Abstract
Objective
Osteoarthritis affects 1 in 8 American adults over the age of 25 and in the US is a leading cause of chronic disability. Therefore, researchers are pursuing investigations on treating this naturally occurring joint disease. Finding the appropriate animal model for translational purposes is of utmost importance. The aim of the present study is to report the occurrence of naturally-occurring osteoarthritis in the domestic rabbit in the context of an appropriate animal model system.
Methods
A six-year radiographic retrospective study of domestic rabbits was conducted to assess the presence and severity of naturally occurring osteoarthritis in four major joints: the hip, knee, shoulder, and elbow.
Results
The rabbit experiences radiographic signs of naturally-occurring osteoarthritis. There was a significant influence of age on the development of the disease. Rabbits begin to have radiographic signs of the disease as early as 1 year of age with older rabbits experiencing over 70% occurrence. The most commonly effected joints were the knee and the hip. In addition, there was certain correlation of weight with the occurrence of osteoarthritis.
Conclusion
Discovery of new osteoarthritis treatment modalities relies on documenting efficacy in a relevant animal model prior to clinical translation. Despite previous claims on the ability of the rabbit’s cartilage to heal spontaneously and readily repair, we demonstrate that radiographically rabbits demonstrate progressive osteoarthritis. We have found that the rabbit is an excellent spontaneously arising model that may allow a predictable translation of results obtained in bioengineering experiments pertaining to the naturally occurring human disease.
Introduction
Animal models are necessary to study the pathogenesis and pathology of the degenerative joint disease osteoarthritis (OA) and to evaluate the success of potential treatment modalities 1,2. A specific limitation of many induced animal models of OA is that the human disease usually takes years to develop. This is a critical difference that limits the utility of induced models, since studies of adaptive processes and treatment modalities must first take this into account before their findings can be extrapolated to humans 2. Moreover, human OA manifests through a complex interplay of disease-specific characteristics such as genetics and environmental and iatrogenic effects that are difficult, if not impossible, to replicate in an induced model 2.
Current animal models used to study the treatment and progression of OA oftentimes lack the spontaneous occurrence of this disease in the model species and thus suffer from the known differences between naturally occurring and traumatically induced OA. In models where OA has been observed to naturally occur, the small size of the animals limits their utility. For instance, many OA animal models are induced by joint damage via meniscectomy or collateral or cruciate ligament transection, essentially provoking traumatic onset of OA 1,2. Although traumatic causes may mimic certain aspects of the pathogenesis and pathology of OA, there are some crucial differences. As an example, unlike the human condition in which the use of an affected limb is restricted following injury, rodents will generally resume near-normal activity soon after induction of experimental traumatic OA 1,2. Therefore, the disease progression in the animal model following surgical induction is much faster than that of naturally occurring OA in human, making it a less realistic model 3,4. In addition, intra-articular injections of arthritis-inducing substances can induce OA but may also result in other undesired changes 2,5,6. In the cases where spontaneous OA has been described, the animal models are either too small (guinea-pig, Syrian hamster) or costly (non-human primates) 1,7–10. One of the inherent disadvantages of using small species (e.g., hamster or mouse) as a model is that in most cases only the knee can be studied and surgical approaches are technically demanding or impossible 2. While the pathology and pathogenesis of OA in the guinea-pig and Syrian hamster is likely to be similar to human OA 1, an intermediate-sized animal model where OA naturally occurs is still conspicuously absent.
Conventional x-radiography is the standard diagnostic modality for imaging evaluation of OA; however, radiographic features of OA are evident only in the presence of moderate to advanced disease 11. In the early stages of OA, imaging techniques such as radiography, ultrasound or magnetic resonance imaging (MRI) are often considered insufficient 11,12. Nonetheless, radiography is the current standard, represents the broadest basis of experience, and is a common initial assessment and monitoring tool for ongoing clinical studies of treatment modalities for OA 11,13–15.
