positive selection of peripheral VH4+JH6+ B cells only in plasmablasts
VH4+ B cells from HC subjects use JH4 segments more frequently than JH6 segments, and VH4+JH6+ B cells from HCs displayed higher SHMs than VH4+JH6+ B cells from HCs (Figure 4c and d). In contrast, VH4+ B cells from MS patients used JH6 segments approximately twofold more frequently than JH4 segments (Figure 4c) but did not display higher SHMs than VH4+ B cells using JH4 segments (Figure 4d). The VH1+ B cells from MS patients also utilized JH6 segments more, and these were positively selected over JH4 segments in addition to accumulating significant positive charge (Supplementary Figure S1). Similar to the CSF VH4+ B-cell patient groups, the peripheral blood VH4+ repertoires did not have any difference in CDR3 charge (Figure 4e) or length (Figure 4f). Contrary to the trend towards more JH4 usage in the TMA memory B-cell pools, the plasmablast B-cell pools from TMA patients using VH4 genes had an increased trend towards JH6 usage, but this did not reach significance. Nevertheless, the VH4+JH6+ plasmablast B-cell pool preferentially accumulated SHMs compared with the VH4+JH4+ plasmablast B-cell pool from the same TMA patients (VH4+JH4+: 6.27% vs VH4+JH6+: 9.40%, P = 0.001; Figure 4d). Peripheral VH4+ memory B cells from TMA patients did not demonstrate this level of selection (VH4+JH4+: 6.22% vs VH4+JH6+: 6.57%, P = 0.611) (Figure 4d). This depth of selection in TMA patients was unique to VH4+ peripheral CD27high plasmablasts, as no selection was observed for VH1+ peripheral CD27high plasmablasts from TMA patients (Supplementary Figure S1).
DISCUSSION
Patients with high risk to convert to MS present with lesions at two different anatomical locations: the optic nerve and the spinal cord. Patients afflicted with TM (lesions in the spinal cord) tend to have worse clinical prognosis than ON (lesions in the optic nerve) if they convert to MS.7,12 CIS patients have higher CSF cell counts than patients with established MS,55 which may indicate that lymphocytes circulate more readily in the central nervous system during the highly inflammatory state of the early acute disease. In fact, although B cells are rare in the CSF of normal HC donors,56,57 MS patients undergoing an attack have an expansion of memory B cells in the CSF24 and a contraction of memory B cells in the periphery.58 This suggests that B cells are recruited to the CSF from the periphery in these patients, and recent data underscore this possibility.59
One of the goals of this study was to determine whether there are any irregularities in the B-cell subpopulations of TM patients compared with ON patients. We found that a subset of TM patients, termed the TMA patient subgroup, had a unique expansion of CD27high plasmablasts in the CSF. The majority of these TMA patients also demonstrated an expansion of plasma-blasts in the periphery. Expansion of plasmablasts was not observed in any of the ON patients in either compartment. The frequency of CD27high plasmablasts is elevated in several autoimmune diseases30,31,34,35 and occurs in the diseased tissues where the putative autoantigens are present.
Clinical evidence of plasmablast activity has been found in patients with NMO,60 rheumatoid arthritis30,61 and systemic lupus erythematosus37,39,62,63 who receive the B-cell-depleting agent rituximab and are more likely to relapse early if their memory B cells, and more importantly their CD27high plasmablasts, return earlier. In addition, mRNA from rheumatoid arthritis patients who are non-responsive to rituximab treatment demonstrate increases in mRNA markers of plasmablasts.61 NMO patients who are positive for anti-aquaporin-4 IgG have increased plasmablasts in the periphery, which are further increased during a relapse.33 Indeed, one of the TMA patients in this present study was diagnosed with NMO and had a high frequency of plasmablasts in both the CSF and periphery. All the remaining TMA patients tested negative for the NMO diagnostic anti-aquaporin-4 IgG reactivity and are at high risk to convert to MS. All these data suggest that the re-emergence of symptoms in autoimmune diseases may be marked or potentially caused by an abnormal increase in CD27high plasmablasts. Future studies may find this expansion in additional autoimmune diseases in which humoral immunity is a component of the pathology.
Furthermore, plasmablasts are correlated with neuroinflammatory disease activity as evidenced by MRI of MS patients.64 Treatment with natalizumab, which blocks the entry of cells into the CNS through VLA-4, was effective in MS patients if they had lower levels of CSF plasmablasts before treatment and maintained low levels post treatment.65 Other studies have found that during a MS relapse, memory B cells are readily recruited to the CSF,58,66 possibly through a VLA-4 dependent mechanism, as memory B cells67 and plasmablasts67,68 express high levels of VLA-4. Trafficking of B cells may be an early disease step, as CIS patients have higher levels of VLA-4 on their transitional B cells than is observed in MS patients.69 Such an initial step would enable B cells to enter the CNS, encounter their autoantigen and undergo germinal center-like reactions as evidenced by SHM accumulation and altered selection of VH genes.
