Abstract
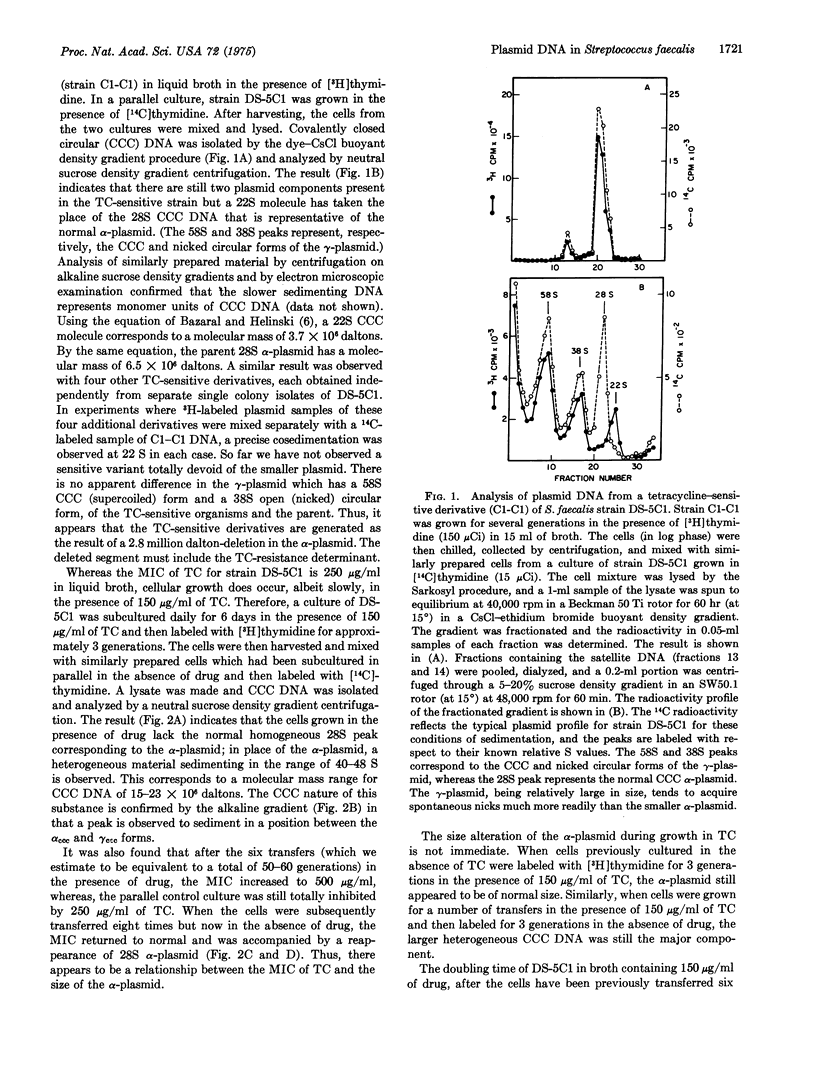
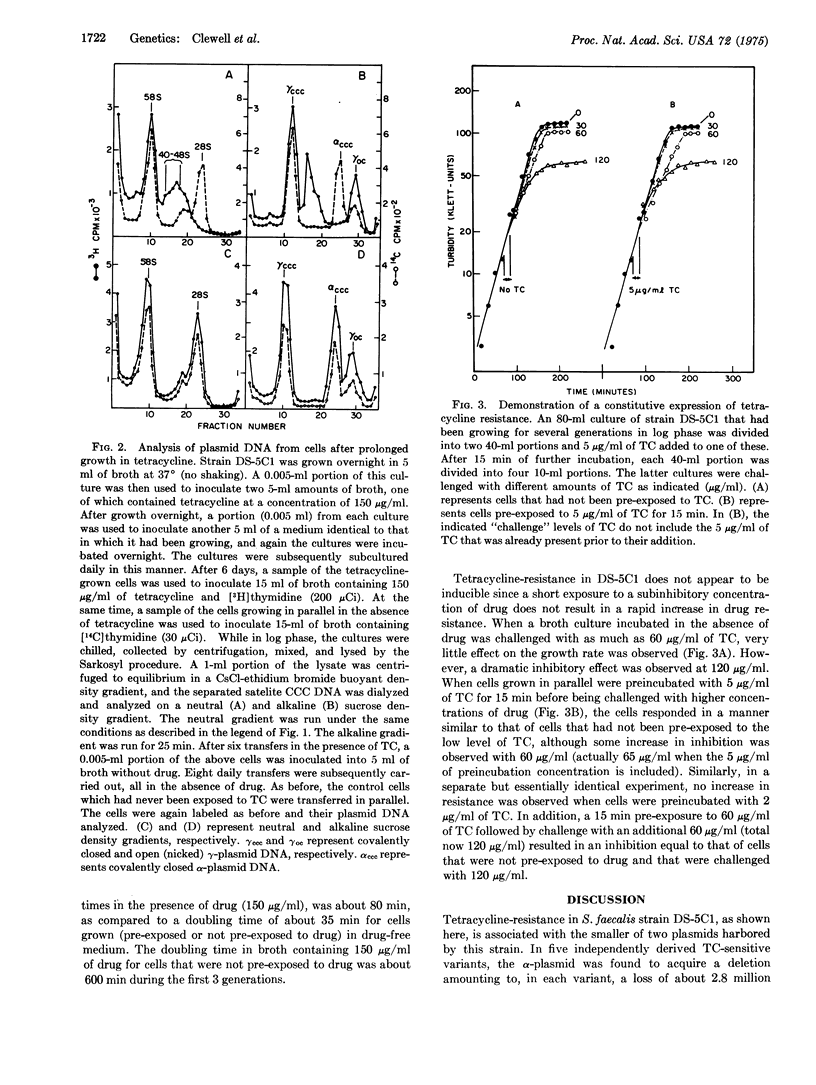
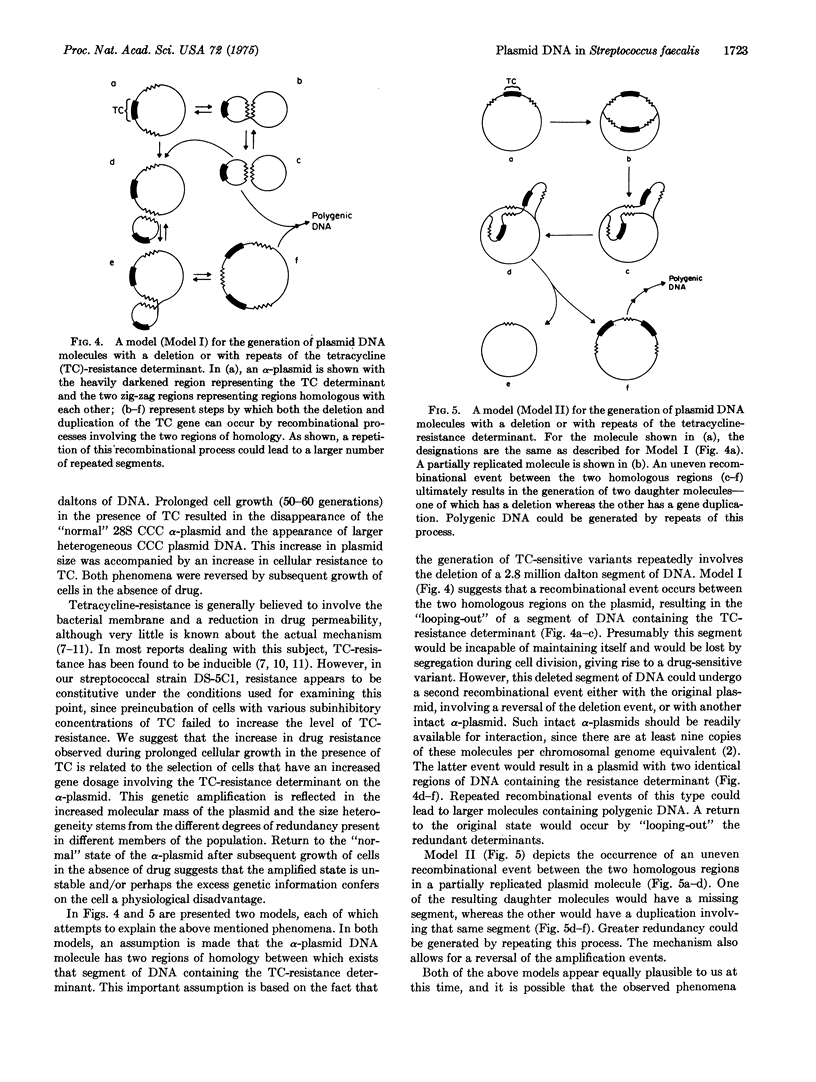
The tetracycline (TG)-resistant Streptococcus faecalis strain DS-5Cl harbors two plasmids designated alpha and gamma with molecular masses of approximately 6 and 35 million daltons, respectively. TC-sensitive variants were derived by storing cells at 45 degrees for 2-3 weeks. Analysis of covalently closed circular DNA from five such variants (derived independently) revealed that in each variant the alpha-plasmid, which normally sediments at 28 S (supercoiled) in a sucrose density gradient, was replaced by a 22S substance. Growth of DS-5Cl in the presence of 150 mug/ml of TC (minimum inhibitory concentration is 250 mug/ml in liquid broth) for a prolonged period of time (50-60 generations) resulted in the disappearance of 28S DNA and the appearance of a heterogeneous covalently-closed circular DNA sedimenting at about 40-48 S. This phenomenon was accompanied by an increase in the level of bacterial TC-resistance, whereby tells were subsequently grown in the absence of TC for 70-80 generations, the heterogeneous DNA disappeared and a typical 28S alpha-plasmid reappeared. The cells also became less resistant to TC, i.e., the minimum inhibitory concentration returned to 250 mug/ml. These data suggest that bacterial growth in the presence of TC results in a reversible gene amplification with respect to