To the best of our knowledge, the existence of naturally-occurring OA in the rabbit has not been previously described. Therefore, the aim of the present study is to report the presence, occurrence, and prevalence of the radiographic signs of naturally-occurring OA in the domestic rabbit, and its possible association with age and weight. The hypotheses of this study are that rabbits develop OA, OA prevalence increases with increasing age and weight, and that due to the anatomic conformation of the species, the knee and the hip are the most commonly affected joints.
Materials and methods
Inclusion Criteria
Skeletally mature (over 1-year-old) domestic rabbits of various breeds admitted to the William R. Pritchard Veterinary Medical Teaching hospital at the University of California Davis (January 2004 – November 2010) for reasons other than orthopedic disease and had radiographic examination that included at least one pair of appendicular joints (hip, knee, shoulder and elbow) were included in this study. Exclusion criteria were presence of lameness, history or gross imaging evidence of orthopedic trauma, systemic disease affecting the skeleton (e.g., neoplasia) or metabolic disease. The rabbits were divided into 4 age groups: group 0 = rabbits aged 1 up to 3, group 1 = rabbits aged 3 and up to 6, group 2 = rabbits aged 6 and up to 9, and group 3 = rabbits over the age of 9.
Image evaluation
Radiographs were acquired using a commercially available digital detector (Eklin Medical system Inc. Santa Clara Inc). All digital images were evaluated and scored by a single blinded observer (ERW) on a medical grade flat-screen monitor, using commercially available software (e.Film work station 2.1.0, e.Film Medical Inc. Toronto, Canada). No image enhancement or contrast agent was applied. A semi-quantitative scoring system was applied to each radiographic image. Scoring (0–3) was defined as 0 = no OA, 1 = mild OA, 2 = moderate OA, 3 = marked OA (Figure 1 and 2). Joints were scored as having mild OA if there was any evidence of meniscal mineralization, mild remodeling of subchondral bone, early signs of periarticular new bone formation, or small, separate osseous bodies within the joint space. Joints were scored as having moderate OA if the previously described features were more pronounced. Joints were scored as having marked OA if the previously described signs were more pronounced or if large separate osseous bodies were evident within the joint space or moderate subluxation was present.
Figure 1.
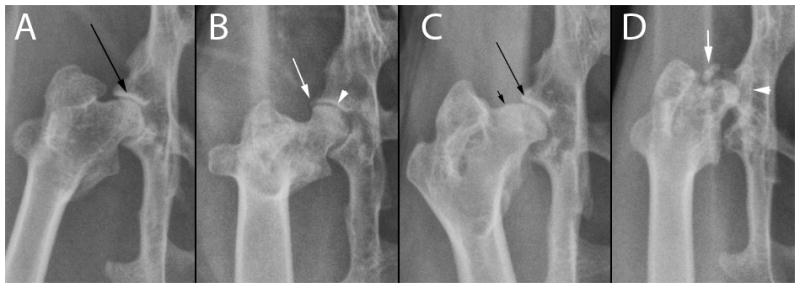
A. Normal coxofemoral joint; B. Mild osteoarthritis. Minimal periarticular new bone formation arising from the distal femur and proximal tibia (arrows); C. Moderate osteoarthritis. Minimal periarticular new bone formation involving the medial aspect of the proximal tibia (right arrow), meniscal mineralization (arrowhead), and a separate osseous body within the joint space; D. Marked osteoarthitis, pronounced periarticular new bone formation involving the distal femur and proximal tibia (arrows, dashed lines), architectural remodeling of subchondral bone in the proximal tibia (arrowhead), subchondral sclerosis of the lateral and medial femoral condyles.
Figure 2.
