Abstract
AIM: To investigate the effects of mesenchymal stem cells (MSCs) on dextran sulfate sodium-induced inflammatory bowel disease (IBD).
METHODS: C57BL/6 mice were fed 3.5% (g/L) dextran sulfate sodium. On day seven, the mice received intraperitoneal injections of 1 × 106 MSCs. The survival rate, disease activity index values, and body weight, were monitored daily. On day ten, colon lengths and histopathologic changes were assessed. In addition, immunoregulatory changes following MSC administration were evaluated by determining the levels of effector T cell responses in the spleen and mesenteric lymph nodes, and the expression levels of inflammatory cytokines in homogenized colons.
RESULTS: Intraperitoneal administration of MSCs did not prevent development of colitis and did not reduce the clinicopathologic severity of IBD. No significant difference was evident in either survival rate or disease activity index score between the control and MSC-treated group. Day ten-sacrificed mice exhibited no significant difference in either colon length or histopathologic findings. Indeed, the MSC-treated group exhibited elevated levels of interleukin (IL)-6 and transforming growth factor-β, and a reduced level of IL-10, in spleens, mesenteric lymph nodes, and homogenized colons. The IL-17 level was lower in the mesenteric lymph nodes of the MSC-treated group (P = 0.0126). In homogenized colons, the IL-17 and tumor necrosis factor-α (P = 0.0092) expression levels were also lower in the treated group.
CONCLUSION: MSC infusion provided no significant histopathologic or clinical improvement, thus representing a limited therapeutic approach for IBD. Functional enhancement of MSCs is needed in further study.
Keywords: Crohn’s disease, Dextran sulfate sodium, Inflammatory bowel disease, Mesenchymal stem cells, Ulcerative colitis
Core tip: We evaluated the effects of mesenchymal stem cells (MSCs) on inflammatory bowel disease (IBD). Although MSCs are considered useful therapeutic agents for treatment of IBD, their efficacy has not been immunologically confirmed. We found that MSCs did not exert significant effects and did not restore immune balance or influence levels of interleukins 6 and 10. Recent studies have shown that MSCs may be effective upon local infusion, or in combination with other agents. Therefore, new approaches toward regulation of the immunoregulatory properties of MSCs are required if such cells are to be used to alleviate IBD.
INTRODUCTION
The inflammatory bowel diseases (IBDs) are principally Crohn’s disease and ulcerative colitis. Although the developmental mechanism remains poorly understood, IBD pathogenesis is characterized by the development of an exaggerated immune response to intestinal bacteria in genetically-susceptible individuals[1,2]. The current therapies for IBD include anti-tumor necrosis factor (TNF)-α and anti-interferon (IFN)-γ antibodies, and anti-α-integrin drugs. Although symptoms are thus relieved, there is no cure. Furthermore, relapse is always a risk. Thus, it is essential to develop more effective therapeutic approaches to treat IBD. Recently, the utility of mesenchymal stem cells (MSCs) has been suggested, because MSCs exert immunosuppressive effects and aid in tissue repair[3-8].
Some preclinical studies explored the utility of MSCs in treatment of IBD[9-12]. MSCs were injected subcutaneously, intravenously, or intraperitoneally, and attenuated IBD. However, the MSCs used had been pretreated; the cells used included interleukin (IL)-12p40-transduced MSCs, autoimmune regulator knock-out MSCs, and MSCs coated with mucosal vascular addressin cell adhesion molecule-1 and vascular cell adhesion protein-1 antibodies.
Some clinical data on the use of MSCs to treat IBD have appeared[13-20]. MSCs were infused intralesionally or through a fistula, thus not systemically. The work confirmed that MSCs improved fistular pathogenesis. Recently, allogeneic MSCs have been used to treat IBD; the cells were delivered via intralesional or intrafistular injection. de la Portilla et al[15] considered that local injection was preferable to systemic infusion when IBD was to be treated.
As MSCs exhibit immunomodulatory effects, we hypothesized that MSCs per se would exert therapeutic effects in IBD models. Although MSCs are used to treat autoimmune diseases such as graft-versus-host disease (GvHD), rheumatoid arthritis (RA)[21-26], and possible rejection of skin allografts[27]; any potential role for MSCs in IBD treatment remains unclear. Thus, in the present study, we explored the anti-inflammatory actions of mouse bone-marrow-derived MSCs used to treat dextran sulfate sodium (DSS)-induced IBD.
MATERIALS AND METHODS
Mice
Eight-to-ten-week-old female C57BL/6 (B6, H-2kb) mice were purchased from OrientBio (Sungnam, Korea) and were maintained under specific pathogen-free conditions in an animal facility in which the humidity was controlled at 55% ± 5%, with a 12 h light/dark cycle and a temperature of 22 °C ± 1 °C. The air in the facility was HEPA-filtered to exclude bacteria and viruses. Mouse chow and tap water were available ad libitum. All protocols used were approved by the Animal Care and Use Committee of the Catholic University of Korea.