We were surprised to find that the CD27high plasmablast expansion could also be detected in the periphery of many TMA patients, as the pathology of the disease is confined to the CNS. The detection of these plasmablasts in the periphery at this early stage of disease may be due to the activation of B cells in the periphery before migration into the CNS where they participate in the formation of ectopic germinal centers.70,71 Alternatively, this activation could occur in the CSF compartment, leading to cell migration into the periphery. Unlike plasma cells, plasmablasts are quite motile,72 but their travel direction in TM patients is unknown from this current study. The occurrence of peripheral expansion of plasmablasts found in TM patients is unique to this particular CIS presentation of an early stage of the MS disease course.
We also found that the percentage of CD27high plasmablasts in the periphery of the TMA group increased the longer the patient had been untreated. This may reflect an accumulation of over-activated B cells, which develop into plasmablasts in a state of extended neuroinflammation. Presumably, lack of treatment can prolong the immune response time to the triggering antigen(s), and the resulting potent inflammatory milieu may promote aberrant cell activation. This correlation was not seen in the CSF compartment, possibly due to plasmablasts entering niches in the inflamed tissue72 or exiting back into the periphery over time after expanding in the CSF.
Next, we analyzed the patient B-cell repertoires for distinctions in antibody genetics. VH4+ B cells were expanded in the CSF of all the patients experiencing both acute (CIS) and chronic (MS) neuroinflammation, which corroborates previous findings.45,46,49 Interestingly, CSF B cells from ON patients had the greatest enrichment of VH4 gene usage and the greatest contraction of VH1 gene usage among the examined patient groups. In addition to the expansion of VH4 genes in the CSF of MS and CIS patients, there was evidence of positive selection of these VH4+ B cells in the CSF of MS and CIS patients. This aberrant selection of VH4+ B cells in the CSF was also observed at the early stages of disease represented by the ON, TMA and TMB patient subgroups.
In contrast, VH4+ gene usage was not increased in peripheral CD27high plasmablasts from TMA patients. However, SHM accumulation in peripheral VH4+ plasmablasts was extensive in comparison to VH3+ plasmablasts in TMA patients. Furthermore, the VH4+ plasmablasts using JH6 segments accounted for this depth of SHM accumulation. VH1+ plasmablasts from the same TMA patients did not demonstrate this level of selection. Taken together, these data suggest that within the peripheral CD27high plasmablast pool of TMA patients, there is a subgroup of VH4+ plasmablasts enriched for JH6 segment use that are undergoing affinity maturation at a faster rate than their VH4+ counterparts that utilize JH4 segments.
High JH6 usage is an indicator of VH receptor editing, which is a process that is estimated to occur in 5–10% of healthy B-cell pools.52,73 Autoreactive B cells often demonstrate skewing to JH6 segment use but do not typically accumulate mutations at a high rate as their selection has been arrested due to self-reactivity.74 Yet the VH4+ plasmablasts in the periphery of TMA patients using JH6 segments are accumulating SHMs at a higher rate than the VH4+JH4+ plasmablasts from the same patients. This suggests that the peripheral plasmablast B-cell pool of TMA patients is both antigen driven and undergoing VH receptor editing in the periphery of TM patients. B cells from ectopic germinal centers of rheumatoid arthritis patients have also demonstrated this phenomenon.75 Interestingly, we also see evidence of VH receptor editing in VH4+ and VH1+ B-cell pools from the periphery of established MS patients. Peripheral B cells from MS patients had a skewed ratio of JH4:JH6 in both the VH4+ and VH1+ B-cell pools due to a significant increase of the JH6 segment usage and accumulation of SHMs but lack the intensity of SHM selection and receptor editing demonstrated only in the VH4+JH6+ plasmablasts from TMA patients.
We were interested in whether we could find genetic evidence of these VH4+JH6+ plasmablasts with high SHMs in the CSF of these TMA patients. Our data suggest that they are not accumulating as VH4+ B cells from the CSF of TMA patients that utilize JH6 segments do not accumulate SHMs to a greater extent than JH4 segment-using VH4+ B cells from the same patients. Instead, there was no selection between VH4+JH4+ and VH4+ JH6+ B cells in the CSF of TMA patients. However, it is possible that they reside within the brain tissue and are not found circulating in the CSF as readily as their memory B-cell counterparts. In either case, receptor editing is not prominent in the CSF B-cell compartment of these groups.
