Abstract
AIM: To analyze the mismatch repair (MMR) status and the ARID1A expression as well as their clinicopathological significance in gastric adenocarcinomas.
METHODS: We examined the expressions of MMR proteins and ARID1A by immunohistochemistry in consecutive 489 primary gastric adenocarcinomas. The results were further correlated with clinicopathological variables.
RESULTS: The loss of any MMR protein expression, indicative of MMR deficiency, was observed in 38 cases (7.8%) and was significantly associated with an older age (68.6 ± 9.2 vs 60.4 ± 11.7, P < 0.001), a female sex (55.3% vs 31.3%, P = 0.004), an antral location (44.7% vs 25.7%, P = 0.021), and a differentiated histology (57.9% vs 39.7%, P = 0.023). Abnormal ARID1A expression, including reduced or loss of ARID1A expression, was observed in 109 cases (22.3%) and was significantly correlated with lymphatic invasion (80.7% vs 69.5%, P = 0.022) and lymph node metastasis (83.5% vs 73.7%, P = 0.042). The tumors with abnormal ARID1A expression more frequently indicated MMR deficiency (47.4% vs 20.2%, P < 0.001). A multivariate analysis identified abnormal ARID1A expression as an independent poor prognostic factor (HR = 1.36, 95%CI: 1.01-1.84; P = 0.040).
CONCLUSION: Our observations suggest that the AIRD1A inactivation is associated with lymphatic invasion, lymph node metastasis, poor prognosis, and MMR deficiency in gastric adenocarcinomas.
Keywords: Adenocarcinoma, ARID1A, Mismatch Repair, Stomach, Immunohistochemistry
Core tip: Inactivation of ARID1A, a key component of the chromatin remodeling complex, has been recently reported in several tumors, including gastric cancer. Previous studies showed a significant relationship between ARID1A mutations and MMR deficiency in gastric cancers. On the other hand, there have been inconsistent reports on the clinicopathological significance of altered ARID1A expression. In the present study, we examined expressions of ARID1A and MMR proteins in a large series of primary gastric adenocarcinomas, and showed their clinicopathological significance.
INTRODUCTION
The incidence of gastric cancer has been declining, but it remains one of the leading causes of death from cancer worldwide[1]. Multiple genetic and epigenetic alterations in oncogenes and tumor suppressor genes are involved in the process of gastric carcinogenesis[2,3]. Defects in the DNA mismatch repair (MMR) system are involved in the development of some tumors including gastric cancers[4,5]. During DNA replication, DNA polymerase makes base pairing errors at a certain rate[4,5]. The MMR system is critical for correcting these errors, and defects in the system lead to an accelerated accumulation of mutations and a predisposition to certain types of cancers[4,5]. For instance, the loss of MLH1 because of promoter hypermethylation is known to be a major cause of MMR defects in sporadic gastrointestinal cancers[6]. Patients with MMR-deficient gastric cancers reportedly exhibit some clinicopathological features, including an older age, a female sex, an antral location and a differentiated histology[2,7-14].
ARID1A, also known as BAF250a, is a key component of the multi-protein SWI/SNF chromatin remodeling complex, and is involved in the regulation of diverse cellular processes, from development and differentiation to proliferation[15-17]. The SWI/SNF complex interacts directly or indirectly with p53 and regulates the transcription of target genes downstream of p53, thereby suggesting that ARID1A plays important roles in tumor suppression[15-18]. Somatic mutations in ARID1A are reportedly present in a nearly half of all ovarian clear cell carcinomas and about 30% of endometrioid carcinomas[19,20]. The prevalence of ARID1A mutations has been reported to vary among tumor types, and recent studies have reported the frequent presence of mutations in tumors of several organs, including gastric cancer[7,21-27]. Some studies have examined clinicopathological significance of ARID1A inactivations[7,23,26,27]; interestingly, a significant relationship between ARID1A mutations and MMR deficiency have been suggested in gastric cancers[7,23,26,27].
The purpose of the present study was to examine the clinicopathological significance and correlation between MMR deficiency and ARID1A abnormality in a large consecutive series of advanced gastric cancers using immunohistochemistry.
MATERIALS AND METHODS
Study population
This study was approved by the ethical committee of the National Cancer Center, Tokyo, Japan. The present study involved a consecutive series of 489 primary gastric cancers with invasion to the muscularis propria or deeper that were treated by gastrectomy at the National Cancer Center Hospital, Tokyo, Japan, between 1999 and 2001. All the cases had been histologically confirmed as adenocarcinoma. Of the 489 cases, 327 were men and 162 were women. The mean age was 61 years. Six patients received adjuvant chemotherapy. Tumors were classified into differentiated type (papillary and tubular adenocarcinoma) and undifferentiated type (poorly differentiated adenocarcinoma and signet ring cell carcinoma). Mucinous adenocarcinomas were subclassified into differentiated type and undifferentiated type, depending on their histology. The pathological stage was determined according to the UICC TNM classification (the 7th edition)[28].