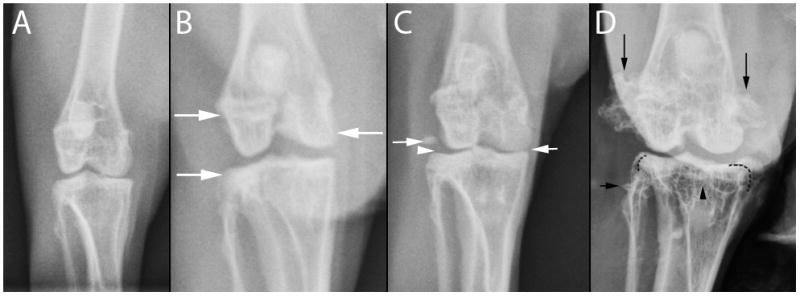
A. Normal femorotibial joint; B. Mild osteoarthritis. Minimal periarticular new bone formation (arrow), regional joint space narrowing (arrowhead); C. Moderate osteoarthritis. Mild suchondral bone sclerosis (arrow), remodeling of the femoral head and neck associated with new bone formation (small arrow); D. Marked osteoarthritis. Pronounced coxafemoral subluxation, large osseous body within the joint space, remodeling of femoral head and neck and acetabulum.
Statistical Analysis
In order to determine the possible association of the presence or absence of OA with increasing age the Cochran – Armitage trend test was applied. The Jonckheere – Terpstra Test was applied to assess the quantitative increase in OA score of the hip or knee joints with increasing age. Binary logistic regression was applied to determine the possible association of body weight with OA, with the result presented as odds ratio (OR) and 95% confidence interval (CI). McNemar’s test was used to compare the joint distribution of OA between the forelimb and hindlimb. Finally, a chi-square test for homogeneity was performed to evaluate the association of rabbit breed with OA. For all tests p<0.05 was considered statistically significant.
Results
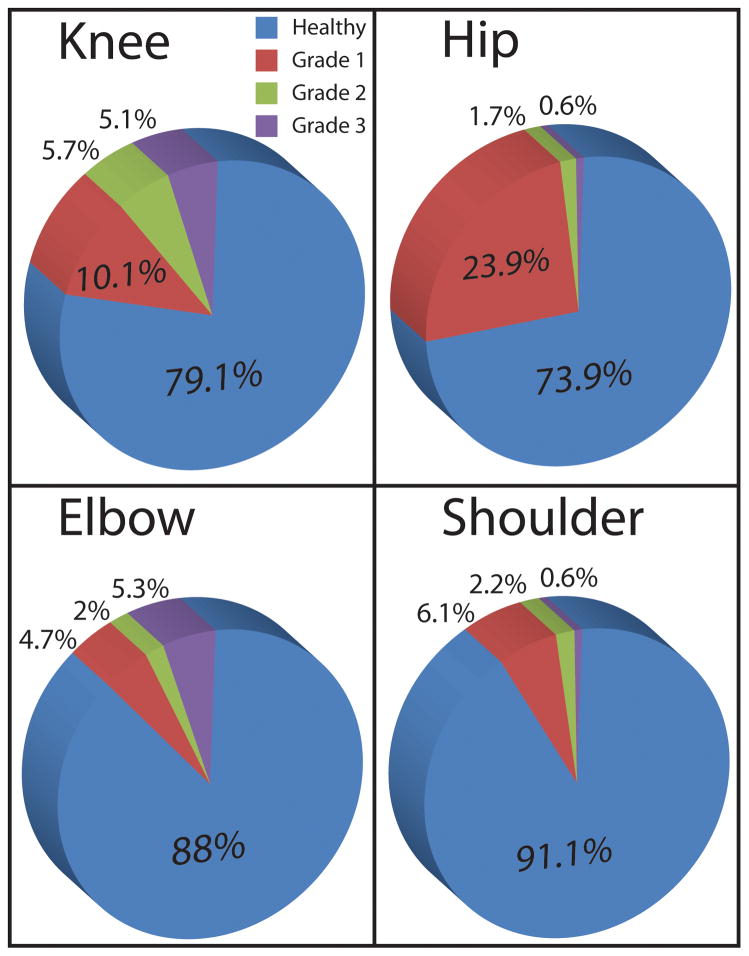
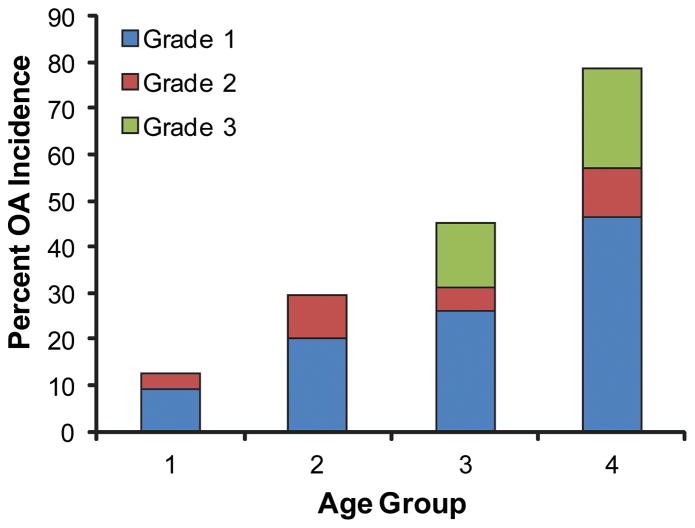
Of 330 rabbits included in the medical record system, 189 met the inclusion criteria. Overall, OA was found in 40.2% of the studied population. The involvement and the severity of radiographic changes of OA in each of the examined joints are expressed in Figure 3. The most commonly affected joints were the hip and the knees (Figure 3). Moreover, out of the affected population, 37% had involvement of two or more pair of joints, and 13% had three or more pair of joints affected. There was a significant association between age and the development of OA (p<0.0001) (Figure 4). In addition, there was strong positive correlation between multiple joint (two or more) involvement and increase in age (p<0.001). The osteoarthritic population was significantly (p<0.0001) more likely to have the hindlimb affected with OA and forelimb without OA versus having the forelimb affected with OA and hindlimb not