In conclusion, we have found a unique phenotype of expanded plasmablasts in a subset of TM patients which is not observed in ON patients. These cells exhibit evidence of heavy chain receptor editing through positive selective pressure of VH4+JH6+ B cells. Additionally, this plasmablast expansion amplifies in the periphery proportionally with time to clinical visit, suggesting that chronic untreated neuroinflammation can expand the VH4+ plasmablasts in these patients. Receptor editing may be a driving force contributing to JH6 selection in the periphery but is a mechanism absent in the CSF compartment where disease pathology is localized and autoreactive B cells continue to expand. Perhaps, this expansion of abnormally selected plasmablasts at the early stage of TM affects the course of neuroinflammation in these patients.
MATERIALS AND METHODS
Patient description and sample acquisition
CSF was obtained by lumbar puncture and peripheral blood by venipuncture on the same day from patients recruited to be in the study at UT Southwestern Medical Center (UTSWMC) in accordance with the UTSWMC Institutional Review Board (IRB). This study includes samples from 11 patients who presented with ON and 22 patients who presented with TM. Of the 11 ON patients,1 of them had definite MS at the time of sampling. The remaining 10 ON patients were defined as CIS with ON. Of the 22 TM patients, 2 of them had definite MS at the time of sampling. The remaining 20 TM patients were defined as CIS with TM.
None of the patients had received immunomodulatory agents for at least 1 month before lumbar puncture. The peripheral blood cells were centrifuged after being underlaid with a polysaccharide ficoll gradient that collects the mononuclear cells in a separate layer; the cells were then washed, counted, stained with fluorescently labeled antibodies and sorted for single CD19+ B cells through a CD45+ lymphocyte gate. CSF cells were collected as a pellet after centrifugation, washed, counted, stained with fluorescently labeled antibodies and sorted for single CD19+ B cells through a CD45+ lymphocyte gate. A second blood sample was obtained from four TMA patients, and these were sorted using CD19 and CD27 as markers in the CD45+ lymphocyte gate to separate the CD19+ CD27+ memory B cell and the CD19+ CD27high plasmablast populations.
Clinical information from CSF diagnostic tests (CSF Ig synthesis rate and CSF Ig index), as well as patient age and disease duration at the time of lumbar puncture sampling, were obtained in accordance with the UTSWMC IRB. At the time of publication, four of the CIS with ON patients had converted to MS. Of the 20 CIS with TM patients, 6 had converted to MS, 1 had converted to PPMS and 1 had converted to NMO. Of the nine TM patients defined as TMA based on CSF CD27high percentages, one was NMO anti-aquaporin-4 IgG positive, while the remaining eight TMA patients were NMO anti-aquaporin-4 IgG negative and are thus at high risk to convert to MS.
Flow cytometry analyses
Data were collected and sorts were performed on either the BD FACSAria flow cytometer (Becton Dickinson, San Jose, CA, USA) or the MoFlo High-Performance Cell Sorter (Cytomation, Ft Collins, CO, USA). During the sort, flow cytometry data were collected, in conjunction with single cell sorting, to measure expression of lymphocyte and B-cell markers of interest as previously described.49 All fluorescently labeled monoclonal antibodies were obtained from BD Biosciences (Becton Dickinson). Events were analyzed with Flowjo Software (Treestar, Ashland, OR, USA). Cells were gated on live cells and single cells based on FSC × SSC (forward light scatter × sideward light scatter) characteristics. A CD45+ lymphocyte gate was created, and all cell population analyses were conducted within this gate.
Single-cell PCR
After the single cell sort and cell lysis, a primer extension preamplification was used to amplify the original genomic DNA for the MS patient samples.48 The original mRNA was reverse transcribed into cDNA for the CIS patient samples in a similar manner as previously described.76 Nested PCR was then performed to further amplify the Ig heavy chain sequence rearrangement in each B cell as described by our group.48 The PCR products were purified, sequenced, catalogued and analyzed for gene and mutation characteristics as described below.
Identification of VH and JH genes
Germline rearrangements were inferred using the IMGT/V-QUEST Ig blasting tool (http://www.imgt.org/IMGT_vquest/share/textes/).77 Sequences were analyzed and compiled into databases containing VH gene, JH gene, CDR3 amino-acid sequence and mutation information using a Perl program developed at UTSWMC78 that utilizes the IMGT/V-QUEST tool as a basis for extracting the sequence information. Sequences with o85% homology to their VH germline gene segment were dismissed to avoid introducing potential VH gene miscalls into the databases.