Immunohistochemical staining
Representative formalin-fixed and paraffin-embedded specimens from each case were cut into 4 μm-thick sections. Antibodies against MLH1 (clone G168-15; diluted 1:100; BD Pharmingen, San Diego, CA, United States), PMS2 (clone A16-9; diluted 1:100; BD Pharmingen, San Diego, CA, United States), MSH2 (clone FE11; diluted 1:200; Caibiochem, La Jolla, CA, United States), MSH6 (clone 44; diluted 1:500; BD Pharmingen, San Diego, CA, United States), and ARID1A (polyclonal, HPA005456; diluted 1:200; Sigma-Aldrich, St Louis, MO, United States) were used as primary antibodies. The sections were deparaffinized and autoclaved at 121 °C for 15 min in Target retrieval solution with a high pH of 9 (Dako, Glostrup, Denmark) and then allowed to cool at room temperature. Endogenous peroxidase was blocked using 0.3% hydrogen peroxide. The slides were incubated for three hours with the primary antibodies and then were reacted for one hour with HRP conjugated secondary antibodies (Dako, Glostrup, Denmark) at room temperature. The signals were visualized using substrate chromogen (Dako liquid DAB chromogen; Dako, Glostrup, Denmark), and counterstaining was performed using Mayer’s hematoxylin.
Non-neoplastic cells, including endothelial cells, fibroblasts, and lymphocytes, typically showed nuclear expression for all five of the antibodies that were used and served as positive controls.
Evaluation of immunohistochemical staining
The tumors were classified into two categories according to the MMR protein expression status as follows: MMR deficient, negative staining for one or more MMR proteins; or MMR intact, positive nuclear staining for all four MMR proteins.
The expression of ARID1A was evaluated based on the intensity and pattern of staining. The staining intensity was classified as loss, weak, and retained. Weak staining was defined by comparison with the staining intensities of the internal controls. The staining patterns were classified into either homogenous or heterogeneous. Heterogeneous expression was defined as a reduced or loss of staining in 10%-90% of the tumor cells. Two observers independently evaluated the staining results. Discrepant cases were reviewed using a multiheaded microscope to achieve consensus.
Statistical analysis
Categorical variables were compared using the Fisher’s exact test. Continuous variables were presented as mean ± SD and compared using the Mann-Whitney U test. Disease specific survival curves were calculated using the Kaplan-Meier method, and the differences in survival times among subgroups were compared using the log-rank test. Univariate and multivariate analyses were performed using the Cox proportional hazard regression model to determine the associations between clinicopathological variables and cancer-related mortality. The factors with P values of < 0.1 in the univariate analyses were included in a multivariate analysis to determine independent prognostic factors. P values of < 0.05 were considered significant.
RESULTS
MMR protein expression and its clinicopathological significance
Of the 489 cases that were analyzed, 33 cases showed the concurrent loss of MLH1 and PMS2, three cases showed the isolated loss of PMS2, one case showed the concurrent loss of MSH2 and MSH6, and one case showed the loss of all four proteins (Figure 1 and Table 1). The remaining 451 cases retained the expressions of all four proteins. Overall, 38 cases (7.8%) were regarded as MMR-deficient. All but one MMR-deficient case showed the homogeneous loss of MMR protein expression in invasive components. Eighteen MMR-deficient lesions were associated with intramucosal components. Among them, 12 cases showed homogeneous loss, whereas three showed heterogeneous loss and three other cases retained the expressions of the MMR proteins in the intramucosal components.
Figure 1.
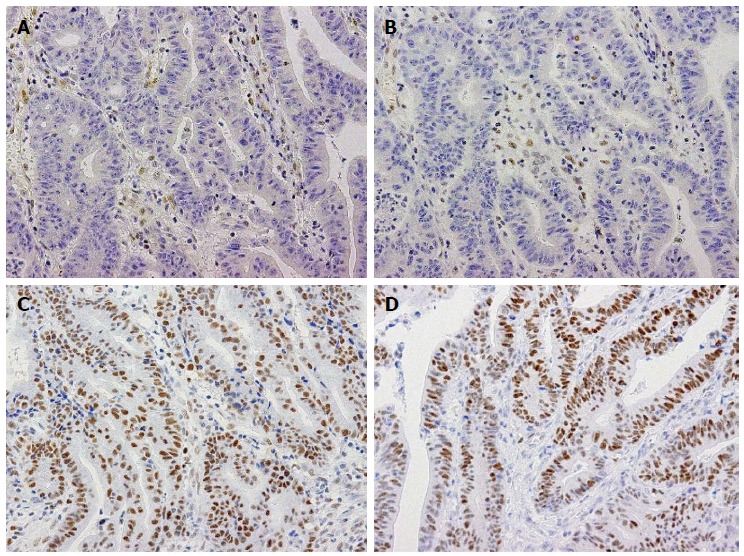
Immunohistochemistry for mismatch repair proteins in a case of gastric cancer with a mismatch repair-deficient phenotype. Immunohistochemistry for MLH1 (A), PMS2 (B), MSH2 (C), and MSH6 (D). MLH1 and PMS2 expression are absent in tumor cells, whereas stromal cells show nuclear expression (A, B). On the other hand, the tumor cells retain MSH2 and MSH6 expression (C, D). These staining patterns were consistent with those caused by MLH1 deficiency.
Table 1.