Induction of DSS-induced colitis
IBD was induced by feeding mice 3.5% DSS (molecular weight 36-50 ku; MP Biomedicals LLC, Santa Ana, CA, United States) dissolved in UV-sterilized tap water, available ad libitum for seven days. On day seven, all animals were returned to plain water. Survival after DSS administration was monitored daily, and disease activity index scores, which evaluate weight loss, stool consistency, and rectal bleeding, were assessed.
Isolation, culture, and administration of MSCs
Donor (C57BL/6, H-2kb) bone marrow cells were collected by flushing mouse femurs and tibias with Dulbecco’s modified Eagle’s medium (Gibco of Thermo Fisher Scientific Inc., Waltham, MA, United States) supplemented with 15% heat-inactivated fetal bovine serum (Gibco). Suspended cells were plated in 95-mm-diameter culture dishes in 1 mL of complete medium, at a density of 2 × 107 cells/mL. Cultures were incubated at 37 °C under 5% CO2 in a humidified chamber. After 3 h, nonadherent cells were removed by changing the medium. Cells at 80% confluency were trypsinized by incubation in 0.5 mL of 0.25% trypsin/1 mmol/L EDTA for 2 min at room temperature. Trypsin was neutralized by addition of 1.5 mL of complete medium. Cells were harvested and expanded in 75-T flasks; cultures were maintained at 37 °C under 5% CO2 in a humidified chamber and subcultured before confluence was attained. After ten passages, the MSCs were surface-stained for c-kit, CD11b, CD34, CD106, CD45, CD31, Sca-1, CD44, and CD29, and were characterized by flow cytometry. Prior to surface staining, the cells were Fc-blocked with 1 μg of mouse spleen and mesenteric lymph nodes 1 × 105 cells for 15 min at room temperature. After blocking, 1 μL amounts of antibody solutions were added and incubation for 30 min at room temperature followed. Unbound antibody was removed by washing the cells in flow cytometry staining buffer. Mice were injected intraperitoneally (ip) with 1 × 106 MSCs one week after DSS induction. Control mice were injected (ip) with equal volumes of PBS (Gibco) at the same time points.
Flow cytometric analysis
Mononuclear cells were immunostained with various combinations of the following fluorescent-label-conjugated antibodies: CD25-APC (eBioscience, San Diego, CA, United States), CD4-Percp (eBioscience), Foxp3-PE (eBioscience), IFN-γ-APC (eBioscience), IL-4-PE (BD PharMingen of Becton, Dickinson and Co., Franklin Lakes, NJ, United States), IL-17-FITC (eBioscience), and IL-6-PE (BioLegend, San Diego, CA, United States). Before staining for intracellular cytokines, the cells were stimulated in culture medium containing phorbol myristate acetate (25 ng/mL; Sigma-Aldrich, St. Louis, MO, United States), ionomycin (250 ng/mL; Sigma-Aldrich), or monensin (GolgiStop, 1 μL/mL; BD PharMingen) in an incubator under 5% CO2 at 37 °C for 4 h. An intracellular staining kit (eBioscience) was used according to the manufacturer’s protocol. Flow cytometry was performed on a fluorescence-activated cell sorting Calibur cytometer (BD PharMingen) running FlowJo software (TreeStar, Ashland, OR, United States).
Histopathologic evaluation
Mice were euthanized for blinded histopathologic analysis of IBD target tissue (the large intestine). Organs were harvested, cryo-embedded, and sectioned. The sections were fixed in 10% buffered formalin (Sigma-Aldrich) and stained with hematoxylin (Sigma-Aldrich) and eosin Y (1% solution; Muto Pure Chemical Co., Ltd, Tokyo, Japan).
ELISAs
Colons were homogenized in buffer [50 mmol/L Tris-HCL (pH 7.4), 250 mmol/L NaCl, 5 mmol/L EDTA] with protease inhibitors (Hoffman La-Roche, Basel, Germany) and centrifuged at 11000 × g for 15 min. The resulting supernatants were collected and subjected to sandwich ELISAs. Solutions of anti-mouse IFN-γ, IL-17, IL-6, IL-10, TNF-α, and TGF-β (RD Systems, Minneapolis, MN, United States) were added to wells of a 96-well plate (Nunc, Roskilde, Denmark) and incubated overnight at 4 °C. Wells were blocked with blocking solution (PBS with 1% bovine serum albumin and 0.05% Tween-20) for 2 h at room temperature. The test samples and standard recombinant IFN-γ, IL-17, IL-6, IL-10, TNF-α, and TGF-β (R and D Systems) were added to separate wells of the 96-well plate and incubated at room temperature for 2 h. The plate was washed, biotinylated antibodies against IFN-γ, IL-17, IL-6, IL-10, and TNF-α, and an anti-TGF-β polyclonal antibody (RD Systems) were added, and reactions allowed to proceed for 2 h at room temperature. The plate was washed, a 2000-fold dilution of ExtrAvidin-alkaline phosphatase (Sigma-Aldrich) was added, and the reaction allowed to proceed for a further 2 h. The plate was washed and 50-μL amounts of P-nitrophenyl phosphate disodium salt (Pierce Chemical Company, Rockford, IL) diluted to 1 mg/mL in diethanolamine buffer were added to each well.