Designation of TMA and TMB CIS patient subgroups
The CIS database was divided into three groups by the initial clinical presentation of disease. The ON group (n = 11) presented with ON and the TM group (n = 22) presented with TM, which was further divided into TMA (Above) and TMB (Below) based on the percentage of CD19+ CD27high cells determined by flow cytometry (Figure 1). The threshold for being in the TMA group was determined by calculating the mean percentage of CD19+ CD27high plasmablasts in ON patients and adding two s.e.m. Patients whose percentage fell below this threshold were designated TMB and those whose percentage fell above this threshold were designated TMA. This grouping was done separately for both the peripheral and CSF compartments (see Figure 1c and d for patient numbers per group for each compartment).
Database generation
VH Ig sequence information from singly sorted B cells was generated using the aforementioned IMGT/V-QUEST and UTSWMC Perl program. Only sequences that were productive were included for analysis. Sequences were considered productive provided that codon 103, the beginning of framework region 4, remained in frame and no stop codons were inserted throughout the length of the V gene. Of the productive sequences, those which contained ⩾2 nucleotide mutations were designated as antigen experienced and were included for further study. This was done to ensure that comparisons could be made to the HC database, which does not exhibit enrichment for memory cells like the diseased repertoires.
The HC peripheral blood database was obtained from previous publications using similar single-cell sequencing methodologies and consists of 217 antibody rearrangements.79,80 The MS database from CSF consists of CD19+ B-cell antibody rearrangements collected from 327 CSF CD19+ B cells isolated from 10 relapsing-remitting MS and 1 primary progressive MS patient. The MS database from peripheral blood consists of 58 peripheral CD19+ B cells from four of the MS patients. The CIS database consists of 518 CD19+ B-cell antibody rearrangements from six ON and 11 TM patients. This was divided into the CIS subgroups ON (six patients, 148 sequences), TMA (six patients, 285 sequences) and TMB (five patients, 85 sequences). The peripheral TMA databases consist of 169 CD19+ CD27+ memory B cells and 223 CD19+ CD27high plasmablasts from 4 TMA patients.
VH rearrangement read length determination and MF
The Kabat codon numbering system was used,81 and the IMGT/V-QUEST definitions present in the blast outputs were converted to Kabat numbering by the aforementioned Perl-based program (with in-house modifications). VH read length was defined as the number of nucleotides from codon 31 to 92 (complementarity determining regions (CDRs) 1 and 2, framework regions (FRs) 2 and 3). The 3’ end of the V gene segment was defined as codon 92 and the CDR3 read contained codons 93–102.
MF was determined by dividing the number of mutations in a given germline VH rearrangement between codons 31 and 92 by the number of nucleotides in that region. For example, a sequence of 206 nucleotides with 1 mutation would have a MF of 1/206 or 0.48%, which can be translated into 99.52% identical to the germline variable gene. FR 1 codons were not included in the analysis.
CDR3 length and charge determination
CDR3 length begins at codon 93 within the V gene, extends through the D segment and ends at codon 102 of the JH segment, as defined by Kabat.81 CDR3 charge was calculated by translating the CDR3 nucleotide sequence into the corresponding amino acids and summing the charges, counting each arginine (R) and lysine (K) as a positive one charge, and each aspartic acid (D) and glutamic acid (E) as a negative one charge. Histidine was not included because it holds relatively no charge at physiological pH.82 This calculation was done using the Perl program developed at UTSWMC.
Statistical analyses
Cell population frequencies, VH and JH gene usages, MF and JH4:JH6 ratio were compared using Chi-square analysis. CDR3 lengths and charge were compared using the Student’s t-test. Pearson’s correlation was used to compare the plasmablast numbers and percentages to patient clinical measures. P-values ⩽0.05 were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the patients who consented to sampling for this study. Bonnie Darnell, Angie Mobley, Julia McClouth, Ann-Marie Schaefer and Elizabeth Curry are thanked for their technical expertise in cell sorting. This study was supported by Grants from the National Multiple Sclerosis Society (NMSS) to NLM (RG3267 and RG4653). AJL, WHR, EMC and CTH were supported by Grant no. NIH NRSA5 T32 AI 005284-28 from NIAID. LGC’s contributions were funded by Grant no. 1R01AI097403-01.
Footnotes
CONFLICT OF INTEREST
EMF has received speaker and consulting fees from TEVA, Biogen Idec, Acorda and Novartis. EMF has received consulting fees from Abbott Laboratories and Genzyme. BG received honoraria from the MSAA and the AAN and has received consulting fees from Biogen Idec, Acorda Therapeutics, Sanofi-Aventis and DioGenix. BG has equity shares in DioGenix. NLM receives funding from MedImmune, Inc., TEVA Neuroscience, DioGenix, Inc. and the National MS Society. NLM is an advisor for Genentech, Inc. All the other authors declare no conflict of interest.