affected. Radiographic changes indicative of OA were already observed in group 0 (12.5%) and increased to over 70% in group 3. It is important to note that around the age of 6 about 50% of the population exhibit radiographic changes of OA in at least one joint. Moreover, as age increased, the maximum OA score for the hip and the knee increased (p<0.001). There was a significant (p = 0.0075) association between rabbit breeds and the presence of OA, with a lower incidence associated with dwarf rabbit breeds. In addition, there was no significant influence of weight or gender on the presence of OA. However, rabbits weighting over 5kg were more likely to exhibit radiographic signs of OA than those 5kg or less (OR = 10.5, 95% CI = 1.4 - ∞; p = 0.019).
Figure 3.
The involvement and the severity of radiographic changes of OA of each of the examined joints. The most commonly affected joints were the hip and the knee.
Figure 4.
The influence of age on the development of OA. As age increased, the prevalence of OA increased (P<0.0001). As age increased, the maximum OA score for the hip and the knee increased (P<0.001).
Discussion
OA is a slowly progressive degenerative joint disease with larger diarthrodial joints being most commonly affected 16,17. Conservatively, OA affects 1 in 8 American adults over the age of 25, is a leading cause of disability in the US, and the estimated cost of this disorder exceeds $65 billion per year (both medical costs and lost wages) 17–19. To investigate OA treatment methods, it is imperative to find an appropriate animal model that will simulate the slowly developing disease process and accurately reflect the pathogenesis and pathology of the disorder. Spontaneous models such as the guinea pig, mouse, hamster and non-human primate offer the best opportunity to study the slow progression of OA that is most characteristic of the human disease 1. However, use of these models suffers from small joint size of small animal models and ethical concerns regarding the use of non-human primates in preliminary animal studies. To our knowledge, the present study is the first to report the occurrence and distribution of naturally occurring OA in the rabbit and points toward its possible application as model for spontaneous OA.