Statistical analysis
Data were analyzed by Student’s t-tests or analyses of variance using GraphPad Prism software (version 5.01, GraphPad Software Inc., La Jolla, CA, United States). Survival was compared between groups by Kaplan-Meier analysis and using the log-rank test. Data are presented as mean ± SD, and a P < 0.05 was considered as significant.
RESULTS
Phenotypes of culture-expanded MSCs
Whole bone marrow cells of C57BL/6 mice were cultured, nonadherent cells removed, and spindle-like cells expanded. Culture-expanded MSCs showed a typical fibroblast-like morphology under the microscope and were uniformly positive for Sca-1, CD44, and CD29, but negative for c-kit, CD11b, CD31, CD34, CD45, CD80, CD86, and CD106 (data not shown).
Clinical outcome of MSC therapy in the DSS-induced IBD model
To explore the effects of MSCs on IBD, we gave single ip injections of donor bone marrow-derived MSCs to DSS-induced IBD mice. C57BL/6 mice were fed 3.5% DSS ad libitum from day zero to day six. On day seven, mice received ip injections of 1 × 106 MSCs. The median survival times were ten days for the control group and eleven days for the MSC-treated group (Figure 1A). Also, the disease activity index scores (Figure 1B) and body weight changes from day zero did not differ between the two groups.
Figure 1.
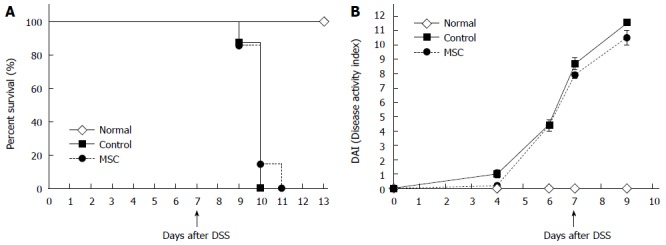
Survival and disease activity index scores. A: Survival after dextran sulfate sodium (DSS) administration (n = 8, all tests were performed in triplicate); B: Disease activity index scores (which consider weight loss, stool consistency, and the extent of rectal bleeding) after DSS administration (n = 10, all tests were performed in triplicate). MSC: Mesenchymal stem cells.
Histopathologic changes following MSC therapy in the DSS-induced IBD model
On day ten, large intestines (from the appendix to the anus) were harvested and colon lengths measured. Generally, shorter colon length correlates with the severity of IBD pathogenesis, and the median colon lengths were 3.8 cm in the control group and 3.7 cm in the MSC-treated group (Figure 2). The median colon length in normal mice not fed DSS was 6.4 cm.
Figure 2.
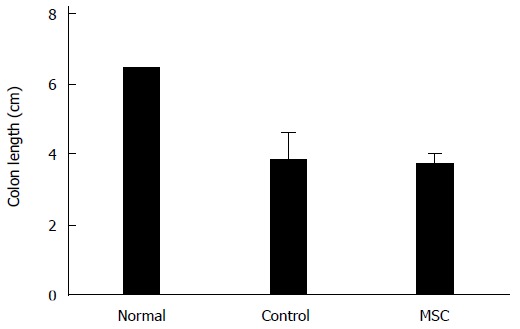
Colon lengths. Colon lengths (from the appendix to the anus) in normal, control and mesenchymal stem cell (MSC)-treated groups (n = 2, all tests were performed in triplicate).
Distal colons were harvested on day ten and stained with hematoxylin and eosin. DSS-induced IBD is characterized by goblet cell loss and inflammatory cell infiltration. Unlike normal mice, the control and MSC-treated groups exhibited epithelial disruption, damage to the lamina muscularis mucosae, and submucosal edema (Figure 3).
Figure 3.
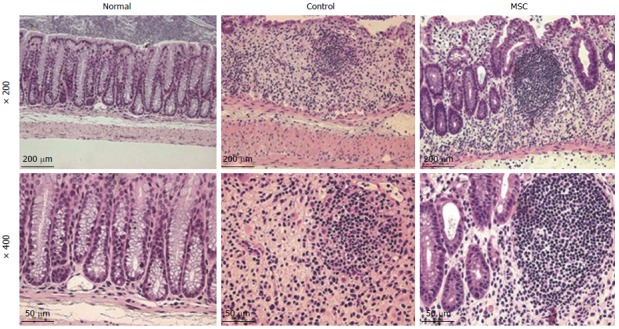
Histopathologic changes in the colon after mesenchymal stem cell infusion. Hematoxylin and eosin staining showed symptoms of colon damage in control and mesenchymal stem cell (MSC)-treated groups, but not in the normal group.