REFERENCES
- 1.Geurts JJ, Bo L, Pouwels PJ, Castelijns JA, Polman CH, Barkhof F. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. Ajnr. 2005;26:572–577. [PMC free article] [PubMed] [Google Scholar]
- 2.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 3.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 4.Bitsch A, Schuchardt J, Bunkowski S, Kuhlmann T, Bruck W. Acute axonal injury in multiple sclerosis. Correlation with demyelination and inflammation. Brain. 2000;123(Pt 6):1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- 5.Cepok S, Jacobsen M, Schock S, Omer B, Jaekel S, Boddeker I, et al. Patterns of cerebrospinal fluid pathology correlate with disease progression in multiple sclerosis. Brain. 2001;124(Pt 11):2169–2176. doi: 10.1093/brain/124.11.2169. [DOI] [PubMed] [Google Scholar]
- 6.Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part I: natural history, pathogenesis, diagnosis, and prognosis. Lancet Neurol. 2005;4:281–288. doi: 10.1016/S1474-4422(05)70071-5. [DOI] [PubMed] [Google Scholar]
- 7.Atkins EJ, Biousse V, Newman NJ. Optic neuritis. Semin Neurol. 2007;27:211–220. doi: 10.1055/s-2007-979683. [DOI] [PubMed] [Google Scholar]
- 8.Kerr DA, Ayetey H. Immunopathogenesis of acute transverse myelitis. Curr Opin Neurol. 2002;15:339–347. doi: 10.1097/00019052-200206000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Frohman EM, Havrdova E, Lublin F, Barkhof F, Achiron A, Sharief MK, et al. Most patients with multiple sclerosis or a clinically isolated demyelinating syndrome should be treated at the time of diagnosis. Arch Neurol. 2006;63:614–619. doi: 10.1001/archneur.63.4.614. [DOI] [PubMed] [Google Scholar]
- 10.Rocca MA, Agosta F, Sormani MP, Fernando K, Tintore M, Korteweg T, et al. A three-year, multi-parametric MRI study in patients at presentation with CIS. J Neurol. 2008;255:683–691. doi: 10.1007/s00415-008-0776-z. [DOI] [PubMed] [Google Scholar]
- 11.Patrucco L, Rojas JI, Cristiano E. Assessing the value of spinal cord lesions in predicting development of multiple sclerosis in patients with clinically isolated syndromes. J Neurol. 2011;259:1317–1320. doi: 10.1007/s00415-011-6345-x. [DOI] [PubMed] [Google Scholar]
- 12.Gajofatto A, Monaco S, Fiorini M, Zanusso G, Vedovello M, Rossi F, et al. Assessment of outcome predictors in first-episode acute myelitis: a retrospective study of 53 cases. Arch Neurol. 2010;67:724–730. doi: 10.1001/archneurol.2010.107. [DOI] [PubMed] [Google Scholar]
- 13.Tintore M, Rovira A, Arrambide G, Mitjana R, Rio J, Auger C, et al. Brainstem lesions in clinically isolated syndromes. Neurology. 2010;75:1933–1938. doi: 10.1212/WNL.0b013e3181feb26f. [DOI] [PubMed] [Google Scholar]
- 14.Kabat EA, Moore DH, Landow H. An electrophoretic study of the protein components in cerebrospinal fluid and their relationship to the serum proteins. J Clin Invest. 1942;21:571–577. doi: 10.1172/JCI101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabat EA, Freedman DA, et al. A study of the crystalline albumin, gamma globulin and total protein in the cerebrospinal fluid of 100 cases of multiple sclerosis and in other diseases. Am J Med Sci. 1950;219:55–64. doi: 10.1097/00000441-195001000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kabat EA, Glusman M, Knaub V. Quantitative estimation of the albumin and gamma globulin in normal and pathologic cerebrospinal fluid by immunochemical methods. Am J Med. 1948;4:653–662. doi: 10.1016/s0002-9343(48)90389-1. [DOI] [PubMed] [Google Scholar]
- 17.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Raine CS, Cannella B, Hauser SL, Genain CP. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann Neurol. 1999;46:144–160. doi: 10.1002/1531-8249(199908)46:2<144::aid-ana3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 20.Esiri MM. Immunoglobulin-containing cells in multiple-sclerosis plaques. Lancet. 1977;2:478. doi: 10.1016/s0140-6736(77)91603-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43:465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 22.Sadaba MC, Tzartos J, Paino C, Garcia-Villanueva M, Alvarez-Cermeno JC, Villar LM, et al. Axonal and oligodendrocyte-localized IgM and IgG deposits in MS lesions. J Neuroimmunol. 2012;247:86–94. doi: 10.1016/j.jneuroim.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 23.Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, et al. Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci USA. 2004;101:11064–11069. doi: 10.1073/pnas.0402455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cepok S, von Geldern G, Grummel V, Hochgesand S, Celik H, Hartung H, et al. Accumulation of class switched IgD-IgM- memory B cells in the cerebrospinal fluid during neuroinflammation. J Neuroimmunol. 2006;180:33–39. doi: 10.1016/j.jneuroim.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Frohman EM, Filippi M, Stuve O, Waxman SG, Corboy J, Phillips JT, et al. Characterizing the mechanisms of progression in multiple sclerosis: evidence and new hypotheses for future directions. Arch Neurol. 2005;62:1345–1356. doi: 10.1001/archneur.62.9.1345. [DOI] [PubMed] [Google Scholar]