Radiographic OA quantification remains the standard diagnostic tool for the evaluation of OA progression due to the positive correlation of radiographic features with gross macroscopic lesions (e.g., cartilage and meniscal degeneration, osteophyte formation and tibial cartilage lesions) 20–22. The clinical diagnosis of OA is usually based on symptoms, confirmed radiographically, and graded using a semi-quantitative scoring system that has been validated in clinical and epidemiological studies 16,21,23. Thus in this study, radiographs were used to semi-quantitatively assess naturally-occurring OA. The major drawback in using radiographs to diagnose OA is that radiography is only able to detect degenerative changes if moderate to advanced lesions are present 11. For example, one study demonstrated that following meniscectomy in rabbits, the changes to the cartilage that occurred up to 40 weeks post surgery were not extensive enough to be detected by radiography 24. Thus, the incidence of OA reported in this study is likely an underestimation of the true prevalence of naturally-occurring OA in the rabbit.
When selecting an animal model, it is critical that it represents a high level of translatability to the human. In the case of OA, researchers should attempt to match features of human OA including spontaneous occurrence, lack of regenerative response, and association of incidence and severity with age and weight. In addition to matching characteristics of the human disease, it is important to consider the practicality of using a particular animal model. These considerations include a large enough joint for surgical interventions and a sufficient amount of tissue to perform in-depth tissue characterization. Spontaneous OA in humans has been shown to be intimately linked to age and obesity 9,25. In this study we observed that rabbits also follow these trends with increasing OA prevalence with age and with body weight greater than the normal range (>5kg). Previous studies have investigated the healing response following damage to the cartilage of adult rabbits and have determined that, similar to humans, only fibrocartilaginous repair tissue is generated 26–28. The small animal models currently used for studying OA include guinea pigs and mice. While these models may be useful in drug discovery, their utility when exploring surgical interventions is severely hindered due to the small size of their joints. As demonstrated by numerous studies employing the rabbit model, complex surgical procedures can be performed on adult rabbit joints and the larger amounts of tissue obtained allow for in-depth tissue characterization to be performed 29–32. In light of the results of this retrospective study, rabbit OA appears to mimic many characteristics of the human disease.
Among adults in the US, nearly 27 million people 25 years of age and older have osteoarthritis, and with the aging of the US population, these estimates are likely to increase in the coming years 17. Moreover, OA prevalence increases with age, and the knees, hips, and hands are the joints most commonly affected. The present study documents significant positive association of age and radiographic signs of OA in the domestic rabbit. As veterinary medicine advances and owner awareness increases, more pet animals are reaching an advanced age, allowing for the prevalence of chronic disorders such as OA to be observed. In addition, body weight in humans and guinea pigs has been found to be an important predisposing factor for the development of spontaneous OA 9,25. This study reveals a similar pattern whereby rabbits weighting up to 5kg do not exhibit significant association with OA; when obesity is present (e.g., rabbits over the weight of 5kg), a positive association with OA is demonstrated. It is plausible that if a vendor is wishing to create a spontaneous OA condition in rabbits, combining older age and heavier weight will result in a high incidence of naturally-occurring disease. The similarities associated with naturally-occurring OA in humans and rabbits could further support the use of the rabbit as a spontaneous model for OA research.
When choosing the appropriate animal model for OA, the age of the animal plays a critical role. To begin with, the physeal closure of the distal femur in New Zealand White rabbits occurs between 4.5–5.7 months and closure of the proximal tibial physis occurs between 5.9–7.6 months 33. In addition, there is wide variability regarding the time when bone maturity is achieved in the rabbits 33. Indeed, when reviewing the radiographs in the present study, we detected few cases in which 9–10 months old rabbits still had open physes in the distal femur and proximal tibia. This fact is of importance both biologically and practically when planning orthopedic experiments in the rabbits 33. Therefore, we highly recommend utilizing only confirmed skeletally mature rabbits for biomedical engineering studies; for example using medical records or preoperative radiographs. Evaluation of cartilage healing in young vs. adult rabbits revealed important and clinically relevant findings 27. First, in the young rabbit (4 weeks old), trauma to cartilage perpendicular to its surface resulted in regression and necrosis of the tissue within 3 days; at 4–6 weeks following the trauma there were demarcating fibers covering and protecting the cartilage fragment 27. In comparison, when the cartilage of adult rabbits (24 months old) was subjected to trauma, tissue splitting was identified by the absence of wound healing potential and the repair process did not occur 27. In addition, no reaction of chondrocytes and lack of mitotic activity were observed in adult rabbits; whereas, in young rabbits these responses were observed 27. Clearly, the maturity of the cartilage is an important factor determining whether cartilage repair occurs and to what extent 34.
