Abstract
Circulating fatty acids (FA) are associated with a multitude of chronic diseases. However, a major gap in establishing such relationships is the lack of accepted fatty acid reference ranges representing healthy individuals. Data on validated FA reference ranges would provide a better understanding of study baseline measures and aid in the evaluation and interpretation of pharmaceutical or dietary interventions. Reference ranges for plasma FA levels have been reported in a few small studies and on a limited number of FA. Therefore, we determined the average and percentiles of a broad set of 61 FA (C14 - C24:1) from plasma total lipids from an ethnically diverse population of healthy young Canadian males and females (Total n = 826). Plasma concentrations of some of the major FA ranged from 0.3 to 4.1 mmol/L for palmitic acid, 0.1 to 1.0 mmol/L for stearic acid, 0.03 to 3.2 mmol/L for oleic acid, 0.2 to 5.0 mmol/L for linoleic acid (LA), 12.0 to 186.9 μmol/L for α-linolenic acid, and 7.2 to 237.5 μmol/L for docosahexaenoic acid (DHA). Males had significantly higher plasma concentrations of γ-linolenic acid (GLA) and n-3 docosapentaenoic acid and lower concentrations of palmitoleic acid, LA and DHA than females. Comparison of FA concentrations between Caucasians, East Asians and South Asians revealed that South Asians had significantly lower levels of palmitoleic acid (p < 0.01) and oleic acid (p = 0.01) while East Asians had lower levels of GLA (p = 0.02) and dihomo-γ-linolenic acid (p = 0.03). Overall, these data provide a comprehensive set of quantitative values that profiles a small cohort of Canadians which highlights the utility of establishing validated FA reference ranges that may be used to understand how deficient, suboptimal, or excess amounts of a given FA may be associated with chronic disease.
Introduction
Currently, there is a fundamental gap in the field of fatty acids (FA) research that hinders the translation and utilization of current knowledge into clinical practice for the prevention and management of chronic diseases. A large body of work has made evident the important influence of dietary and circulating FA in health and disease. FA are implicated in chronic diseases such as cardiovascular and heart disease, cancer, inflammation and autoimmune disease [1–7]; however, despite their recognized ability to modify the risk of disease, “normal” levels of circulating fatty acids are yet to be defined. The lack of established reference ranges for saturated, trans, monounsaturated and polyunsaturated FA has resulted in the poor interpretability of human research [8]. Clinical reference values, obtained by objective clinical measures and not estimated from dietary assessment, are established for many types of lipids including LDL-cholesterol, HDL-cholesterol, total cholesterol, triglycerides, and total free fatty acids. FA linked to these lipids are just as important in relation to short and long term health. As such, a recent study reported associations between serum FA and certain types of ischemic strokes [9]. Clinical reference ranges of FA will allow definitive identification of deficiencies or excesses associated with poor health and would make it possible to establish healthy targets. Yet, the identification of such abnormalities requires first knowledge of the normal distribution of individual circulating FA concentrations. Thus, measurement of FA concentrations in young healthy adults will provide a distribution of values from which identification of age- and disease-related changes is attainable. In that regard, we sought to determine the average concentrations (μmol/L) of 61 FA in total plasma of young healthy Canadians in a cross-sectional study.
Subjects and Methods
Study population
Participants were recruited as part of the cross-sectional Toronto Nutrigenomics and Health (TNH) Study [10] between September 2004 and July 2009. Participants ethno-cultural groups were self reported and these included Caucasian, Asian, African, South Asian, Middle eastern, Hispanic, Native American and Jewish. Ages of participants ranged between 20 and 29 years old and written informed consent was obtained from all of those who participated. Participants were a random sample of free living subjects consuming their usual diet. Anthropometric measurements were recorded for all participants and health, lifestyle, and food frequency questionnaires were completed by subjects. Standard clinical procedure was followed for the measurement of glucose, insulin, total-, LDL- and HDL-cholesterol, triglycerides, and free fatty acids [10] (S1 Table). HOMA-IR was calculated using the homeostasis model assessment method [11] (S1 Table). Total energy intake from fat and physical activity scores were calculated from completed questionnaires as previously described [10,12] (S1 Table). Women who were pregnant or breastfeeding were not included in the study. Exclusion criteria for the analysis consisted of: smoking, underlying health problems and use of hormonal contraceptives. The study protocol was approved by the Research Ethics Boards at the University of Toronto and University of Guelph.