Immunohistochemical expression of mismatch repair proteins
| Immunohistochemical expression | n = 489 |
| Loss of MLH1 and PMS2 | 33 |
| Loss of PMS2 | 3 |
| Loss of MSH2 and MSH6 | 1 |
| Loss of all four proteins | 1 |
| Retention of all four proteins | 451 |
The clinicopathological features according to the MMR status are shown in Table 2. MMR deficiency was significantly associated with an older age (P < 0.001), a female sex (P = 0.004), an antral location (P = 0.021), and a differentiated histology (P = 0.023).
Table 2.
Clinicopathologic features of the 489 patients with gastric cancers according to mismatch repair status n (%)
| Total (n = 489) |
MMR |
P value | ||
| Deficient (n = 38) | Intact (n = 451) | |||
| Age (yr) | < 0.0011 | |||
| mean ± SD | 61.0 ± 11.8 | 68.6 ± 9.2 | 60.4 ± 11.7 | |
| Gender | 0.0042 | |||
| Male | 327 (66.9) | 17 (44.7) | 310 (68.7) | |
| Female | 162 (33.1) | 21 (55.3) | 141 (31.3) | |
| Serum CEA (ng/mL) | 0.9101 | |||
| mean ± SD | 36.3 ± 315.7 | 4.4 ± 6.0 | 39.0 ± 328.7 | |
| Tumor size (mm) | 0.8311 | |||
| mean ± SD | 80.3 ± 45.1 | 79.0 ± 38.9 | 80.4 ± 45.6 | |
| Tumor location | 0.0212 | |||
| Fundus and Corpus | 356 (72.8) | 21 (55.3) | 335 (74.3) | |
| Antrum | 133 (27.2) | 17 (44.7) | 116 (25.7) | |
| Histology | 0.0232 | |||
| Differentiated type | 193 (39.5) | 22 (57.9) | 171 (37.9) | |
| Undifferentiated type | 296 (60.5) | 16 (42.1) | 280 (62.1) | |
| Lymphatic invasion | 1.0002 | |||
| Absent | 137 (28.0) | 10 (26.3) | 127 (28.2) | |
| Present | 352 (72.0) | 28 (73.7) | 324 (71.8) | |
| Venous invasion | 0.6092 | |||
| Absent | 281 (57.5) | 20 (52.6) | 261 (57.9) | |
| Present | 208 (42.5) | 18 (47.4) | 190 (42.1) | |
| Primary tumor | 0.6122 | |||
| T2, 3 | 241 (49.3) | 22 (57.9) | 219 (48.6) | |
| T4 | 248 (50.7) | 16 (42.1) | 232 (51.4) | |
| Lymph node involvement | 0.4382 | |||
| N0 | 118 (24.1) | 11 (28.9) | 107 (23.7) | |
| N1, 2, 3 | 371 (75.9) | 27 (71.1) | 344 (76.3) | |
| Distant metastasis | 0.2022 | |||
| M0 | 336 (68.7) | 30 (78.9) | 306 (67.8) | |
| M1 | 153 (31.3) | 8 (21.1) | 145 (32.2) | |
| Stage | 0.8602 | |||
| Stage I, II | 172 (35.2) | 14 (36.8) | 158 (35.0) | |
| Stage III, IV | 317 (64.8) | 24 (63.2) | 293 (65.0) | |
| Residual disease | 0.1612 | |||
| Negative | 375 (76.7) | 33 (86.8) | 342 (75.8) | |
| Positive | 114 (23.3) | 5 (13.2) | 109 (24.2) | |
Mann-Whitney U test;
Fisher’s exact test. MMR: Mismatch repair; CEA: Carcinoembryonic antigen.
ARID1A expression and its clinicopathological significance
Abnormal ARID1A expression was observed in 109 cases (22.3%). These cases included homogeneous loss (43 cases, 8.8%), heterogeneous loss (29 cases, 5.9%), homogeneously weak expression (21 cases, 4.3%), and heterogeneously weak expression (16 cases, 3.3%; Figure 2). Among the 45 cases that showed heterogeneous ARID1A expression, 34 cases showed heterogeneity within the invasive component. In remaining 11 cases, ARID1A expression was homogenously lost or weakened in the invasive component; and in the intramucosal component, the expression was heterogeneous in 8 cases and retained in 3 cases. ARID1A expression was retained in the remaining 380 cases (77.7%). Among the clinicopathological factors that were examined, lymphatic invasion (P = 0.022) and lymph node metastasis (P = 0.042) were significantly correlated with abnormal ARID1A expression (Table 3).
Figure 2.