Reciprocal regulation of IL-6 and IL-10 in spleens of mice with DSS-colitis upon MSC therapy
To confirm that MSCs exerted immunoregulatory effects on T cells, we measured the levels of proinflammatory cytokines and helper T cell cytokines, and that of regulatory T (Treg) cells, by flow cytometry. The cytokine levels in the MSC-treated group were similar to those in the control group (Figure 4A). The median percentages of IFN-γ in the control and MSC-treated groups were 1.10 and 1.02%, respectively. The median percentages of IL-4, IL-17, IL-6, IL-10, and Treg cells in the control group were 5.64%, 2.63%, 1.49%, 1.46%, and 22.20%, respectively, and 5.97%, 2.41%, 1.88%, 1.65%, and 21.30%, respectively, in the MSC-treated group. Figure 4B shows the percentages of cytokines in mesenteric lymph nodes; the average percentages of IFN-γ, IL-4, IL-6, IL-10, and Treg cells in the control group were 2.24%, 4.02%, 2.19%, 0.89%, and 22.65%, respectively, and 2.53%, 6.11%, 2.82%, 1.57%, and 21.15%, respectively, in the MSC-treated group. The mean percentage of IL-17 was significantly higher in the control group compared to the MSC-treated group (2.61% vs 1.92%, P = 0.0126). Although the level of IL-17 in mesenteric lymph nodes was slightly higher in the MSC-treated group, the levels of most other cytokines, including IFN-γ, IL-4, IL-6, and IL-10, and Treg cells, did not differ significantly between the groups. Interestingly, in the spleen, the percentage of IL-6 was elevated and that of IL-10 reduced in the MSC-treated group.
Figure 4.
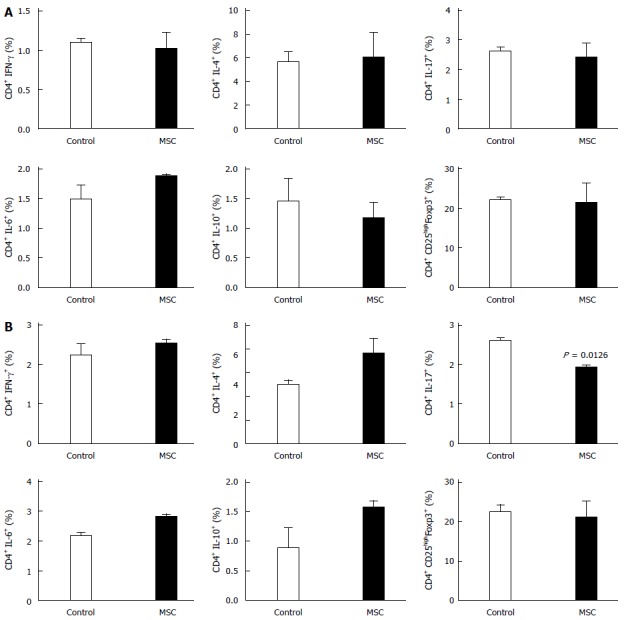
Changes in cytokine expression in the spleen and mesenteric lymph nodes. The percentages of cells expressing interferon (IFN)-γ, interleukin (IL)-4, IL-6, IL-10, and IL-17, and Treg cells in the Spleen (A) and Mesenteric lymph nodes (B) (n = 2, all tests were performed in triplicate). MSC: Mesenchymal stem cells.
Changes in the cytokine profile in homogenized colonic tissue of DSS-treated mice after MSC therapy
Finally, we analyzed homogenized colon supernatants via ELISA to explore the local immunoregulatory effects of MSCs. Mouse colons were harvested on day ten after DSS treatment, feces were removed and the colons homogenized in a buffer containing protease inhibitors. The levels of IFN-γ, IL-17, and TGF-β were lower in the MSC-treated compared to the control group (201.51, 6.99, and 246.21 pg/mL vs 4010.58, 14.42, and 269.96 pg/mL, respectively), and TNF-α levels were significantly lower (14.25 pg/mL vs 24.94 pg/mL, P = 0.0092) (Figure 5). The level of IL-4 in the MSC-treated group was 34.15 pg/mL and 22.18 pg/mL in the control group; the level thus decreased upon MSC treatment. As also found in the spleen, the level of IL-6 increased and that of IL-10 decreased in the MSC-treated group. In the control group, the level of IL-6 was 387.48 pg/mL and that of IL-10 156.25 pg/mL. In the MSC-treated group, the level of IL-6 was 546.71 pg/mL and that of IL-10 35.89 pg/mL. Interestingly, the level of IL-10 decreased by more than fourfold in the MSC-treated group.
Figure 5.