Gas chromatography analysis
Subjects were required to fast overnight for a minimum of 12 h prior to blood collection, separation of plasma and subsequent freezing of samples at -80°C. Frozen plasma samples were thawed on ice for 30 min and a mixture of chloroform: methanol (2:1 v/v) was added to a 50 μl aliquot and analyzed as described previously [13]. In brief, free fatty acid C17:0 was used as an internal standard (5 μg of 1 mg/ml stock). Samples were flushed with nitrogen gas prior to storage over night at 4°C. The next day, samples were subjected to a double extraction. The lower organic phase containing lipids were dried down under a gentle stream nitrogen then saponified by KOH in methanol for 1 hour and subsequently methylated by boron trifluoride (14%) for 1 hour. Fatty acid methyl esters (FAME) were quantified as previously described by gas chromatography [14]. FA peak areas were determined using EZChrom Elite software (Version 3.3.2) [15]. The internal standard was used to calculate FA concentrations (μmol/L) (S1 Table). The responsiveness of the detector was routinely checked against the composition of a commercial mixture of FAME standards.
Statistical analysis of Data
Results are expressed as mean ± standard deviation (SD). All data was analyzed using JMP genomics software V5 (SAS Institute, Cary, NC). A Tukey’s Honestly Significant Difference test was used to determine significant differences in mean biomarkers of health and FA concentrations between sexes and ethnicities. P values were adjusted for age, BMI, sex/ethnicity, % energy from dietary fat and physical activity in linear regression models. A p-value of ˂ 0.05 was considered statistically significant and Bonferroni correction was used to account for multiple testing.
Results and Discussion
In this study we determined, average concentrations of 61 FA in total plasma of young healthy and ethnoculturally-diverse Canadians. We also identified differences in FA concentrations between males and females and between Caucasian, East Asian, and South Asian Canadians. The general characteristics of the study population are presented in Table 1 and are compared by sex and ethnicity in Tables 1 and 2, respectively. Concentrations and percentiles for FA were determined in 826 healthy young individuals (Table 3) and examples of the normal distribution of the wide range of fatty acid concentrations are demonstrated in Fig 1. In 1994, Sera et al determined reference ranges for lauric acid, myristic acid, palmitic acid, palmitoleic acid, stearic acid, oleic acid, linoleic acid, linolenic, homo-γ-linolenic acid and arachidonic acid in American males and females aged 18–55 (n = 128) [16]. Reference range values from these limited FA are similar; however the larger sample size in the present study reveals an even greater range of values. This wider range of concentrations forms a normal distribution as shown in Fig 1. Lower minimums are likely due to the greater sample size in the present study or potentially reflects dietary changes over the past two decades. In contrast with the current study, the vast majority of studies examining circulating FA levels have measured a smaller subset of FA, and reported values of FA as percent composition (summarized in Table 4). The problem of presenting findings in such a manner is the difficulty in comparing results since percent composition values depend on the basket of FA investigated. A study investigating the validity of reporting FA as concentrations compared to weight percentages found that using the latter method of reporting led to the loss of significant differences in FA profiles between groups [17]. Thus, FA concentrations are more useful for facilitating comparisons between studies; hence, we recommend that future studies examining FA levels determine FA concentrations. It is worth noting that studies that have claimed to report FA reference ranges have done so by determining FA concentrations in healthy populations without determining whether these FA are associated with the risk of specific disease [16,18]. Thus, validated reference ranges are yet to be truly established. Also, in establishing high quality reference values it will be important to use multiple internal standards to account for differential responses by FA of different chain length. Thus the inclusion of C17:0 and C21:0 may be appropriate for quantifying long and very long chain FA. Another important consideration when selecting relative or concentrations for establishing references is how these values are used. Studies using concentration values may potentially be more appropriate than relative FA values in association studies with chronic disease biomarkers. Concentrations values are not dependent upon the relative abundance of other FA which is the case when FA are reported as mol% or wt% (area under curve). Reporting of relative FA values is common in nutrition studies and easier to determine than a quantitative approach. Therefore, the association of a relative value to a biomarker is influenced by other FA. Therefore, concentration values which are measured independent of other FA would reflect a direct relationship between what is measured and a given biomarker or outcome.