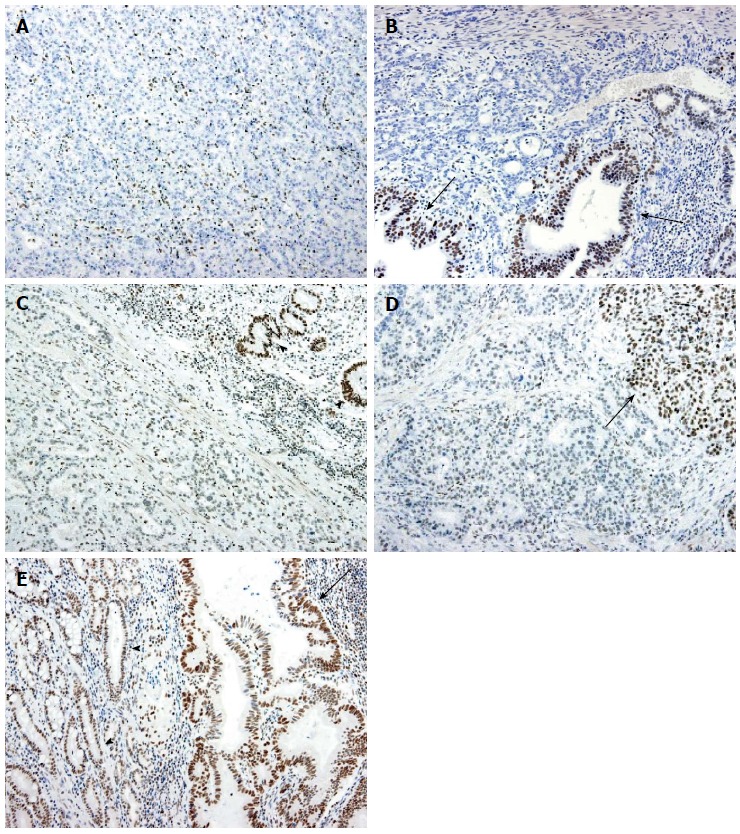
Immunohistochemistry for ARID1A. A: Homogeneous loss of expression. All the tumor cells show no expression, whereas the stromal cells retain the nuclear expression of ARID1A; B: Heterogeneous loss of expression. Most of the tumor cells show no expression, whereas some of the gland-forming tumor cells retain nuclear expression (arrows); C: Homogeneously weak expression. The tumor cells show the reduced expression of ARID1A. Non-neoplastic gastric glandular cells retain the expression (arrowheads); D: Heterogeneously weak expression. Most of the tumor cells exhibit reduced expression, but a subset of tumor cells retain nuclear expression (arrow); E: Retained expression: Tumor cells (arrow) show strong nuclear ARID1A expression, similar to non-neoplastic glandular cells (arrowheads).
Table 3.
Clinicopathological features of 489 patients with gastric cancer according to ARID1A expression n (%)
|
ARID1A expression |
P value | ||
| Loss/weak (n = 109) | Retained (n = 380) | ||
| Age (yr) | 0.2121 | ||
| mean ± SD | 62.1 ± 10.9 | 60.7 ± 12.0 | |
| Gender | 0.4192 | ||
| Male | 69 (63.3) | 258 (67.9) | |
| Female | 40 (36.7) | 122 (32.1) | |
| Serum CEA (ng/mL) | 0.9371 | ||
| mean ± SD | 39.7 ± 363.4 | 35.3 ± 301.3 | |
| Tumor size (mm) | 0.3361 | ||
| mean ± SD | 83.9 ± 46.1 | 79.2 ± 44.8 | |
| Tumor location | 0.0672 | ||
| Fundus and Corpus | 87 (79.8) | 269 (70.8) | |
| Antrum | 22 (20.2) | 111 (29.2) | |
| Histology | 0.7392 | ||
| Differentiated type | 41 (37.6) | 152 (40.0) | |
| Undifferentiated type | 68 (62.4) | 228 (60.0) | |
| Lymphatic invasion | 0.0222 | ||
| Absent | 21 (19.3) | 116 (30.5) | |
| Present | 88 (80.7) | 264 (69.5) | |
| Venous invasion | 0.4432 | ||
| Absent | 59 (54.1) | 222 (58.4) | |
| Present | 50 (45.9) | 158 (41.6) | |
| Primary tumor | 0.0652 | ||
| T2, 3 | 45 (41.3) | 184 (48.4) | |
| T4 | 64 (58.7) | 196 (51.6) | |
| Lymph node involvement | 0.0422 | ||
| N0 | 18 (16.5) | 100 (26.3) | |
| N1, 2, 3 | 91 (83.5) | 280 (73.7) | |
| Distant metastasis | 0.7252 | ||
| M0 | 73 (67.0) | 263 (69.2) | |
| M1 | 36 (33.0) | 117 (30.8) | |
| Stage | 0.1112 | ||
| Stage I, II | 31 (28.4) | 141 (37.1) | |
| Stage III, IV | 78 (71.6) | 239 (62.9) | |
| Residual disease | 0.7982 | ||
| Negative | 85 (78.0) | 290 (76.3) | |
| Positive | 24 (22.0) | 90 (23.7) | |
Mann-Whitney U test;
Fisher’s exact test. CEA: Carcinoembryonic antigen.
Survival analysis
The median follow-up period of the patients was 44 months. The disease specific survival curves according to the MMR and ARID1A expression statuses did not show any significant differences (Figure 3). A multivariate analysis revealed several factors to be associated with a poorer prognosis, including a female sex, a higher serum CEA level, a larger tumor size, an undifferentiated-type histology, a higher pathological stage, a positive residual disease status and abnormal ARID1A expression (Table 4).
Figure 3.