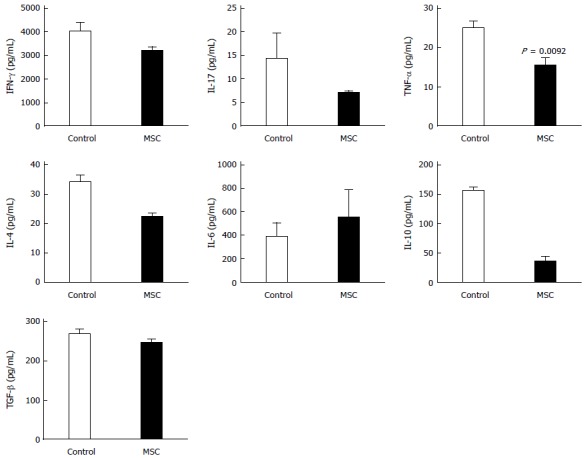
Changes in cytokine levels in the colon. Mice colons were harvested and the levels of interferon (IFN)-γ, interleukin (IL)-4, IL-6, IL-10, IL-17, and TNF-α were measured by ELISA (n = 2; all tests were performed in triplicate).
DISCUSSION
Currently, MSCs are used to treat chronic inflammatory diseases, including GvHD, RA, and IBD[28]. However, no clear therapeutic effect of MSCs on IBD has been shown. Considerable progress has been made in understanding the mechanisms by which MSCs exert immunomodulatory functions. MSCs exert immunosuppressive and anti-inflammatory effects and are considered to suppress T cell proliferation[29-31]. MSCs suppress T cells in a manner independent of variations in the major histocompatibility complex[32], and exert effects on lymphocytes involved in both the innate and adaptive immune systems. Our goal was to treat IBD with MSCs; we hypothesized that MSCs would exert immunomodulatory effects that would be of clinical value in improving the pathogenesis of IBD. However, we found, for the first time, that MSCs were not helpful in IBD treatment; the cells were not clinically efficacious. MSCs were unable to restore the immune balance. Both our in vivo and ex vivo data indicate that MSCs were not effective. Also, the ex vivo data indicate that MSCs did not influence the balance between IL-6 and IL-10 levels.
Although MSCs are known to possess immunomodulatory properties, these have recently been shown to not be constitutive, being rather highly dependent on inflammatory conditions. “Licensing” by acute inflammatory Th1-type cytokines[33,34], especially the proinflammatory cytokine IFN-γ[35,36], is required. Polchert et al[37] evaluated the roles played by IFN-γ-activated MSCs, and the role of IFN-γ in such activation. MSCs were activated in the presence of IFN-γ. However, in a chronic inflammatory environment, MSCs may aggravate inflammation. It is thought that, in autoimmune environments such as IBD, GvHD, and RA, MSCs secrete cytokines that aggravate the Th17 condition. Examples of MSCs aggravating, or having no effect on, autoimmune diseases such as RA are extant[22,26,38,39]. Also, in a previous study, we showed that MSCs were ineffective for treating Th17-mediated collagen-induced arthritis[25]. Those data, and our present findings, suggest that MSCs are unable to exert immunomodulatory functions when a Th17 response is in play.
Also, MSCs are known to produce IL-6 and TGF-β, which play important roles in regulating the differentiation of naïve T cells into Th17 or Treg cells[22,40,41]. MSCs produce TGF-β in the absence of stimulatory cytokines; but synthesize high levels of IL-6 in the presence of proinflammatory cytokines such as IFN-γ or TNF-α. Although TGF-β promotes the differentiation of naïve T cells into anti-inflammatory Treg cells, TGF-β and IL-6 (acting together) polarize T cells into proinflammatory Th17 cells[42-44]. Several studies, including our previous work, confirmed that MSCs can promote the expansion of Th17 cells under appropriate conditions[25,45]. In our present work, we found that MSCs did not exert significant effects in a DSS-induced IBD model. The high levels of IL-6 and TGF-β developing in the colon after DSS induction may have allowed the MSCs to induce Th17 cell proliferation and expansion.
Some preclinical studies on the use of MSCs for treatment of IBD have appeared (Table 1). However, some authors did not use MSCs alone, rather subjecting the MSCs to IL-12p40 transduction[11], autoimmune regulator knockout[10], or coating with antibodies against MAdCAM-1 and VCAM-1[9]. Other authors did use MSCs alone to treat IBD. However, although one report claimed that multiple MSC infusion improved the clinical symptoms of IBD, the levels of only TNF-α and IL-1β were shown[10].
Table 1.