Table 1. General characteristics of study population.
| Total Population | Males | Females | p-value | |
|---|---|---|---|---|
| Population (#) | 826 | 327 | 499 | |
| Age (yrs) | 22.6 ± 2.5 | 22.8 ± 2.5 | 22.5 ± 2.5 | 0.08 |
| BMI (kg/m2) | 22.8 ± 3.4 | 23.6 ± 3.3 | 22.2 ± 3.3 | ˂ 0.01* |
| HOMA-IR | 1.4 ± 1.3 | 1.4 ± 1.1 | 1.4 ± 1.3 | 0.59 |
| Glucose (mmol/L) | 4.8 ± 0.4 | 4.9 ± 0.4 | 4.8 ± 0.4 | ˂ 0.01* |
| Insulin (pmol/L) | 46.2 ± 38.2 | 44.0 ± 30.4 | 47.6 ± 42.6 | 0.19 |
| Total cholesterol (mmol/L) | 4.2 ± 0.7 | 4.1 ± 0.7 | 4.2 ± 0.7 | ˂ 0.01* |
| HDL-cholesterol (mmol/L) | 1.5 ± 0.4 | 1.3 ± 0.3 | 1.6 ± 0.4 | ˂ 0.01* |
| LDL-cholesterol (mmol/L) | 2.3 ± 0.6 | 2.3 ± 0.7 | 2.3 ± 0.6 | 0.27 |
| Triglycerides (mmol/L) | 0.9 ± 0.4 | 1.0 ± 0.5 | 0.8 ± 0.3 | ˂ 0.01* |
| Free fatty acids (μmol/L) | 474.6 ± 251.7 | 457.5 ± 252.5 | 485.9 ± 250.8 | 0.11 |
| % Energy from dietary fat | 27.0 ± 6.0 | 26.8 ± 6.0 | 27.1 ± 6.1 | 0.43 |
Data represented as Mean±SD.
*A p-value < 0.05, determined by Tukey’s HSD for differences between males and females, was considered statistically significant.
Table 2. General characteristics of study population compared by ethnicity.
| Fatty Acid | Caucasians | East Asians | South Asians | p- value |
|---|---|---|---|---|
| Population (#) | 287 | 353 | 107 | |
| Age (yrs) | 23.0 ± 2.5 | 22.1 ± 2.3 | 22.4 ± 2.5 | |
| BMI (kg/m2) | 23.5 ± 3.3a | 21.8 ± 2.6b | 23.3 ± 3.9a | ˂ 0.01* |
| HOMA-IR | 1.3 ± 1.5b | 1.3 ± 1.0b | 1.9 ± 1.3a | ˂ 0.01* |
| Glucose (mmol/L) | 4.8 ± 0.4b | 4.8 ± 0.4b | 5.0 ± 0.4a | ˂ 0.01* |
| Insulin (pmol/L) | 42.2 ± 46.2b | 43.7 ± 28.4b | 61.7 ± 40.4a | ˂ 0.01* |
| Total cholesterol (mmol/L) | 4.1 ± 0.7b | 4.3 ± 0.7a | 4.1 ± 0.8a, b | 0.03* |
| HDL-cholesterol (mmol/L) | 1.5 ± 0.4b | 1.6 ± 0.4a | 1.3 ± 0.3c | ˂ 0.01* |
| LDL-cholesterol (mmol/L) | 2.2 ± 0.6b | 2.3 ± 0.6a, b | 2.4 ± 0.7a | 0.04* |
| Triglycerides (mmol/L) | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.9 ± 0.4 | 0.29 |
| Free fatty acids (μmol/L) | 464.2 ± 253.8a, b | 503.4 ± 265.0a | 420.5 ± 214.8b | 0.02* |
| % Energy from dietary fat | 27.8 ± 6.6a | 26.3 ± 5.1b | 26.6 ± 7.3a, b | ˂ 0.01* |
Data represented as Mean±SD. A p-value < 0.05, determined by Tukey’s HSD, was considered statistically significant. Different letters (a/b) denote values that are significantly different between groups.
* denote p-values that are significant.