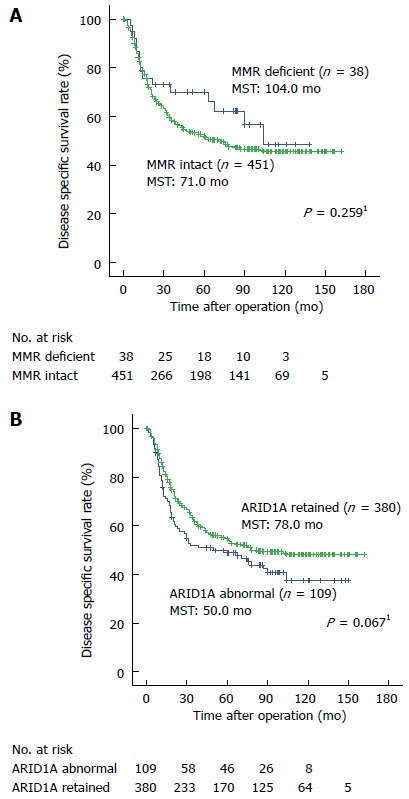
Kaplan-Meier estimates of disease specific survival for patients with gastric cancer according to the mismatch repair (A) and ARID1A expression (B) statuses. The disease specific survival curves according to the MMR and ARID1A expression statuses did not show significant differences. 1The log-rank test. MST: Median survival time; MMR: Mismatch repair.
Table 4.
Cox’s proportional hazard model analysis of prognostic factors in 489 patients with gastric cancers
| Variables |
Univariate analysis |
Multivariate analysis |
||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (yr) | ||||||
| ≥ 60/≤ 59 | 1.22 | 0.94-1.59 | 0.123 | |||
| Sex | ||||||
| Male/female | 0.72 | 0.56-0.94 | 0.015 | 0.72 | 0.55-0.95 | 0.020 |
| Serum CEA (ng/mL) | ||||||
| ≥ 5.0/< 5.0 | 1.75 | 1.33-2.33 | < 0.001 | 1.54 | 1.15-2.06 | 0.004 |
| Tumor size (mm) | ||||||
| ≥ 50/< 50 | 3.70 | 2.50-5.26 | < 0.001 | 1.88 | 1.25-2.83 | 0.002 |
| Histology | ||||||
| Undifferentiated/differentiated type | 1.54 | 1.12-2.01 | 0.002 | 1.64 | 1.24-2.16 | 0.001 |
| Lymphatic invasion | ||||||
| Present/absent | 3.23 | 2.22-4.55 | < 0.001 | 1.48 | 0.98-2.22 | 0.062 |
| Venous invasion | ||||||
| Present/absent | 1.89 | 1.45-2.44 | < 0.001 | 1.21 | 0.92-1.60 | 0.171 |
| Stage | ||||||
| Stage III, IV/I, II | 9.09 | 5.56-14.29 | < 0.001 | 3.77 | 2.30-6.17 | 0.023 |
| Residual disease | ||||||
| Positive/negative | 6.25 | 5.00-8.33 | < 0.001 | 3.79 | 2.85-5.03 | < 0.001 |
| MMR status | ||||||
| Deficient/intact | 0.74 | 0.44-1.25 | 0.264 | |||
| ARID1A status | ||||||
| Abnormal/retained | 1.30 | 0.98-1.75 | 0.070 | 1.36 | 1.01-1.84 | 0.040 |
HR: Hazards ratio; CEA: Carcinoembryonic antigen; MMR: Mismatch repair.
Relationship between the MMR status and ARID1A expression
Among the 38 MMR-deficient cases, 18 cases (47.4%) showed abnormal ARID1A expression. On the other hand, among the 451 cases with intact MMR protein expression, only 91 cases (20.2%) indicated abnormal ARID1A expression (Table 5). A statistical analysis showed a significant correlation between the ARID1A expression and the MMR statuses (P < 0.001).
Table 5.
Relationship between mismatch repair protein and ARID1A expression n (%)
|
ARID1A expression |
||||||
|
Loss |
Weak |
|||||
| Homo (n = 43) | Hetero (n = 29) | Homo (n = 21) | Hetero (n = 16) | Retained (n = 380) | P value | |
| MMR status | < 0.0011 | |||||
| Deficient | 10 (26.3) | 4 (10.5) | 2 (5.3) | 2 (5.3) | 20 (52.6) | |
| (n = 38) | ||||||
| Intact | 33 (7.3) | 25 (5.6) | 19 (4.2) | 14 (3.1) | 360 (79.8) | |
| (n = 451) | ||||||
Extended Fisher’s exact test. MMR: Mismatch repair; Homo: Homogeneous; Hetero: Heterogeneous.
