Abstract
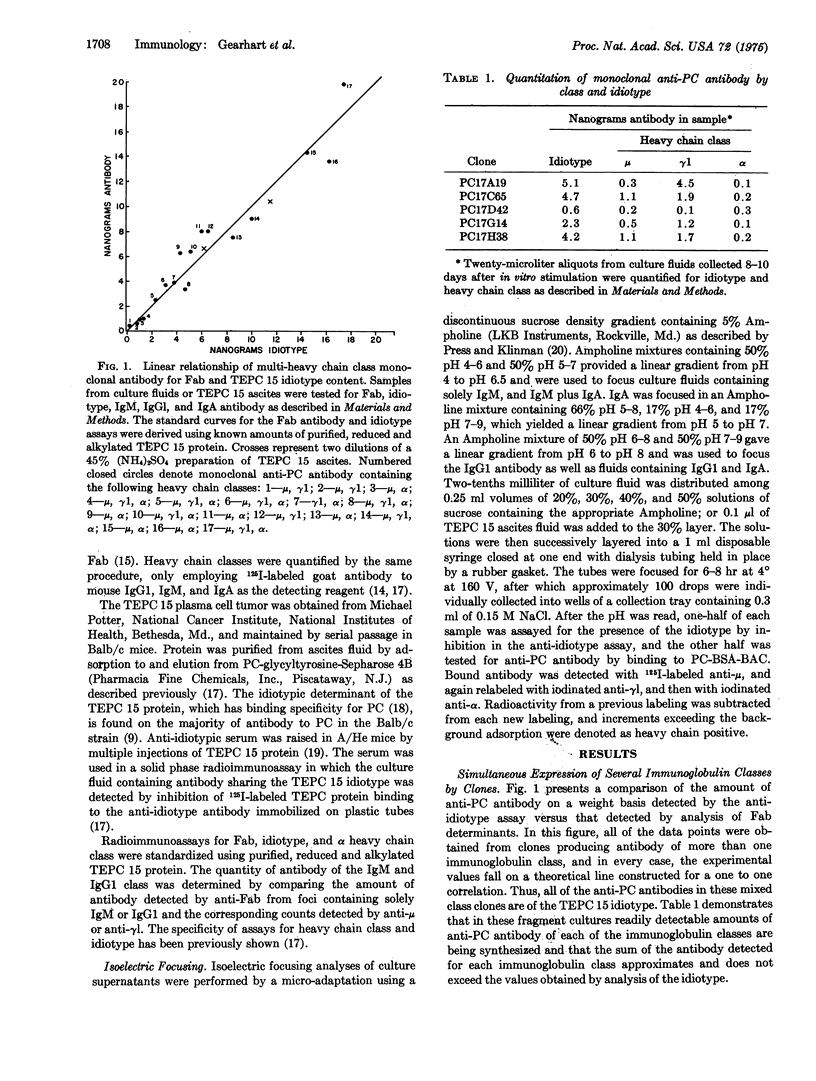
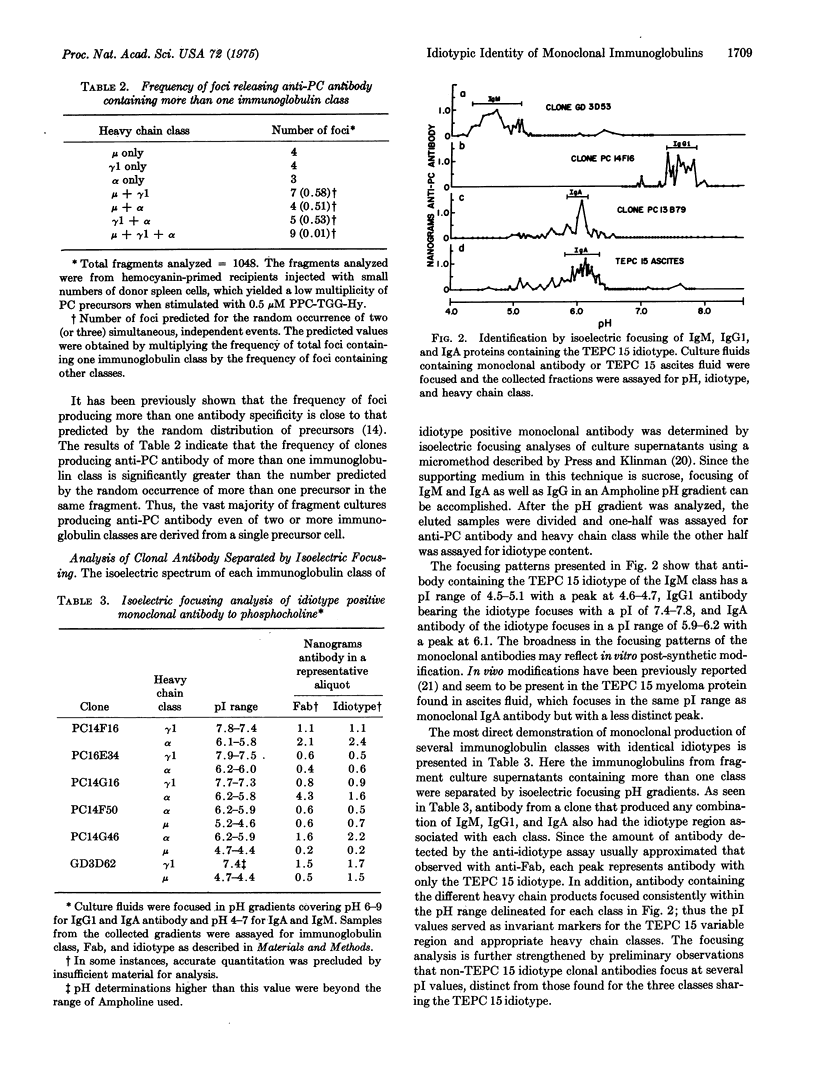
The availability of anti-phosphocholine antibody of the TEPC 15 idiotype from the clonal progeny of a single precursor cell, stimulated in vitro, permitted the demonstration of monoclonal antibodies with as many as three immunoglobulin classes with identical variable regions. This demonstration was dependent on sensitive radioimmunoassays which showed a one to one relationship between the total anti-phosphocholine antibody produced by a clone and the sum of anti-phosphocholine antibody of the different classes as well as the amount of antibody of the TEPC 15 idiotype. The class distribution was confirmed by isoelectric focusing identification of IgM, Igta, and IgGl antibodies of the TEPC 15 idiotype produced by single clones which showed characteristic pI values for each immunoglobulin class. Thus, within the generative phase of a single antibody-producing cell clone, various heavy chain constant regions can be linked to the same variable region, and single precursor cells have the capacity to generate progeny expressing at least three distinct immunoglobulin classes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. II. Idiotypic specificity and binding characteristics of anti-phosphorylcholine antibodies. J Immunol. 1974 May;112(5):1747–1756. [PubMed] [Google Scholar]
- Cosenza H., Köhler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science. 1972 Jun 2;176(4038):1027–1029. doi: 10.1126/science.176.4038.1027. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C. Partial deletion in the heavy chain disease protein ZUC. Nature. 1969 Nov 8;224(5219):597–599. doi: 10.1038/224597a0. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Sigal N. H., Klinman N. R. Heterogeneity of the BALB/c antiphosphorylcholine antibody response at the precursor cell level. J Exp Med. 1975 Jan 1;141(1):56–71. doi: 10.1084/jem.141.