Preclinical studies of mesenchymal stem cells therapy for inflammatory bowel disease
| Ref. | Model | Induction method | MSC source | Dose | Injection route | Time | Characteristic | Outcome |
| Kim et al[11] | C57BL/6 | 2% DSS | Xenogeneic: Rat (Sprague-Dawley) BM | 1 × 105 | sc | 0, 3, 6 d | IL-12p40-transduced | Positive |
| 6, 9, 12 d | ||||||||
| He et al[10] | BALB/c | 4% DSS | Syngeneic: Mouse (BALB/c) BM | 1 × 106 | iv | 2, 5, 8 d | Multiple injection | Positive |
| Parekkadan et al[12] | C57BL/6 RAG-1-/- | CD4+CD45RBhi 5 × 105 | Allogeneic: Mouse (Aire-/-) BM | 2.5 × 105 | iv | 0, 3 wk | Aire-/- injection | Positive |
| iv injection | ||||||||
| Ko et al[9] | C57BL/6 | 5% DSS | Allogeneic: Mouse (C57BL-10 × CBA/CA) BM | 1 × 106 | iv | 2 d | MAdCAM-1- VCAM-1-Ab-coated | Positive |
| Gonzalez-Rey et al[49] | C57BL/6 | a: 5% DSS | Xenogeneic: Human adipose | a: 1-50 × 105 hMSC, 1 × 106 mMSC c: 1 × 106 hMSC | ip | a: 2 d | hMSC | Positive |
| c: 3% DSS | Syngeneic: Mouse (C57BL/6) BM | c: 7 d of each cycle | ||||||
| Allogeneic: Mouse (BALB/c) BM |
a: Acute; BM: Bone marrow; c: Chronic; DSS: Dextran sulfate sodium; hMSC: Human mesenchymal stem cells; MAdCAM-1: Mucosal addressin cell adhesion molecule-1; mMSC: Mouse mesenchymal stem cells; MSC: Mesenchymal stem cells; VCAM-1: Vascular cell adhesion molecule-1.
Some clinical studies have explored the effects of adipose-derived stem cells (ASCs) and MSCs in IBD patients (Table 2). ASCs and MSCs were used to treat fistulas. In all studies, stem cells were injected after tract curettage and closing. Although all studies reported improvements in IBD symptoms, the basic pathogenesis of IBD was not affected.
Table 2.
Clinical trials of adipose-derived stem cells and mesenchymal stem cells inflammatory bowel disease therapies
| Ref. | Disease | Phase | No. of patients | Stem cell source | Route | Outcome |
| de la Portilla et al[15] | Complex perianal fistula | I/II | 24 | ASC; Allo-adipose | Intralesional | Improved |
| Lee et al[20] | Crohn’s fistula | II | 43 | ASC; Allo-adipose | Intralesional | Improved |
| Cho et al[13] | Crohn’s fistula | I | 10 | ASC; Allo-adipose | Intrafistula | Improved |
| Herreros et al[19] | Complex cryptoglandular perianal fistula | III | 200 | ASC; Allo-adipose | Intrafistula | Improved |
| Guadalajara et al[18] | Complex perianal fistula | II | 24 | ASC; Allo-liposuction | Intrafistula | Improved |
| Ciccocioppo et al[14] | Crohn’s fistula | I/II | 12 | MSC; Allo-BM | Intrafistula | Improved |
| Garcia-Olmo et al[17] | Complex perianal fistula | II | 14 | ASC; Auto-adipose | Intrafistula | Improved |
| García-Olmo et al[16] | Crohn’s fistula | I | 10 | ASC; Allo-adipose | Intrafistula | Improved |
ASC: Adipose-derived stem cells; MSC: Mesenchymal stem cells.
Thus, MSC infusion alone may not be sufficient to re-establish the immune balance in IBD patients. MSCs may require other factors to exert polarized immunomodulatory functions in an established chronic inflammatory environment[46]. Several studies have shown that MSCs treated in various ways improved IBD pathogenesis[9,11,12]. In our earlier work, we found that TGF-β-transduced MSCs improved collagen-induced arthritis symptoms[25]. Also, we evaluated combinatorial cell therapy (MSCs and Treg cells) of skin allografts[27] and GvHD[47], and explored whether such therapy enhanced solid-organ transplant tolerance[48]. We also found that administration of MSCs with Treg cells, or IL-10-producing Tr1 cells, was an efficient therapeutic approach in the DSS-induced IBD model; the IL-10 level in the colon was reduced in MSC-treated groups. Therefore, MSCs in combination with other factors, and/or multiple injections of MSCs, may be effective for treatment of IBD. We suggest that further studies are needed to explore the localization and survival of MSCs administered by intraperitoneal infusion in the murine IBD model.
COMMENTS
Background
Inflammatory bowel disease (IBD) is an inflammatory autoimmune disorder of the colon caused by colonic microbes, dietary habits, and/or genetic factors. IBD is common in Western countries. IBD patients present with clinical symptoms of diarrhea, and, histopathologically, immune cell infiltration of the colon is evident. Complete recovery is rare; relapses are common. Recently, mesenchymal stem cells (MSCs) have emerged as a new therapeutic approach for autoimmune diseases accompanied by inflammatory changes.
Research frontiers
MSCs are under preclinical and clinical investigation as a new treatment for autoimmune diseases.
Innovations and breakthroughs
IBD has been treated using MSCs combined with other interventions, such as genetic modification of MSCs. In the present study, the authors report, for the first time, that MSCs alone do not exert significant effects on IBD. MSCs were unable to regulate the balance between interleukin (IL)-6 and IL-10 levels.