Table 3. Range, mean and percentiles of FA concentrations (μmol/L) of plasma total lipids.
| Fatty Acid | Min | Mean±SD | Max | 10 | 25 | 50 | 75 | 90 |
|---|---|---|---|---|---|---|---|---|
| 14:0 (Myristic acid) | 16.2 | 63.6 ± 37.1 | 325.7 | 29.8 | 39.2 | 54.0 | 76.2 | 104.7 |
| 15:0 (Pentadyclic acid) | t | 17.8 ± 6.7 | 56.1 | 10.8 | 13.2 | 17.1 | 21.3 | 26.3 |
| 16:0 (Palmitic acid) | 285.4 | 1631.1 ± 459.3 | 4064.5 | 1140.2 | 1339.7 | 1569.5 | 1839.2 | 2182.2 |
| 18:0 (Stearic acid) | 110.2 | 489.5 ± 124.3 | 1013.7 | 353.3 | 406.9 | 474.2 | 556.8 | 650.9 |
| 19:0 | t | 4.3 ± 4.2 | 25.7 | t | 1.1 | 3.1 | 6.6 | 10.2 |
| 20:0 (Arachidic acid) | t | 4.9 ± 3.7 | 29.8 | t | 2.6 | 4.6 | 6.4 | 9.4 |
| 21:0 | t | 1.5 ± 1.9 | 10.0 | t | T | 0.3 | 2.9 | 4.1 |
| 22:0 (Behenic acid) | t | 6.7 ± 5.3 | 39.0 | t | 3.4 | 6.0 | 9.4 | 14.0 |
| 24:0 (Lignoceric acid) | t | 1.4 ± 2.4 | 15.7 | t | T | t | 2.4 | 5.1 |
| 14:1 (Myristoleic acid) | t | 2.7 ± 4.1 | 31.3 | t | T | t | 4.2 | 7.7 |
| 15:1 c10 | t | 0.1 ± 0.3 | 2.7 | t | T | t | t | t |
| 16:1 c9 (Palmitoleic acid) | 27.7 | 133.0 ± 67.2 | 555.9 | 67.8 | 88.5 | 119.5 | 156.9 | 211.4 |
| 17:1 c10 | t | 10.5 ± 7.4 | 45.2 | t | 6.2 | 10.7 | 14.7 | 19.1 |
| 18:1 c9 (Oleic acid) | 178.7 | 1285.5± 416.7 | 3210.5 | 858.6 | 1007.1 | 1226.3 | 1472.0 | 1808.7 |
| 18:1 c11 (Vaccenic acid) | 11.4 | 129.2 ± 59.5 | 562.9 | 81.5 | 96.6 | 114.3 | 141.5 | 185.1 |
| 18:1 c12 | t | 18.7 ± 13.6 | 101.8 | 6.6 | 9.0 | 14.9 | 22.7 | 38.3 |
| 18:1 c13 | t | 3.5 ± 3.5 | 18.1 | t | 1.2 | 2.5 | 4.5 | 8.9 |
| 18:1 c14 | t | 2.2 ± 1.9 | 11.6 | t | 0.6 | 2.0 | 3.4 | 5.0 |
| 19:1 c10 | t | 0.5 ± 1.1 | 8.2 | t | T | t | t | 1.9 |
| 20:1 c5 | t | 4.8 ± 3.1 | 26.9 | 2.2 | 3.1 | 4.2 | 5.6 | 7.6 |
| 20:1 c8 | t | 1.3 ± 1.8 | 10.5 | t | T | 0.4 | 2.1 | 4.1 |