Combining age-relevant aspects of the model animals with naturally occurring OA suggests that mature rabbits with naturally-occurring disease may be the ideal OA model. At present, bioengineering efforts to induce healing and regeneration of cartilage are directed toward enhancing natural healing potential of cartilage or replacing the damaged cartilage with engineered tissues 35. These approaches, although promising, are far from reliable and are not yet sufficiently refined to be employed in the clinical setting. A key to successful identification of the ideal treatment modalities will be reliant on the selection of an animal model that mimics the natural OA progression of humans 35.
Previous studies determined that an articular cartilage defect in the rabbit has the ability to heal spontaneously 36,37. A number of review articles have claimed that rabbit articular cartilage is able to readily repair unlike human cartilage, and due to this the rabbit model has lost favor 38,39. In addition, it was also demonstrated that the pattern of healing depends largely on the size of the defect and the time from cartilage insult to sacrifice 36,37,40. Contrary to these observations and claims, the present study clearly demonstrates that when viewing a larger population of rabbits, if a healing response does occur, it is only temporary and, if allowed to live longer, rabbits exhibit cartilage deterioration and OA as observed in humans. These findings are in agreement with other investigations that demonstrate that articular cartilage defects may undergo repair with either fibrous or fibrocartilaginous tissue and that this tissue is prone to degeneration over long term 28,41. With time, the repaired tissue exhibits loss of matrix cellularity and proteoglycans, as well as loss of physical integrity of the surface 28,41.
In conclusion, the rabbit is a commonly used model for the study of articular cartilage repair and changes associated with defect creation or chemical insult 42. This creates an OA-like condition but may not mimic the slowly progressing disease as seen in humans. This study has documented that rabbit OA shares a similar progression pattern with over 10% demonstrating radiographic signs of OA at a young age and about 50% exhibiting radiographic signs of the spontaneous disease by the age of six. In addition to the age factor, this study also identified a link between rabbit OA and obesity, similar to that seen in humans. The size of rabbits is amenable to surgical application of bioengineering efforts such as implantation of engineered cartilage or cartilage modifying agents, while the use of smaller models such as the hamster or the mice may be technique limiting and time consuming. This study therefore recommends that future efforts should be directed toward validation of the rabbit as a spontaneous model for OA and its consideration as a leading animal model for studying treatment modalities.
Acknowledgments
This work was supported by NIH R01 AR053286 and an Innovative Research Grant from the Arthritis Foundation.
Footnotes
No financial support or benefits were derived from commercial sources, and there is no conflict of interest of any of the authors.