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granato D., Braun D. G., Vassalli P. Induction of anti-DNP antibodies: suppressive effect of circulating anti-idiotypic antibodies to mouse myeloma protein MOPC 315. J Immunol. 1974 Jul;113(1):416–420. [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Kindt T. J., Klapper D. G., Waterfield M. D. An idiotypic cross-reaction between allotype a3 and allotype a negative rabbit antibodies to streptococcal carbohydrate. J Exp Med. 1973 Mar 1;137(3):636–648. doi: 10.1084/jem.137.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R. Purification and analysis of "monofocal" antibody. J Immunol. 1971 May;106(5):1345–1352. [PubMed] [Google Scholar]
- Klinman N. R. Regain of homogeneous binding activity after recombination of chains of "mono- focal" antibody. J Immunol. 1971 May;106(5):1330–1337. [PubMed] [Google Scholar]
- Klinman N. R. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972 Aug 1;136(2):241–260. doi: 10.1084/jem.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Cosenza H., Köhler H. Clonal restriction of the immune response to phosphorylcholine. Nature. 1974 Jan 4;247(5435):55–57. doi: 10.1038/247055a0. [DOI] [PubMed] [Google Scholar]
- Lieberman R., Humphrey W., Jr Association of H-2 types with genetic control of immune responsiveness to IgA allotypes in the mouse. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2510–2513. doi: 10.1073/pnas.68.10.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Warner N. L., Lewis H. Incidence of cells simultaneously secreting IgM and IgG antibody to sheep erythrocytes. Cell Immunol. 1971 Feb;2(1):41–53. doi: 10.1016/0008-8749(71)90024-4. [DOI] [PubMed] [Google Scholar]
- Oudin J., Michel M. Idiotypy of rabbit antibodies. II. Comparison of idiotypy of various kinds of antibodies formed in the same rabbits against Salmonella typhi. J Exp Med. 1969 Sep 1;130(3):619–642. doi: 10.1084/jem.130.