| 20:1 c11 (Gondoic acid) | t | 8.2 ± 4.9 | 29.5 | 2.2 | 4.8 | 8.0 | 11.1 | 14.1 |
| 22:1 c13 (Erucic acid) | t | 3.9 ± 5.9 | 48.0 | t | T | t | 7.1 | 12.2 |
| 24:1 c15 (Nervonic acid) | t | 4.0 ± 5.3 | 30.0 | t | T | 1.8 | 6.3 | 11.8 |
| 16:1 t9 (Palmitoleic acid) | t | 17.0 ± 9.1 | 65.2 | t | 12.1 | 17.3 | 22.0 | 28.2 |
| 18:1 t4 | t | 5.2 ± 5.3 | 30.7 | t | T | 4.1 | 8.3 | 12.3 |
| 18:1 t5 (Thalictric acid) | t | 1.7 ± 2.9 | 19.0 | t | T | 0.5 | 1.7 | 5.6 |
| 18:1 t6–8 (Petroselaidic acid) | t | 7.5 ±5.9 | 56.0 | 2.5 | 3.8 | 5.7 | 9.5 | 14.8 |
| 18:1 t9 (Elaidic acid) | t | 16.5 ± 11.3 | 88.0 | 6.4 | 9.0 | 13.1 | 20.0 | 32.9 |
| 18:1 t10 | t | 17.0 ± 11.3 | 71.1 | 6.4 | 8.8 | 13.7 | 21.8 | 32.4 |
| 18:1 t11 (Transvaccenic acid) | t | 14.0 ± 8.1 | 74.2 | 5.9 | 8.7 | 12.3 | 18.0 | 24.7 |
| 18:1 t12 | t | 9.6 ± 5.7 | 42.8 | 4.0 | 5.6 | 8.4 | 12.3 | 17.1 |
| 18:1 t13 or c6 | t | 12.5 ± 17.1 | 175.7 | t | 5.7 | 9.3 | 13.9 | 21.1 |
| 18:2 tt | t | 3.5 ± 4.3 | 38.7 | t | 1.1 | 2.3 | 4.5 | 7.4 |
| 18:2 t9t12 (Linoelaidic acid) | t | 2.1 ± 3.0 | 20.9 | t | T | 1.2 | 2.5 | 6.7 |
| 18:2 c9t13 | t | 8.5 ± 8.0 | 69.3 | t | 2.1 | 7.7 | 11.9 | 17.7 |
| 18:2 c9t12 | t | 15.4 ± 6.4 | 45.2 | 8.4 | 10.9 | 14.2 | 18.9 | 24.0 |
| 18:2 t9c12 | t | 9.8 ± 4.3 | 31.5 | 5.3 | 6.9 | 9.0 | 12.2 | 15.6 |
| 18:2 c9c14 | t | 3.0 ± 7.7 | 62.1 | t | t | t | t | 10.0 |
| 18:2 c9c15 (Mangiferic acid) | t | 3.0 ± 3.9 | 39.1 | t | t | 2.0 | 4.6 | 7.2 |
| 18:2 c9t11 CLA | t | 14.4 ± 6.2 | 42.7 | 7.5 | 10.1 | 13.3 | 17.3 | 22.4 |
| 18:2 c11t13 CLA | t | 2.1 ± 1.8 | 12.3 | t | t | 2.2 | 3.0 | 4.2 |
| 18:2 t10c12 CLA | t | 4.3 ± 2.5 | 17.9 | 2.0 | 2.9 | 3.8 | 5.2 | 7.3 |
| 18:2 c/c CLA1 | t | 0.8 ± 1.3 | 8.5 | t | t | t | 1.4 | 2.6 |
| 18:2 c/c CLA2 | t | 0.5 ± 1.0 | 6.6 | t | t | t | t | 1.9 |
| 18:2 tt CLA | t | 6.5 ± 4.4 | 23.2 | t | 3.2 | 6.5 | 9.5 | 11.9 |