Reference List
- 1.Bendele AM. Animal models of osteoarthritis in an era of molecular biology. J Musculoskelet Neuronal Interact. 2002;2(6):501–503. [PubMed] [Google Scholar]
- 2.Dinser R. Animal models for arthritis. Best Pract Res Clin Rheumatol. 2008;22(2):253–267. doi: 10.1016/j.berh.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Bendele AM. Progressive chronic osteoarthritis in femorotibial joints of partial medial meniscectomized guinea pigs. Vet Pathol. 1987;24(5):444–448. doi: 10.1177/030098588702400512. [DOI] [PubMed] [Google Scholar]
- 4.Bendele AM, White SL. Early histopathologic and ultrastructural alterations in femorotibial joints of partial medial meniscectomized guinea pigs. Vet Pathol. 1987;24(5):436–443. doi: 10.1177/030098588702400511. [DOI] [PubMed] [Google Scholar]
- 5.van der Kraan PM, Vitters EL, van de Putte LB, et al. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol. 1989;135(6):1001–1014. [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kraan PM, Vitters EL, van Beuningen HM, et al. Degenerative knee joint lesions in mice after a single intra-articular collagenase injection. A new model of osteoarthritis. J Exp Pathol (Oxford) 1990;71(1):19–31. [PMC free article] [PubMed] [Google Scholar]
- 7.Bendele AM, Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;31(4):561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- 8.Bendele AM, White SL, Hulman JF. Osteoarthrosis in guinea pigs: histopathologic and scanning electron microscopic features. Lab Anim Sci. 1989;39(2):115–121. [PubMed] [Google Scholar]
- 9.Bendele AM, Hulman JF. Effects of body weight restriction on the development and progression of spontaneous osteoarthritis in guinea pigs. Arthritis Rheum. 1991;34(9):1180–1184. doi: 10.1002/art.1780340916. [DOI] [PubMed] [Google Scholar]
- 10.Silberberg RSJSG. Degenerative jointdisease in Syrian hamsters. 1952;11:427–432. [Google Scholar]
- 11.Coan P, Wagner A, Bravin A, et al. In vivo x-ray phase contrast analyzer-based imaging for longitudinal osteoarthritis studies in guinea pigs. Phys Med Biol. 2010;55(24):7649–7662. doi: 10.1088/0031-9155/55/24/017. [DOI] [PubMed] [Google Scholar]
- 12.Mollenhauer J, Aurich ME, Zhong Z, et al. Diffraction-enhanced X-ray imaging of articular cartilage. Osteoarthritis Cartilage. 2002;10(3):163–171. doi: 10.1053/joca.2001.0496. [DOI] [PubMed] [Google Scholar]
- 13.Dieppe PA. Recommended methodology for assessing the progression of osteoarthritis of the hip and knee joints. Osteoarthritis Cartilage. 1995;3(2):73–77. doi: 10.1016/s1063-4584(05)80040-8. [DOI] [PubMed] [Google Scholar]
- 14.Muraki S, Akune T, Oka H, et al. Association of radiographic and symptomatic knee osteoarthritis with health-related quality of life in a population-based cohort study in Japan: the ROAD study. Osteoarthritis Cartilage. 2010;18(9):1227–1234. doi: 10.1016/j.joca.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Szebenyi B, Hollander AP, Dieppe P, et al. Associations between pain, function, and radiographic features in osteoarthritis of the knee. Arthritis Rheum. 2006;54(1):230–235. doi: 10.1002/art.21534. [DOI] [PubMed] [Google Scholar]
- 16.Kinds MB, Welsing PM, Vignon EP, et al. A systematic review of the association between radiographic and clinical osteoarthritis of hip and knee. Osteoarthritis Cartilage. 2011 doi: 10.1016/j.joca.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.Jackson DW, Simon TM, Aberman HM. Symptomatic articular cartilage degeneration: the impact in the new millennium. Clin Orthop Relat Res. 2001;(391 Suppl):S14–S25. [PubMed] [Google Scholar]