- 26.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 27.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. doi: 10.1016/j.jneuroim.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin Mdel P, Cravens PD, Winger R, Kieseier BC, Cepok S, Eagar TN, et al. Depletion of B lymphocytes from cerebral perivascular spaces by rituximab. Arch Neurol. 2009;66:1016–1020. doi: 10.1001/archneurol.2009.157. [DOI] [PubMed] [Google Scholar]
- 29.Schneider S, Bruns A, Moewes B, Holzknecht B, Hausdorf G, Riemekasten G, et al. Simultaneous cytometric analysis of (auto)antigen-reactive T and B cell proliferation. Immunobiology. 2002;206:484–495. doi: 10.1078/0171-2985-00196. [DOI] [PubMed] [Google Scholar]
- 30.Sellam J, Rouanet S, Hendel-Chavez H, Abbed K, Sibilia J, Tebib J, et al. Blood memory B cells are disturbed and predict the response to rituximab in patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:3692–3701. doi: 10.1002/art.30599. [DOI] [PubMed] [Google Scholar]
- 31.Hansen A, Odendahl M, Reiter K, Jacobi AM, Feist E, Scholze J, et al. Diminished peripheral blood memory B cells and accumulation of memory B cells in the salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum. 2002;46:2160–2171. doi: 10.1002/art.10445. [DOI] [PubMed] [Google Scholar]
- 32.Jacobi AM, Odendahl M, Reiter K, Bruns A, Burmester GR, Radbruch A, et al. Correlation between circulating CD27high plasma cells and disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1332–1342. doi: 10.1002/art.10949. [DOI] [PubMed] [Google Scholar]
- 33.Chihara N, Aranami T, Sato W, Miyazaki Y, Miyake S, Okamoto T, et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA. 2011;108:3701–3706. doi: 10.1073/pnas.1017385108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Q, Gu JR, Li TW, Zhang FC, Lin ZM, Liao ZT, et al. Value of the peripheral blood B-cells subsets in patients with ankylosing spondylitis. Chin Med J (Engl) 2009;122:1784–1789. [PubMed] [Google Scholar]
- 35.Tarlton NJ, Green CM, Lazarus NH, Rott L, Wong AP, Abramson ON, et al. Plasmablast frequency and trafficking receptor expression are altered in pediatric ulcerative colitis. Inflamm Bowel Dis. 2012;18:2381–2391. doi: 10.1002/ibd.22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 37.Yang DH, Chang DM, Lai JH, Lin FH, Chen CH. Significantly higher percentage of circulating CD27(high) plasma cells in systemic lupus erythematosus patients with infection than with disease flare-up. Yonsei Med J. 2010;51:924–931. doi: 10.3349/ymj.2010.51.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167:2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 39.Boekel ET, Prins M, Vrielink GJ, de Kieviet W, Siegert CE. Longitudinal studies of the association between peripheral CD27+ + plasma cells and systemic lupus erythematosus disease activity: preliminary results. Ann Rheum Dis. 2011;70:1341–1342. doi: 10.1136/ard.2010.133959. [DOI] [PubMed] [Google Scholar]
- 40.de Graaf M, de Beukelaar J, Bergsma J, Kraan J, van den Bent M, Klimek M, et al. B and T cell imbalances in CSF of patients with Hu-antibody associated PNS. J Neuroimmunol. 2008;195:164–170. doi: 10.1016/j.jneuroim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Pranzatelli MR, Travelstead AL, Tate ED, Allison TJ, Verhulst SJ. CSF B-cell expansion in opsoclonus-myoclonus syndrome: a biomarker of disease activity. Mov Disord. 2004;19:770–777. doi: 10.1002/mds.20125. [DOI] [PubMed] [Google Scholar]
- 42.Blaes F, Tschernatsch M. Paraneoplastic neurological disorders. Expert Rev Neurother. 2010;10:1559–1568. doi: 10.1586/ern.10.134. [DOI] [PubMed] [Google Scholar]
- 43.Owens GP, Kraus H, Burgoon MP, Smith-Jensen T, Devlin ME, Gilden DH. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol. 1998;43:236–243. doi: 10.1002/ana.410430214. [DOI] [PubMed] [Google Scholar]
