Abstract
Lenalidomide is an effective therapy against malignant plasma cells and a potent agent against proinflammatory and proangiogenic cytokines. The use of lenalidomide in POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein with plasma cells, skin changes) has been reported, but its benefit in long-term use is not well established. A 55-year-old man with POEMS and debilitating polyneuropathy was treated with lenalidomide and dexamethasone followed by maintenance lenalidomide. He remains in haematologic remission and in complete recovery of functional status 3.5 years after diagnosis. This case supports the long-term use of lenalidomide in patients with POEMS syndrome.
Keywords: Lenalidomide, POEMS
Introduction
POEMS syndrome, first described by Bardwick et al., is a constellation of symptoms associated with a rare plasma cell disorder [1]. As described in the acronym, patients present with polyneuropathy, organomegaly (Castleman’s disease), endocrinopathy, monoclonal gammopathy and skin changes. Misdiagnosis of POEMS syndrome is not uncommon, as it is often indolent with unusual symptoms. Other accompanying clinical features and laboratory findings include papilloedema, volume overload, sclerotic bony lesions, thrombocytosis, elevated vascular endothelial growth factor (VEGF) and tendency for thromboembolic events. Castleman’s disease, a lymphoproliferative disorder, is present in about 11%–30% of POEMS patients [2]. Elevation of angiogenic cytokines such as VEGF is thought to be the pathogenic association of Castleman’s to POEMS syndrome [2].
The treatment for POEMS syndrome is mostly derived from regimens that have shown efficacy in other plasma cell dyscrasia; for example, the use of autologous stem cell transplant (ASCT) in multiple myeloma patients [3]. In addition to being a powerful immune modulator against malignant plasma cells, lenalidomide has been shown to reduce proinflammatory and proangiogenic cytokines, which are known cellular disturbances in POEMS syndrome [2]. Here we describe a patient with POEMS syndrome who had a dramatic clinical improvement after the long-term use of lenalidomide without an ASCT.
Methods
A 55-year-old man presented with progressive polyneuropathy over a one-year period. He was initially diagnosed with chronic inflammatory polyneuropathy. He received rituximab, high-dose intravenous immunoglobulin and approximately one month of dexamethasone without any improvement. He also received two cycles of rituximab, cyclophosphamide, adriamycin, vincristine and prednisone after a diagnosis of Castleman’s disease. He was transferred to our institution due to progressive severe motor dysfunction.
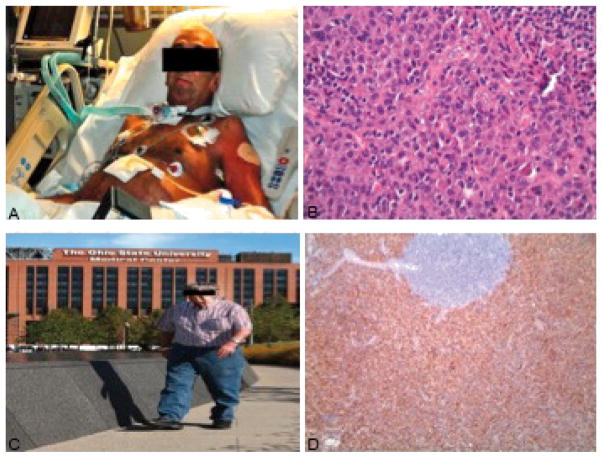
Physical examination was remarkable for skin discoloration, pitting of the nail beds and profound motor deficit (Fig. 1a).
Figure 1.
(a) Picture taken at diagnosis. (b) Haematoxylin and eosin stains at 40× demonstrating sheets of mature and atypical plasma cells with rare germinal centres. (c) CD138 stain indicating plasma cells. (d) Picture taken 3 years after starting treatment.
His only appreciable movement on examination was left second finger flexion. Laboratory studies revealed hypothyroidism and hypogonadism. Serum protein electrophoresis showed the presence of monoclonal protein. Serum lambda light chain was elevated. HIV, hepatitis B, HHV-8 and EBV were negative. VEGF was not performed. Other relevant laboratory data and physical exam findings are shown in Table 1.