- 20.Altman R, Brandt K, Hochberg M, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996;4(4):217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 21.Boulocher CB, Viguier ER, Cararo RR, et al. Radiographic assessment of the femorotibial joint of the CCLT rabbit experimental model of osteoarthritis. BMC Med Imaging. 2010;10:3. doi: 10.1186/1471-2342-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovati LC. Radiographic assessment. Introduction: existing methodology. Osteoarthritis Cartilage. 1999;7(4):427–429. doi: 10.1053/joca.1998.0233. [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ, Felson DT. Osteoarthritis. BMJ. 2006;332(7542):639–642. doi: 10.1136/bmj.332.7542.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messner K, Fahlgren A, Persliden J, et al. Radiographic joint space narrowing and histologic changes in a rabbit meniscectomy model of early knee osteoarthrosis. Am J Sports Med. 2001;29(2):151–160. doi: 10.1177/03635465010290020701. [DOI] [PubMed] [Google Scholar]
- 25.Davis MA, Ettinger WH, Neuhaus JM, et al. Sex differences in osteoarthritis of the knee. The role of obesity. Am J Epidemiol. 1988;127(5):1019–1030. doi: 10.1093/oxfordjournals.aje.a114878. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell N, Shepard N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. J Bone Joint Surg Am. 1976;58(2):230–233. [PubMed] [Google Scholar]
- 27.Verwoerd-Verhoef HL, ten Koppel PG, van Osch GJ, et al. Wound healing of cartilage structures in the head and neck region. Int J Pediatr Otorhinolaryngol. 1998;43(3):241–251. doi: 10.1016/s0165-5876(98)00003-2. [DOI] [PubMed] [Google Scholar]
- 28.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Yang X, Liao Y, et al. MRI and histologic analysis of collagen type II sponge on repairing the cartilage defects of rabbit knee joints. J Biomed Mater Res B Appl Biomater. 2011;96(2):267–275. doi: 10.1002/jbm.b.31762. [DOI] [PubMed] [Google Scholar]
- 30.Isaac DI, Meyer EG, Kopke KS, et al. Chronic changes in the rabbit tibial plateau following blunt trauma to the tibiofemoral joint. J Biomech. 2010;43(9):1682–1688. doi: 10.1016/j.jbiomech.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Shirai T, Kobayashi M, Nishitani K, et al. Chondroprotective effect of alendronate in a rabbit model of osteoarthritis. J Orthop Res. 2011 doi: 10.1002/jor.21394. [DOI] [PubMed] [Google Scholar]
- 32.Vaseenon T, Tochigi Y, Heiner AD, et al. Organ-level histological and biomechanical responses from localized osteoarticular injury in the rabbit knee. J Orthop Res. 2011;29(3):340–346. doi: 10.1002/jor.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaweblum M, Aguilar MC, Blancas E, et al. Histological and radiographic determination of the age of physeal closure of the distal femur, proximal tibia, and proximal fibula of the New Zealand white rabbit. J Orthop Res. 1994;12(5):747–749. doi: 10.1002/jor.1100120519. [DOI] [PubMed] [Google Scholar]
- 34.Silver FH, Glasgold AI. Cartilage wound healing. An overview. Otolaryngol Clin North Am. 1995;28(5):847–864. [PubMed] [Google Scholar]
- 35.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80(12):1795–1812. [PubMed] [Google Scholar]
- 36.Lietman SA, Miyamoto S, Brown PR, et al. The temporal sequence of spontaneous repair of osteochondral defects in the knees of rabbits is dependent on the geometry of the defect. J Bone Joint Surg Br. 2002;84(4):600–606. doi: 10.1302/0301-620x.84b4.11631. [DOI] [PubMed] [Google Scholar]
- 37.Otsuka Y, Mizuta H, Takagi K, et al. Requirement of fibroblast growth factor signaling for regeneration of epiphyseal morphology in rabbit full-thickness defects of articular cartilage. Dev Growth Differ. 1997;39(2):143–156. doi: 10.1046/j.1440-169x.1997.t01-1-00003.x. [DOI] [PubMed] [Google Scholar]
- 38.Little CB, Smith MM. Anonymous. 4. Oak Park: Bentham Science Publishers Ltd; 2008. Animal models of osteoarthritis. [Google Scholar]
- 39.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75(4):532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Wei X, Gao J, Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res. 1997;34(1):63–72. doi: 10.1002/(sici)1097-4636(199701)34:1<63::aid-jbm9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Aroen A, Heir S, Loken S, et al. Articular cartilage defects in a rabbit model, retention rate of periosteal flap cover. Acta Orthop. 2005;76(2):220–224. doi: 10.1080/00016470510030607. [DOI] [PubMed] [Google Scholar]