- 44.Cameron EM, Spencer S, Lazarini J, Harp CT, Ward ES, Burgoon M, et al. Potential of a unique antibody gene signature to predict conversion to clinically definite multiple sclerosis. J Neuroimmunol. 2009;213:123–130. doi: 10.1016/j.jneuroim.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bennett JL, Haubold K, Ritchie AM, Edwards SJ, Burgoon M, Shearer AJ, et al. CSF IgG heavy-chain bias in patients at the time of a clinically isolated syndrome. J Neuroimmunol. 2008;199:126–132. doi: 10.1016/j.jneuroim.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owens GP, Winges KM, Ritchie AM, Edwards S, Burgoon MP, Lehnhoff L, et al. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J Immunol. 2007;179:6343–6351. doi: 10.4049/jimmunol.179.9.6343. [DOI] [PubMed] [Google Scholar]
- 47.Zuckerman NS, Hazanov H, Barak M, Edelman H, Hess S, Shcolnik H, et al. Somatic hypermutation and antigen-driven selection of B cells are altered in autoimmune diseases. J Autoimmun. 2010;35:325–335. doi: 10.1016/j.jaut.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Monson NL, Brezinschek HP, Brezinschek RI, Mobley A, Vaughan GK, Frohman EM, et al. Receptor revision and atypical mutational characteristics in clonally expanded B cells from the cerebrospinal fluid of recently diagnosed mutiple sclerosis patients. J Neuroimmunol. 2005;158:170–181. doi: 10.1016/j.jneuroim.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 49.Harp C, Lee J, Lambracht-Washington D, Cameron E, Olsen G, Frohman E, et al. Cerebrospinal fluid B cells from multiple sclerosis patients are subject to normal germinal center selection. J Neuroimmunol. 2007;183:189–199. doi: 10.1016/j.jneuroim.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Briney BS, Willis JR, McKinney BA, Crowe JE., Jr High-throughput antibody sequencing reveals genetic evidence of global regulation of the naive and memory repertoires that extends across individuals. Genes Immun. 2012;13:469–473. doi: 10.1038/gene.2012.20. [DOI] [PubMed] [Google Scholar]
- 51.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 52.Kalinina O, Doyle-Cooper CM, Miksanek J, Meng W, Prak EL, Weigert MG. Alternative mechanisms of receptor editing in autoreactive B cells. Proc Natl Acad Sci USA. 2011;108:7125–7130. doi: 10.1073/pnas.1019389108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brezinschek HP, Brezinschek RI, Lipsky PE. Analysis of the heavy chain repertoire of human peripheral B cells using single-cell polymerase chain reaction. J Immunol. 1995;155:190–202. [PubMed] [Google Scholar]
- 54.Meffre E, Milili M, Blanco-Betancourt C, Antunes H, Nussenzweig MC, Schiff C. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J Clin Invest. 2001;108:879–886. doi: 10.1172/JCI13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rot U, Ledinek AH, Jazbec SS. Clinical, magnetic resonance imaging, cerebrospinal fluid and electrophysiological characteristics of the earliest multiple sclerosis. Clin Neurol Neurosurg. 2008;110:233–238. doi: 10.1016/j.clineuro.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 56.de Graaf MT, Smitt PA, Luitwieler RL, van Velzen C, van den Broek PD, Kraan J, et al. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom. 2011;80:43–50. doi: 10.1002/cyto.b.20542. [DOI] [PubMed] [Google Scholar]
- 57.Svenningsson A, Andersen O, Edsbagge M, Stemme S. Lymphocyte phenotype and subset distribution in normal cerebrospinal fluid. J Neuroimmunol. 1995;63:39–46. doi: 10.1016/0165-5728(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 58.Haas J, Bekeredjian-Ding I, Milkova M, Balint B, Schwarz A, Korporal M, et al. B cells undergo unique compartmentalized redistribution in multiple sclerosis. J Autoimmun. 2011;37:289–299. doi: 10.1016/j.jaut.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 59.von Budingen HC, Kuo TC, Sirota M, van Belle CJ, Apeltsin L, Glanville J, et al. B cell exchange across the blood-brain barrier in multiple sclerosis. J Clin Invest. 2012;122:4533–4543. doi: 10.1172/JCI63842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SH, Kim W, Li XF, Jung IJ, Kim HJ. Repeated treatment with rituximab based on the assessment of peripheral circulating memory B cells in patients with relapsing neuromyelitis optica over 2 years. Arch Neurol. 2011;68:1412–1420. doi: 10.1001/archneurol.2011.154. [DOI] [PubMed] [Google Scholar]