Table 1.
Progression of clinical features following therapy in a patient with POEMS syndrome
| Date | |||
|---|---|---|---|
| September 2010 | September 2011 | June 2013 | |
| Nail bed changes | Present | None | None |
| Hyperpigmentation | Present | None | None |
| Oedema | ++++ | Trace | None |
| Performance status (ECOG) | 4 | 2 | 1 |
| IgG (600–1500 mg/dl) | 1470 | 204 | 594 |
| IgA (82–453 mg/dl) | 186 | 38.5 | 122 |
| Kappa (3.3–19.4 mg/dl) | 60.8 | <2.7 | <2.7 |
| Lambda (5.7–26.3 mg/dl) | 118.00 | <2.35 | 5.37 |
| K/L ratio | 0.52 | Not calculated | Not calculated |
| Beta 2 microglobulin (0.60–2.11 mg/l) | 4.9 | 4.5 | 3.1 |
| Monoclonal protein IgG lambda (mg/dl) | 775 | 25 | 73 |
| Bone marrow | 10% plasma cells | – | – |
| Cytogenetics | Normal | – | – |
| VEGF (31–86 pg/ml) | – | 74 | <31 |
| TSH (0.55–4.78 μIU/ml) | 19.257 | 5.629 | 2.133 |
| Testosterone, total (241–827 ng/dl) | 55 | 624 | 457 |
| Free testosterone (4.00–27.00 pg/ml) | 0.1 | 20.3 | – |
| 24-h urine immunofixation | Negative | Negative | Negative |
| Respiratory status | Tracheostomy 40% FiO2 | 2L oxygen | Room air; tracheostomy closed |
| Motor polyneuropathy | Flaccid muscle weakness | Ambulate with assistance | Ambulate without assistance |
A computed tomography scan of the chest and abdomen showed diffuse body wall oedema, periportal oedema, ascites, bilateral pleural effusions and multiple retroperitoneal and para-aortic lymph nodes including an enlarged portal caval lymph node measuring 4.9×4.3 cm. Biopsy of the dominant portal caval lymph node demonstrated sheets of mature and atypical plasma cells with rare germinal centres (Fig. 1b, c), hyalinized blood vessels and HHV8 negative consistent with HHV8 negative Castleman’s lymphadenopathy.
Bone marrow biopsy showed a mildly increased predominance of plasma cells producing lambda light chains (10% by CD138 immunostain). Electromyography demonstrated severe sensory motor neuropathy with prominent features of demyelination and secondary axonal degeneration. The patient had to be intubated for neuromuscular respiratory failure and eventually received a tracheotomy.
Results
The patient received five sessions of plasmapheresis without any improvement. He was then started on lenalidomide (25 mg, days 1–21) and dexamethasone (40 mg weekly) in October 2010. The lenalidomide dose was reduced (15 mg, days 1–21 every 28 days) after hospitalization for neutropenic fever and bacteraemia. With intensive physical and occupational therapy, there was steady improvement of the patient’s motor function. After four cycles of treatment, his lambda light chain normalized. Monoclonal protein declined to 5.2 mg/dl from 775 mg/dl. He was evaluated for ASCT but unfortunately failed stem cell mobilization. After 12 months of treatment, his respiratory function returned close to baseline. Testosterone and thyroid function normalized and there was resolution of lymphadenopathy. His treatment was switched to lenalidomide alone 5 mg daily after 15 months of combination therapy.
His clinical symptoms continued to recover; in particular, his motor function made the most remarkable recovery. He was able to ambulate and performed get-up-and-go without any assistance (Fig. 1d). In addition to regaining his functional capabilities, several early physical findings, such as the nail bed changes and anasarca, were completely resolved. The trends in his laboratory data are shown in Table 1. At the time of manuscript submission, he remains on lenalidomide single agent at 5 mg daily.