DISCUSSION
In the present study, we used immunohistochemistry for four MMR proteins to analyze the MMR status. While microsatellite instability (MSI) testing has been widely used to examine the MMR status[29,30], the immunohistochemical detection of MMR proteins has been proved to be as sensitive and specific as MSI testing and is being increasingly used to screen for colorectal cancer with MMR deficiency[31-33]. An excellent correlation between the results of MSI testing and immunohistochemistry has also been reported for gastric cancer[6,8,34]. The majority of MMR deficiencies in gastric cancer is thought to arise from the hypermethylation of the MLH1 promoter[6]. In our study, 33 cases showed the concurrent loss of MLH1 and PMS2 expression, consistent with the consequences of defects in MLH1[31,32,35]. The previously reported prevalence of MMR deficiency in gastric cancers has been variable, ranging from 7.7%-25.2%[2,7-14,34]. Geographical differences in the prevalence of MMR-deficient gastric cancers seem to exist. In general, studies from Western countries have reported higher frequencies of MMR deficiency in gastric cancer, whereas those from Asian countries usually report a prevalence of less than 10%, similar to the present result. This difference may be caused by epidemiological differences, such as the prevalence of Helicobacter pylori infection[36].
In tumors defined as MMR-deficient, the loss of MMR protein was mostly homogeneous within the respective tumors, including the majority of intramucosal components. This suggests that MMR deficiency occurs at an early stage of gastric carcinogenesis. Some previous studies have similarly reported that defects in MMR are an early event during gastric carcinogenesis[3,37,38].
MMR deficiency was significantly associated with several clinicopathological features, including an older age, a female sex, an antral location, and a differentiated histology; however, no prognostic significance was observed. These observations are mostly in agreement with some previous large-scale studies[2,7-14]. The clinicopathological features of MMR-deficient colorectal cancers are well recognized: an older age, a female sex, a proximal location, an undifferentiated histology, a lower clinical stage, and a better prognosis[39,40]. Among these, an older age and a female sex are common to the clinicopathological characteristics of gastric cancer with MMR deficiency, whereas the histology associated with the MMR status differed between gastric and colorectal cancers.
We examined ARID1A expression using immunohistochemistry. Of note, previous studies demonstrated a good correlation between genetic defects in ARID1A and immunohistochemically detected ARID1A expression[19,41]. A previous study showed that either the loss of or the weak expression of ARID1A was indicative of the presence of ARID1A mutations in gastric cancers[23]. In the present study, a loss of ARID1A expression was observed in 14.7% and weak ARID1A expression was observed in 7.6% of the cases that were examined. Among the previous immunohistochemical studies of ARID1A expression, five studies defined only the loss of expression as an abnormal pattern and reported prevalence of 11%[7], 11%[22], 14%[21], 21.7%[26] and 51%[27], respectively. Another study reported the loss of and the weak expression of ARID1A as 20.2% and 7.3%, respectively[23]. While some variability exists, the prevalence of abnormal ARID1A expression seems to agree roughly among the studies excluding one study[27].
In ovarian clear cell carcinomas, ARID1A mutations are thought to occur during the early stage of tumorigenesis, since the loss of ARID1A expression is consistently homogeneous and is also observed in their precursor lesion, atypical endometriosis[19,42]. In contrast, the loss of or the weak expression of ARID1A was more commonly heterogeneous within the respective tumors in our study. Even though several immunohistochemical studies examining ARID1A expression in gastric cancer have previously been reported, most of the studies have never described heterogeneous expression[7,21-23]. This circumstance is probably because the previous studies used tissue microarrays in their analyses. The frequent heterogeneous expression of ARID1A suggests that defects in ARID1A occur often during the later stage of tumorigenesis in gastric adenocarcinomas, unlike in ovarian cancers.
Our study showed that abnormal ARID1A expression was significantly associated with lymphatic invasion and lymph node metastasis. Furthermore, abnormal ARID1A expression was significantly associated with a poor prognosis in a multivariate analysis. Three previous studies have shown several clinicopathological features of abnormal ARID1A expression in gastric cancers[7,26,27], including fundus and corpus locations[7], an undifferentiated histology[27], lymphatic invasion[7], venous invasion[7], lymph node involvement[26], and tumor infiltration[7,26,27]. Regarding prognosis, three studies have reported that ARID1A abnormalities were associated with a poorer prognosis in multivariate analyses[7,26,27]; however, ARID1A abnormalities were associated with a better prognosis in a stage-independent manner in one study[23]. In our study, cases with abnormal ARID1A expression had a significantly worse prognosis in a multivariate analysis. This discrepancy might be due to the different parameters analyzed in the multivariate analyses. The previous study reporting ARID1A abnormalities as a better prognostic factor analyzed only clinical stage, MMR status, and histology in their multivariate analysis[23]. Moreover, this study involved a relatively limited number of cases compared with the other studies including ours[23].
The current studies confirmed the previously reported correlation between MMR deficiency and the loss of ARID1A expression[7,23-26]. ARID1A contains many short repeats of 4-7 mononucleotides in its coding region, which is prone to insertion/deletion mutations in MMR-deficient tumors. Indeed, previous studies have shown that the majority of ARID1A mutations occur in its repeating sequence, leading to frameshift mutations and the complete loss of ARID1A proteins, in gastric cancers with MMR-deficiency[23,24].
In conclusion, the present study showed the clinicopathological significance of MMR deficiency and ARID1A abnormalities and the correlation of these two conditions in gastric cancers. Furthermore abnormal ARID1A expression was independently associated with an unfavorable prognosis. We also confirmed the previously reported association between MMR deficiency and abnormal ARID1A expression.
ACKNOWLEDGMENTS
We are grateful to Ms. Sachiko Miura and Ms. Chizu Kina for technical assistance.