- 61.Owczarczyk K, Lal P, Abbas AR, Wolslegel K, Holweg CT, Dummer W, et al. A plasmablast biomarker for nonresponse to antibody therapy to CD20 in rheumatoid arthritis. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002432. 101ra92. [DOI] [PubMed] [Google Scholar]
- 62.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. 2011;63:3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 63.Lazarus MN, Turner-Stokes T, Chavele KM, Isenberg DA, Ehrenstein MR. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology (Oxford) 2012;51:1208–1215. doi: 10.1093/rheumatology/ker526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J, et al. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 65.Villar LM, Garcia-Sanchez MI, Costa-Frossard L, Espino M, Roldan E, Paramo D, et al. Immunological markers of optimal response to natalizumab in multiple sclerosis. Arch Neurol. 2012;69:191–197. doi: 10.1001/archneurol.2011.971. [DOI] [PubMed] [Google Scholar]
- 66.Niino M, Hirotani M, Miyazaki Y, Sasaki H. Memory and naive B-cell subsets in patients with multiple sclerosis. Neurosci Lett. 2009;464:74–78. doi: 10.1016/j.neulet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Kleine TO, Benes L. Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A. 2006;69:147–151. doi: 10.1002/cyto.a.20225. [DOI] [PubMed] [Google Scholar]
- 68.Planas R, Jelcic I, Schippling S, Martin R, Sospedra M. Natalizumab treatment perturbs memory- and marginal zone-like B-cell homing in secondary lymphoid organs in multiple sclerosis. Eur J Immunol. 2011;42:790–798. doi: 10.1002/eji.201142108. [DOI] [PubMed] [Google Scholar]
- 69.Lee-Chang C, Top I, Zephir H, Dubucquoi S, Trauet J, Dussart P, et al. Primed status of transitional B cells associated with their presence in the cerebrospinal fluid in early phases of multiple sclerosis. Clin Immunol. 2011;139:12–20. doi: 10.1016/j.clim.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14:164–174. doi: 10.1111/j.1750-3639.2004.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Corcione A, Aloisi F, Serafini B, Capello E, Mancardi GL, Pistoia V, et al. B-cell differentiation in the CNS of patients with multiple sclerosis. Autoimmun Rev. 2005;4:549–554. doi: 10.1016/j.autrev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–1621. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z, Zemlin M, Wang YH, Munfus D, Huye LE, Findley HW, et al. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19:21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 74.Barbas SM, Ditzel HJ, Salonen EM, Yang WP, Silverman GJ, Burton DR. Human autoantibody recognition of DNA. Proc Natl Acad Sci USA. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Itoh K, Meffre E, Albesiano E, Farber A, Dines D, Stein P, et al. Immunoglobulin heavy chain variable region gene replacement as a mechanism for receptor revision in rheumatoid arthritis synovial tissue B lymphocytes. J Exp Med. 2000;192:1151–1164. doi: 10.1084/jem.192.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tiller T, Meffre E, Yurasov S, Tsuiji M, Nussenzweig MC, Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J Immunol Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lefranc MP. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 2001;29:207–209. doi: 10.1093/nar/29.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ligocki AJ, Lovato L, Xiang D, Guidry P, Scheuermann RH, Willis SN, et al. A unique antibody gene signature is prevalent in the central nervous system of patients with multiple sclerosis. J Neuroimmunol. 2010;226:192–193. doi: 10.1016/j.jneuroim.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brezinschek HP, Foster SJ, Brezinschek RI, Dorner T, Domiati-Saad R, Lipsky PE. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5(+)/IgM+ and CD5(−)/IgM + B cells. J Clin Invest. 1997;99:2488–2501. doi: 10.1172/JCI119433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian C, Luskin GK, Dischert KM, Higginbotham JN, Shepherd BE, Crowe JE., Jr Evidence for preferential Ig gene usage and differential TdT and exonuclease activities in human naive and memory B cells. Mol Immunol. 2007;44:2173–2183. doi: 10.1016/j.molimm.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kabat EA, Wu TT, Bilofsky H, Reid-Miller M, Perry H. Sequences of Proteins of Immunological Interest. Washington, DC, USA: United States Department of Health and Human Services; 1983. [Google Scholar]
- 82.Champe PC, Harvey RA. Lippincott’s Illustrated Reviews: Biochemistry. 2nd edn. Lippincott-Raven Publishers; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.