Applications
This study provides the first evidence that the use of MSCs alone to treat IBD may be inadequate. If MSCs are to be used, their immunomodulatory function must be improved by addition of other factors; for example Treg cells or IL-10-producing Tr1 cells, and/or genetic modification.
Terminology
MSCs are self-renewing multipotent progenitor cells with the potential to differentiate into many other cell types of mesodermal origin. Also, MSCs exert immunosuppressive effects that do not depend on major histocompatibility complex matching. Therefore, MSCs are used in therapeutic approaches for inflammatory and autoimmune diseases.
Peer-review
This is a good study. The authors document the poor efficacy of MSCs used to treat autoimmune diseases, employing a dextran sulfate sodium-induced IBD model. The results are interesting in that MSCs did not exhibit the expected immunomodulatory functions in a chronic inflammatory environment.
Footnotes
Supported by Korea Healthcare Technology R and D Project No. HI12C0193 (A120241), the Ministry for Health, Welfare, and Family Affairs, South Korea.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 13, 2014
First decision: August 27, 2014
Article in press: October 15, 2014
P- Reviewer: Lakatos PL S- Editor: Qi Y L- Editor: AmEditor E- Editor: Ma S
References
- 1.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–466. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Dexter TM, Spooncer E, Schofield R, Lord BI, Simmons P. Haemopoietic stem cells and the problem of self-renewal. Blood Cells. 1984;10:315–339. [PubMed] [Google Scholar]
- 4.Imaeda T. Ultrastructure of L-phase variants isolated from a culture of Mycobacterium phlei. J Med Microbiol. 1975;8:389–395. doi: 10.1099/00222615-8-3-389. [DOI] [PubMed] [Google Scholar]
- 5.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/ multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 8.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. doi: 10.1146/annurev-pathol-011110-130230. [DOI] [PubMed] [Google Scholar]
- 9.Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365–1372. doi: 10.1038/mt.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57:3136–3144. doi: 10.1007/s10620-012-2290-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim DJ, Kim KS, Song MY, Seo SH, Kim SJ, Yang BG, Jang MH, Sung YC. Delivery of IL-12p40 ameliorates DSS-induced colitis by suppressing IL-17A expression and inflammation in the intestinal mucosa. Clin Immunol. 2012;144:190–199. doi: 10.1016/j.clim.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Parekkadan B, Fletcher AL, Li M, Tjota MY, Bellemare-Pelletier A, Milwid JM, Lee JW, Yarmush ML, Turley SJ. Aire controls mesenchymal stem cell-mediated suppression in chronic colitis. Mol Ther. 2012;20:178–186. doi: 10.1038/mt.2011.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant. 2013;22:279–285. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- 14.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 15.de la Portilla F, Alba F, García-Olmo D, Herrerías JM, González FX, Galindo A. Expanded allogeneic adipose-derived stem cells (eASCs) for the treatment of complex perianal fistula in Crohn’s disease: results from a multicenter phase I/IIa clinical trial. Int J Colorectal Dis. 2013;28:313–323. doi: 10.1007/s00384-012-1581-9. [DOI] [PubMed] [Google Scholar]
- 16.García-Olmo D, García-Arranz M, Herreros D, Pascual I, Peiro C, Rodríguez-Montes JA. A phase I clinical trial of the treatment of Crohn's fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423. doi: 10.1007/s10350-005-0052-6. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 18.Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595–600. doi: 10.1007/s00384-011-1350-1. [DOI] [PubMed] [Google Scholar]
- 19.Herreros MD, Garcia-Arranz M, Guadalajara H, De-La-Quintana P, Garcia-Olmo D. Autologous expanded adipose-derived stem cells for the treatment of complex cryptoglandular perianal fistulas: a phase III randomized clinical trial (FATT 1: fistula Advanced Therapy Trial 1) and long-term evaluation. Dis Colon Rectum. 2012;55:762–772. doi: 10.1097/DCR.0b013e318255364a. [DOI] [PubMed] [Google Scholar]
- 20.Lee WY, Park KJ, Cho YB, Yoon SN, Song KH, Kim do S, Jung SH, Kim M, Yoo HW, Kim I, et al. Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells. 2013;31:2575–2581. doi: 10.1002/stem.1357. [DOI] [PubMed] [Google Scholar]