| 18:2 c9c12 (LA) | 279.7 | 2233.8± 622.6 | 4970.5 | 1540.1 | 1853.0 | 2208.2 | 2596.0 | 2962.0 |
| 18:3 c6c9c12 (γ-linolenic acid) | 1.4 | 23.5 ± 13.8 | 93.3 | 9.0 | 13.8 | 20.8 | 29.4 | 41.4 |
| 20:2 c11c14 (Dihomo linolenic acid) | t | 13.1 ± 5.0 | 37.3 | 7.8 | 9.8 | 12.5 | 16.0 | 19.7 |
| 20:3 c8c11c14 (Homo-γ-linolenic acid) | 7.9 | 74.3 ± 30.4 | 222.1 | 41.5 | 53.0 | 68.2 | 90.4 | 113.4 |
| 20:4 c5c8c11c14 (AA) | 42.7 | 393.0 ± 119.1 | 882.8 | 254.8 | 313.3 | 385.6 | 461.4 | 548.2 |
| 22:2 c13c16 (Docosadienoic acid) | t | 3.1 ± 3.3 | 18.4 | t | t | 2.8 | 4.9 | 7.3 |
| 22:4 c7c10c13c16 (Adrenic acid) | t | 15.4 ± 23.0 | 158.4 | 1.9 | 5.6 | 8.5 | 12.5 | 36.5 |
| 22:5 c4c7c10c13c16 (n-6 DPA) | t | 8.0 ± 5.9 | 41.1 | t | 4.7 | 7.4 | 10.3 | 15.1 |
| 18:3 c9c12c15 (LNA) | 12.0 | 54.4 ± 25.1 | 186.9 | 29.1 | 37.9 | 48.6 | 65.4 | 87.6 |
| 18:4 c6c9c12c15 (Stearidonic acid) | t | 0.2 ± 0.5 | 4.3 | t | t | t | t | 0.6 |
| 20:3 c11c14c17 (Dihomolinoleic acid) | t | 1.6 ± 2.5 | 17.9 | t | t | t | 2.9 | 4.8 |
| 20:5 c5c8c11c14c17 (EPA) | 4.4 | 40.3 ± 28.3 | 215.4 | 16.0 | 23.4 | 32.4 | 47.5 | 73.3 |
| 22:3 c13c16c19 | t | 0.6 ± 1.9 | 15.1 | t | t | t | t | 2.6 |
| 22:5 c7c10c13c16c19 (n-3 DPA) | t | 23.9 ± 10.0 | 88.5 | 14.0 | 17.7 | 22.1 | 27.8 | 36.5 |
| 22:6 c4c7c10c13c16c19 (DHA) | 7.2 | 88.8 ± 36.8 | 237.5 | 47.8 | 62.7 | 82.0 | 110.6 | 138.0 |
| Total FA | 1251.1 | 6947.6±1816.2 | 16225.3 | 5052.5 | 5780.4 | 6745.4 | 7947.8 | 9108.9 |
Abbreviation: FA, fatty acids; t, trace. N = 826.
Fig 1. Distribution of total plasma fatty acid concentrations of selected fatty acids.
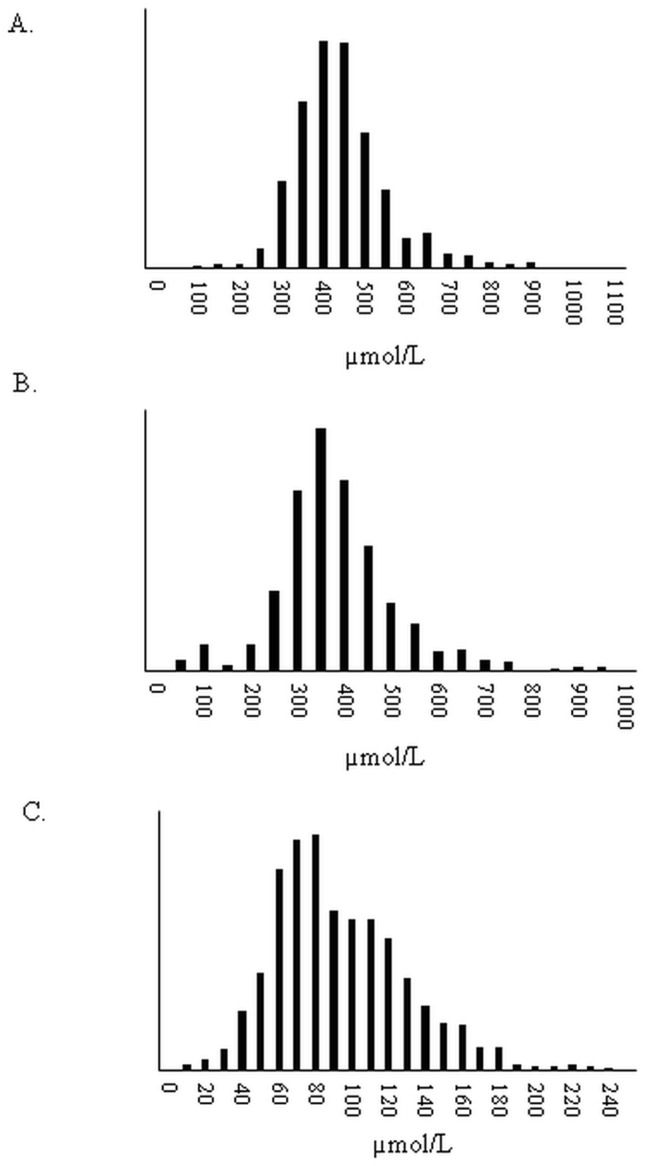
A. 16:0 (Palmitic acid); B. 18:1 c9 (Oleic acid); C. 22:6n-3 (DHA).
Table 4. Studies reporting circulating FA levels (Concentration vs. % composition) in the past 5 years.