Discussion
The recent advancement in the understanding of POEMS’s pathogenesis has given new insights into developing a novel therapeutic strategy [4]. Proangiogenic cytokine, such as VEGF, is believed to play a central role in the clinical manifestations of POEMS syndrome and Castleman’s disease. VEGF, likely originating from abnormal plasma cells, has been associated with vasopermeability, leading to the transudate effusions commonly seen in these patients [2].
Management of POEMS is largely derived from management of other plasma cell dyscrasias, specifically multiple myeloma. While there is no standard of care treatment for patients with POEMS, high-dose chemotherapy with ASCT has routinely been used in eligible patients [3]. D’Souza et al. recently published a long-term outcome study of POEMS patients who underwent ASCT [5]. The 5-year progression-free survival (PFS) and overall survival (OS) were 75% and 94%, respectively, with a median follow-up of 45 months. Our patient failed to mobilize stem cells, likely due to exposure to the initial chemotherapy predating the POEMS diagnosis.
While there has been use of novel agents, such as bortezomib and lenalidomide [6–8], the length of treatment is not well established for POEMS patients who are not ASCT candidates, and there is limited experience in the use of maintenance therapy. Our rationale of maintenance strategy is derived from the observed PFS and OS benefits in multiple myeloma patients receiving maintenance lenalidomide [9].
The treatment for POEMS syndrome is certainly evolving. We are developing new therapeutic strategies as we begin to understand the underlying mechanism of this complex disease. The shared characteristics with other plasma cell dyscrasias provided the backbone for our current treatment strategy for this rare syndrome. Use of lenalidomide and dexamethasone and the maintenance single-agent lenalidomide has markedly improved our patient’s symptoms and functional status. In conclusion, while further study is required to establish its role in routine practice, long-term use of lenalidomide is a viable treatment option for patients with POEMS syndrome.
Learning Points.
Lenalidomide and dexamethasone provided a substantial response in a patient with POEMS.
Long-term use of lenalidomide in POEMS is feasible.
Acknowledgments
Funding provided in part by the National Institute of Health Translational Training Grant in Experimental Therapeutics K 12 CA133250-01.
Footnotes
Conflicts of Interests: The authors declare that they have no conflicts of interest related to this research
References
- 1.Bardwick PA, Zvaifler NJ, Gill GN, Newman D, Greenway GD, Resnick DL. Plasma cell dyscrasia with polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes: the POEMS syndrome. Report on two cases and a review of the literature. Medicine. 1980;59:311–322. doi: 10.1097/00005792-198007000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Dispenzieri A. POEMS syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol. 2012;87:804–814. doi: 10.1002/ajh.23288. [DOI] [PubMed] [Google Scholar]
- 3.Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119:5650–5658. doi: 10.1182/blood-2012-03-378992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zhou DB. New advances in the diagnosis and treatment of POEMS syndrome. Br J Haematol. 2013;161:303–315. doi: 10.1111/bjh.12236. [DOI] [PubMed] [Google Scholar]
- 5.D’Souza A, Lacy M, Gertz M, Kumar S, Buadi F, Hayman S, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience. Blood. 2012;120:56–62. doi: 10.1182/blood-2012-04-423178. [DOI] [PubMed] [Google Scholar]
- 6.Tomas JF, Giraldo P, Lecumberri R, Nistal S. POEMS syndrome with severe neurological damage clinically recovered with lenalidomide. Haematologica. 2012;97:320–322. doi: 10.3324/haematol.2011.041897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dispenzieri A, Klein CJ, Mauermann ML. Lenalidomide therapy in a patient with POEMS syndrome. Blood. 2007;110:1075–1076. doi: 10.1182/blood-2007-03-082354. [DOI] [PubMed] [Google Scholar]
- 8.Tang X, Shi X, Sun A, Qiu H, Gu B, Zhou H, et al. Successful bortezomib-based treatment in POEMS syndrome. Eur J Haematol. 2009;83:609–610. doi: 10.1111/j.1600-0609.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med. 2012;366:1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]