a TC-resistant determinant residing on the alpha-plasmid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Dunny G. M., Birch N., Hascall G., Clewell D. B. Isolation and characterization of plasmid deoxyribonucleic acid from Streptococcus mutans. J Bacteriol. 1973 Jun;114(3):1362–1364. doi: 10.1128/jb.114.3.1362-1364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Foster S. J. Effect of osmotic shock on tetracycline resistance in Escherichia coli bearing an R-factor. Biochem J. 1971 Jan;121(2):287–292. doi: 10.1042/bj1210287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Rownd R. R-factor-mediated resistance to tetracycline in Proteus mirabilis. J Bacteriol. 1973 Jul;115(1):235–242. doi: 10.1128/jb.115.1.235-242.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. F., Rownd R. Transition of the R factor R12 in Proteus mirabilis. J Bacteriol. 1974 Jun;118(3):867–879. doi: 10.1128/jb.118.3.867-879.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Kasamatsu H., Mickel S. The molecular nature and replication of drug resistance factors of the Enterobacteriaceae. Ann N Y Acad Sci. 1971 Jun 11;182:188–206. doi: 10.1111/j.1749-6632.1971.tb30656.x. [DOI] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Tomura T., Hirano T., Ito T., Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group D streptococci. Jpn J Microbiol. 1973 Nov;17(6):445–452. doi: 10.1111/j.1348-0421.1973.tb00930.x. [DOI] [PubMed] [Google Scholar]