| Study | # Subjects | Age range | # FA‡ | % / Conc | Health status |
|---|---|---|---|---|---|
| Articles reporting FA concentrations | |||||
| Klein CJ et al. 2013; 28:87–94.[38] | 10 | ˂ 1 | 10 | Conc | Hypebilirubineamia |
| Sauerwald UC et al. 2012 Mar;54(3):353–63 [39]. | 66 | ˂ 1 | 12 | Conc | Preterm |
| El-Ansary AK et al. 2011; 10:62 [40]. | 52 | 4–12 | 4 | Conc | Normal and autistic |
| Neggers YH et al. 2009;18(1):22–8 [41]. | 62 | ˂ 13 | 19 | Conc | Healthy & mental retardation |
| Mehmetoqlu I et al. 2012;21(4):519–25. | 161 | 21–60 | na $ | Conc | Normal & severe obesity |
| Khaw KT et al. 2012; 9(7): e1001255[42]. | 10000 | 40–79 | 22 | Conc | Healthy and CHD |
| Cunnane SC et al. 2012; 29(3):691–7[43]. | 36 | ns | 8 | Conc | Cognitive impairment and Alzheimer’s disease |
| Articles reporting % composition | |||||
| Meldrum SJ et al. 2012 Jun;86(6):233–9 [44]. | 420 | ˂ 1 | 1 | % | Healthy |
| Miller MR et al. 2010 Oct;6(4):338–46 [45]. | 110 | ˂ 1 | 2 | % | Healthy |
| Sabel KG et al. 2009 Jun 10;8:20 [46]. | 91 | Infants, >40 | 4 | % | Healthy |
| Chien et al. 2011; 10:33[47]. | 1986 | 5 | 2 | % | Healthy & Met S |
| Steer CD et al. 2012 Apr 1;21(7):1504–12 [48]. | 5632 | 7 | 16 | % | Healthy |
| Zhou YE et al. 2009;58(2):158–66 [49]. | 178 | 12–16 | 14 | % | Healthy |
| Bokor S et al. 2010 Aug;51(8):2325–33 [50]. | 1144 | 13–16 | 4 | % | Healthy |
| Gallo S et al. 2010 May;95(5):2410–7 [51]. | 180 | 13–17 | 6 | % | Healthy |
| Wheeler SJ et al. 2011;105(4):601–10 [52]. | 283 | 14–18 | 16 | % | Healthy pregnant |
| Bradbury KE et al. 2011; 3: 152–163 [18]. | 2793 | 15->65 | 13 | % | Healthy |
| Garneau V et al. 2012 Jul 9;11:46 [20]. | 198 | 18–55 | 4 | % | Healthy |
| Garneau V et al. 2013; 38(3):243–8 [20]. | 100 | 18–55 | 3 | % | Healthy |
| Ottestad I et al. 2012 Jul;108(2):315–26 [53]. | 54 | 18–50 | 6 | % | Healthy |
| Glew RH et al. 2010; 28 (2): 159–166 [54]. | 51 | >18 | 26 | % | Healthy |
| Chorell E et al. 2012 Apr;8(4):1187–96 [55]. | 29 | 19–33 | 8 | % | Healthy |
| Telle-Hansen VH et al. 2012 Feb;47(2):151–60 [56]. | 38 | 20–40 | 7 | % | Healthy |
| Schuchardt JP et al. 2011 Aug 22;10:145 [57]. | 12 | 20–50 | 2 | % | Healthy |
| Mathias RA et al. 2011 May 20;12:50 [58]. | 155 | 25–50 | 4 | % | MetS |
| Buydens-Branchey L et al. 2011 Aug 15;188(3):422–7. [59] | 25 | 30–45 | 6 | % | Cocaine Abuse |
| Kim JY et al. 2010 Sep 3;9(9):4368–75 [60]. | 60 | 30–50 | 13 | % | Lean and overweight/obese |
| Park Y et al. 2009 Aug;12(4):803–8 [61]. | 136 | 30–60 | na $ | % | Hypertriglyceridemia |
| Tanaka T et al. 2009 Jan; 5(1): e1000338 [24]. | 2151 | 30–85 | 6 | % | Healthy |
| Kawashima A et al. 2009;55(5):400–6 [62]. | 94 | 35–70 | 11 | % | MetS and abdominal obesity |
| Perez-Martinez P et al. 2012 Feb;56(2):309–15 [63]. | 452 | 35–70 | na $ | % | MetS |
| Lee S et al. 2012 Feb;107(4):567–72 [64]. | 926 | 40–49 | 3 | % | Healthy |