- 21.Bouffi C, Bony C, Courties G, Jorgensen C, Noël D. IL-6-dependent PGE2 secretion by mesenchymal stem cells inhibits local inflammation in experimental arthritis. PLoS One. 2010;5:e14247. doi: 10.1371/journal.pone.0014247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen B, Hu J, Liao L, Sun Z, Han Q, Song Z, Zhao RC. Flk-1+ mesenchymal stem cells aggravate collagen-induced arthritis by up-regulating interleukin-6. Clin Exp Immunol. 2010;159:292–302. doi: 10.1111/j.1365-2249.2009.04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, Guo J, et al. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R210. doi: 10.1186/ar3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park MJ, Park HS, Cho ML, Oh HJ, Cho YG, Min SY, Chung BH, Lee JW, Kim HY, Cho SG. Transforming growth factor β-transduced mesenchymal stem cells ameliorate experimental autoimmune arthritis through reciprocal regulation of Treg/Th17 cells and osteoclastogenesis. Arthritis Rheum. 2011;63:1668–1680. doi: 10.1002/art.30326. [DOI] [PubMed] [Google Scholar]
- 26.Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. doi: 10.1186/ar2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Jeon EJ, Kim N, Nam YS, Im KI, Lim JY, Kim EJ, Cho ML, Han KT, Cho SG. The synergistic immunoregulatory effects of culture-expanded mesenchymal stromal cells and CD4(+)25(+)Foxp3+ regulatory T cells on skin allograft rejection. PLoS One. 2013;8:e70968. doi: 10.1371/journal.pone.0070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EJ, Kim N, Cho SG. The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Exp Mol Med. 2013;45:e2. doi: 10.1038/emm.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 30.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 32.Stagg J, Pommey S, Eliopoulos N, Galipeau J. Interferon-gamma-stimulated marrow stromal cells: a new type of nonhematopoietic antigen-presenting cell. Blood. 2006;107:2570–2577. doi: 10.1182/blood-2005-07-2793. [DOI] [PubMed] [Google Scholar]
- 33.Kim N, Im KI, Lim JY, Jeon EJ, Nam YS, Kim EJ, Cho SG. Mesenchymal stem cells for the treatment and prevention of graft-versus-host disease: experiments and practice. Ann Hematol. 2013;92:1295–1308. doi: 10.1007/s00277-013-1796-z. [DOI] [PubMed] [Google Scholar]
- 34.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33:593–602. doi: 10.1007/s00281-011-0267-7. [DOI] [PubMed] [Google Scholar]
- 35.Dazzi F, Marelli-Berg FM. Mesenchymal stem cells for graft-versus-host disease: close encounters with T cells. Eur J Immunol. 2008;38:1479–1482. doi: 10.1002/eji.200838433. [DOI] [PubMed] [Google Scholar]
- 36.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 37.Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, Genrich K, Mehrotra S, Setty S, Smith B, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi JJ, Yoo SA, Park SJ, Kang YJ, Kim WU, Oh IH, Cho CS. Mesenchymal stem cells overexpressing interleukin-10 attenuate collagen-induced arthritis in mice. Clin Exp Immunol. 2008;153:269–276. doi: 10.1111/j.1365-2249.2008.03683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noël D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595–1603. doi: 10.1002/art.21012. [DOI] [PubMed] [Google Scholar]
- 40.Eljaafari A, Tartelin ML, Aissaoui H, Chevrel G, Osta B, Lavocat F, Miossec P. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 2012;64:2147–2157. doi: 10.1002/art.34391. [DOI] [PubMed] [Google Scholar]
- 41.Guo Z, Zheng C, Chen Z, Gu D, Du W, Ge J, Han Z, Yang R. Fetal BM-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol. 2009;39:2840–2849. doi: 10.1002/eji.200839070. [DOI] [PubMed] [Google Scholar]
- 42.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 43.Park JS, Park MK, Lee SY, Oh HJ, Lim MA, Cho WT, Kim EK, Ju JH, Park YW, Park SH, et al. TWEAK promotes the production of Interleukin-17 in rheumatoid arthritis. Cytokine. 2012;60:143–149. doi: 10.1016/j.cyto.2012.06.285. [DOI] [PubMed] [Google Scholar]
- 44.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Svobodova E, Krulova M, Zajicova A, Pokorna K, Prochazkova J, Trosan P, Holan V. The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem Cells Dev. 2012;21:901–910. doi: 10.1089/scd.2011.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med. 2013;28:387–402. doi: 10.3904/kjim.2013.28.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim JY, Park MJ, Im KI, Kim N, Jeon EJ, Kim EJ, Cho ML, Cho SG. Combination cell therapy using mesenchymal stem cells and regulatory T-cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of TH1/TH2 and th17/treg cells in a murine acute graft-versus-host disease model. Cell Transplant. 2014;23:703–714. doi: 10.3727/096368913X664577. [DOI] [PubMed] [Google Scholar]
- 48.Im KI, Park MJ, Kim N, Lim JY, Park HS, Lee SH, Nam YS, Lee ES, Lee JH, Cho ML, et al. Induction of mixed chimerism using combinatory cell-based immune modulation with mesenchymal stem cells and regulatory T cells for solid-organ transplant tolerance. Stem Cells Dev. 2014;23:2364–2376. doi: 10.1089/scd.2013.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939. doi: 10.1136/gut.2008.168534. [DOI] [PubMed] [Google Scholar]