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn G. M., Kunkel H. G., Grey H. M. Sharing of individual antigenic determinants between a gamma G and a gamma M protein in the same myeloma serum. Proc Soc Exp Biol Med. 1970 Dec;135(3):660–665. doi: 10.3181/00379727-135-35116. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Amante L. Immunoglobulins as cell receptors. Ann N Y Acad Sci. 1971 Dec 31;190:420–431. doi: 10.1111/j.1749-6632.1971.tb13552.x. [DOI] [PubMed] [Google Scholar]
- Potter M., Lieberman R. Common individual antigenic determinants in five of eight BALB-c IgA myeloma proteins that bind phosphoryl choline. J Exp Med. 1970 Oct 1;132(4):737–751. doi: 10.1084/jem.132.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press J. L., Klinman N. R. Isoelectric analysis of neonatal monofocal antibody. Immunochemistry. 1973 Sep;10(9):621–627. doi: 10.1016/0019-2791(73)90164-x. [DOI] [PubMed] [Google Scholar]
- ROCKEY J. H., KLINMAN N. R., KARUSH F. EQUINE ANTIHAPTEN ANTIBODY. I. 7S BETA-2A- AND 1OS GAMMA-1- GLOBULIN COMPONENTS OF PURIFIED ANTI-BETA-LACTOSIDE ANTIBODY. J Exp Med. 1964 Oct 1;120:589–609. doi: 10.1084/jem.120.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Cohn M. Inheritance of an idiotype associated with the immune response of inbred mice to phosphorylcholine. Eur J Immunol. 1972 Aug;2(4):319–326. doi: 10.1002/eji.1830020405. [DOI] [PubMed] [Google Scholar]
- TODD C. W. Allotypy in rabbit 19S protein. Biochem Biophys Res Commun. 1963 May 3;11:170–175. doi: 10.1016/0006-291x(63)90329-2. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Wilson K. S., Hopper J. E., Fudenberg H. H., Nisonoff A. Evidence for control of synthesis of the varible regions of the heavy chains of immunoglobulins G and M by the same gene. Proc Natl Acad Sci U S A. 1970 Jun;66(2):337–343. doi: 10.1073/pnas.66.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. R., Salaman M. R., Kreth H. W. Microheterogeneity and allomorphism of proteins. Ann N Y Acad Sci. 1973 Jun 15;209:210–224. doi: 10.1111/j.1749-6632.1973.tb47530.x. [DOI] [PubMed] [Google Scholar]