COMMENTS
Background
ARID1A plays a role in the regulation of diverse cellular processes, from development and differentiation to proliferation. Recently, ARID1A mutations have also been reported in some tumors, including gastric cancer.
Research frontiers
Some studies have examined clinicopathological significance of ARID1A inactivations; interestingly, a significant relationship between ARID1A mutations and mismatch repair deficiency have been suggested in gastric cancers.
Innovations and breakthroughs
The authors showed that abnormal ARID1A expression was independently associated with an unfavorable prognosis in a large consecutive series of advanced gastric cancers using immunohistochemistry, and the authors also confirmed the association between MMR deficiency and abnormal ARID1A expression.
Applications
The present study suggests that ARID1A inactivation could be a potentially negative prognostic factor in gastric cancers.
Terminology
ARID1A is a key component of the multi-protein SWI/SNF chromatin remodeling complex, and is involved in the regulation of diverse cellular processes, from development and differentiation to proliferation.
Peer-review
The manuscript written by Inada et al analyzed ARID1A expression and its correlation with DNA mismatch repair status in a large series of primary gastric adenocarcinoma. They found that ARID1A inactivation is associated with lymphatic invasion, lymph node metastasis and MMR deficiency. The data are important and provide novel information in the management of patients with gastric adenocarcinoma. However, there are some concerns that need to be addressed.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 2, 2014
First decision: July 21, 2014
Article in press: September 30, 2014
P- Reviewer: Grizzi F, KayambaV, Kwon OD, Shimizu Y, Torres MI S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Oki E, Kakeji Y, Zhao Y, Yoshida R, Ando K, Masuda T, Ohgaki K, Morita M, Maehara Y. Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann Surg Oncol. 2009;16:2510–2515. doi: 10.1245/s10434-009-0580-8. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Rashid A, Hamilton SR, et al. Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol. 2002;161:611–618. doi: 10.1016/S0002-9440(10)64216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jascur T, Boland CR. Structure and function of the components of the human DNA mismatch repair system. Int J Cancer. 2006;119:2030–2035. doi: 10.1002/ijc.22023. [DOI] [PubMed] [Google Scholar]
- 5.Maehara Y, Egashira A, Oki E, Kakeji Y, Tsuzuki T. DNA repair dysfunction in gastrointestinal tract cancers. Cancer Sci. 2008;99:451–458. doi: 10.1111/j.1349-7006.2007.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC. hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res. 1999;59:159–164. [PubMed] [Google Scholar]
- 7.Abe H, Maeda D, Hino R, Otake Y, Isogai M, Ushiku AS, Matsusaka K, Kunita A, Ushiku T, Uozaki H, et al. ARID1A expression loss in gastric cancer: pathway-dependent roles with and without Epstein-Barr virus infection and microsatellite instability. Virchows Arch. 2012;461:367–377. doi: 10.1007/s00428-012-1303-2. [DOI] [PubMed] [Google Scholar]
- 8.Beghelli S, de Manzoni G, Barbi S, Tomezzoli A, Roviello F, Di Gregorio C, Vindigni C, Bortesi L, Parisi A, Saragoni L, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery. 2006;139:347–356. doi: 10.1016/j.surg.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Ahn BK, Nam YS, Pyo JY, Oh YH, Lee KH. Microsatellite instability is associated with the clinicopathologic features of gastric cancer in sporadic gastric cancer patients. J Gastric Cancer. 2010;10:149–154. doi: 10.5230/jgc.2010.10.4.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505–511. doi: 10.1002/ijc.26399. [DOI] [PubMed] [Google Scholar]
- 11.Seo HM, Chang YS, Joo SH, Kim YW, Park YK, Hong SW, Lee SH. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99:143–147. doi: 10.1002/jso.21220. [DOI] [PubMed] [Google Scholar]
- 12.Lee HS, Choi SI, Lee HK, Kim HS, Yang HK, Kang GH, Kim YI, Lee BL, Kim WH. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002;15:632–640. doi: 10.1038/modpathol.3880578. [DOI] [PubMed] [Google Scholar]
- 13.Falchetti M, Saieva C, Lupi R, Masala G, Rizzolo P, Zanna I, Ceccarelli K, Sera F, Mariani-Costantini R, Nesi G, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–932. doi: 10.1016/j.humpath.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 14.Arai T, Sakurai U, Sawabe M, Honma N, Aida J, Ushio Y, Kanazawa N, Kuroiwa K, Takubo K. Frequent microsatellite instability in papillary and solid-type, poorly differentiated adenocarcinomas of the stomach. Gastric Cancer. 2013;16:505–512. doi: 10.1007/s10120-012-0226-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11:481–492. doi: 10.1038/nrc3068. [DOI] [PubMed] [Google Scholar]
- 16.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weissman B, Knudsen KE. Hijacking the chromatin remodeling machinery: impact of SWI/SNF perturbations in cancer. Cancer Res. 2009;69:8223–8230. doi: 10.1158/0008-5472.CAN-09-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan B, Wang TL, Shih IeM. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 2011;71:6718–6727. doi: 10.1158/0008-5472.CAN-11-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones S, Wang TL, Shih IeM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA, Vogelstein B, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;330:228–231. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, Steidl C, Wiseman SM, Gascoyne RD, Gilks B, et al. Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol. 2011;224:328–333. doi: 10.1002/path.2911. [DOI] [PubMed] [Google Scholar]
- 22.Guan B, Mao TL, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL, Shih IeM. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol. 2011;35:625–632. doi: 10.1097/PAS.0b013e318212782a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY, et al. Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet. 2011;43:1219–1223. doi: 10.1038/ng.982. [DOI] [PubMed] [Google Scholar]
- 24.Jones S, Li M, Parsons DW, Zhang X, Wesseling J, Kristel P, Schmidt MK, Markowitz S, Yan H, Bigner D, et al. Somatic mutations in the chromatin remodeling gene ARID1A occur in several tumor types. Hum Mutat. 2012;33:100–103. doi: 10.1002/humu.21633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang ZJ, Cutcutache I, Poon SL, Zhang SL, McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A, et al. Exome sequencing of gastric adenocarcinoma identifies recurrent somatic mutations in cell adhesion and chromatin remodeling genes. Nat Genet. 2012;44:570–574. doi: 10.1038/ng.2246. [DOI] [PubMed] [Google Scholar]
- 26.Wiegand KC, Sy K, Kalloger SE, Li-Chang H, Woods R, Kumar A, Streutker CJ, Hafezi-Bakhtiari S, Zhou C, Lim HJ, et al. ARID1A/BAF250a as a prognostic marker for gastric carcinoma: a study of 2 cohorts. Hum Pathol. 2014;45:1258–1268. doi: 10.1016/j.humpath.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang DD, Chen YB, Pan K, Wang W, Chen SP, Chen JG, Zhao JJ, Lv L, Pan QZ, Li YQ, et al. Decreased expression of the ARID1A gene is associated with poor prognosis in primary gastric cancer. PLoS One. 2012;7:e40364. doi: 10.1371/journal.pone.0040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 29.Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116:1453–1456. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L. Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part II. The utility of microsatellite instability testing. J Mol Diagn. 2008;10:301–307. doi: 10.2353/jmoldx.2008.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shia J, Tang LH, Vakiani E, Guillem JG, Stadler ZK, Soslow RA, Katabi N, Weiser MR, Paty PB, Temple LK, et al. Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol. 2009;33:1639–1645. doi: 10.1097/PAS.0b013e3181b15aa2. [DOI] [PubMed] [Google Scholar]
- 32.Shia J, Ellis NA, Klimstra DS. The utility of immunohistochemical detection of DNA mismatch repair gene proteins. Virchows Arch. 2004;445:431–441. doi: 10.1007/s00428-004-1090-5. [DOI] [PubMed] [Google Scholar]
- 33.Poynter JN, Siegmund KD, Weisenberger DJ, Long TI, Thibodeau SN, Lindor N, Young J, Jenkins MA, Hopper JL, Baron JA, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leite M, Corso G, Sousa S, Milanezi F, Afonso LP, Henrique R, Soares JM, Castedo S, Carneiro F, Roviello F, et al. MSI phenotype and MMR alterations in familial and sporadic gastric cancer. Int J Cancer. 2011;128:1606–1613. doi: 10.1002/ijc.25495. [DOI] [PubMed] [Google Scholar]
- 35.Ward RL, Turner J, Williams R, Pekarsky B, Packham D, Velickovic M, Meagher A, O’Connor T, Hawkins NJ. Routine testing for mismatch repair deficiency in sporadic colorectal cancer is justified. J Pathol. 2005;207:377–384. doi: 10.1002/path.1851. [DOI] [PubMed] [Google Scholar]
- 36.Prinz C, Schwendy S, Voland P. H pylori and gastric cancer: shifting the global burden. World J Gastroenterol. 2006;12:5458–5464. doi: 10.3748/wjg.v12.i34.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JH, Park SJ, Abraham SC, Seo JS, Nam JH, Choi C, Juhng SW, Rashid A, Hamilton SR, Wu TT. Frequent CpG island methylation in precursor lesions and early gastric adenocarcinomas. Oncogene. 2004;23:4646–4654. doi: 10.1038/sj.onc.1207588. [DOI] [PubMed] [Google Scholar]
- 38.Isogaki J, Shinmura K, Yin W, Arai T, Koda K, Kimura T, Kino I, Sugimura H. Microsatellite instability and K-ras mutations in gastric adenomas, with reference to associated gastric cancers. Cancer Detect Prev. 1999;23:204–214. doi: 10.1046/j.1525-1500.1999.99020.x. [DOI] [PubMed] [Google Scholar]
- 39.Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 40.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 41.Maeda D, Mao TL, Fukayama M, Nakagawa S, Yano T, Taketani Y, Shih IeM. Clinicopathological significance of loss of ARID1A immunoreactivity in ovarian clear cell carcinoma. Int J Mol Sci. 2010;11:5120–5128. doi: 10.3390/ijms11125120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto S, Tsuda H, Takano M, Tamai S, Matsubara O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod Pathol. 2012;25:615–624. doi: 10.1038/modpathol.2011.189. [DOI] [PubMed] [Google Scholar]