| Woods MN et al. 2009 Apr;89(4):1180–7 [65]. | 70 | 40–55 | 15 | % | HIV & hypertriglyceridemia |
| Rasic-Milutinovic Z et al. 2012 Jan;43(1):75–82 [66]. | 36 | 40–65 | 11 | % | Healthy & Type2 diabetes |
| Kwak JH et al. 2011 Jan;214(1):94–100 [67]. | 1646 | 40–79 | 10 | % | Healthy and CAD |
| Steffen BT et al. 2012 Jun;36(6):797–804 [68]. | 2848 | 45–48 | 3 | % | Healthy |
| Wilk JB et al. 2012; 96: 882–8 [6]. | 1575 | 45–65 | 1 | % | Healthy |
| Park Y et al. 2009 Dec;29(12):825–30 [69]. | 68 | 45–65 | 16 | % | Ischemia and hemorrhagic stroke |
| Sergeant S et al. 2012 Feb;107(4):547–55 [70]. | 166 | 50–75 | 4 | % | Healthy & Type 2 diabetes |
| Tan ZS et al. 2012;78(9); 658–64 [71]. | 1575 | 55–70 | 2 | % | Healthy |
| Zulyniak MA et al. 2012 Oct;37(5):1003–7 [72]. | 20 | ns | 14 | % | Healthy & HI & HG |
| Holub BJ et al. 2009 Dec 24;8:58 [73]. | 2053 | ns | 1 | % | Healthy |
Abbreviations: FA: fatty acids; Conc: concentration; CHD: coronary heart disease; MetS: metabolic syndrome; CAD: coronary artery disease; HG: hyperglycemia; HI: hyperinsulinemia; ns: not specified; na: not applicable.
ǂ # of FA does not include sums of FA groups but only individual FA of which levels have been reported.
$Articles reported sums of FA groups but not levels of individual FA.
In the present study concentrations of circulating FA were compared by sex and ethnicity (Tables 5 and 6, respectively). From the 61 FA investigated five were significantly different between males and females: palmitoleic acid, linoleic acid (LA) and γ-linolenic acid (GLA) and docosapentaenoic acid (n-3 DPA) and docosahexaenoic acid (DHA) (Table 5). Our findings show that males had significantly higher GLA and DPA concentrations than females (p ˂ 0.05) while females had significantly higher palmitoleic acid, LA and DHA concentrations than males (p < 0.05). Previously, in a self-selected dietary intake study, in 29 healthy adults from the U.S aged 20–59 years old, higher plasma LA concentrations were reported in females compared to males [19]. To the best of our knowledge no other studies have reported increased GLA concentrations in males compared to females. The significantly increased DPA concentrations in males observed in this study was also reported previously in a study of 200 subjects, from Quebec city, aged 18–55 years old [20]; however, DPA levels were measured in the plasma phospholipid fraction, not total lipids. Increased levels of DHA and decreased levels in DPA in females compared to males have been attributed to higher rate of DHA synthesis in females [21,22]. Giltay et al have attributed higher DHA concentrations in females to estrogen [23]. The differences observed in FA concentrations between sexes in this study may also be attributed to differences in hormones or genetic variations [24].
Table 5. Concentration (μmol/L) of select FA in males and females.
| FA | Males | Females | p- value |
|---|---|---|---|
| n = 327 | n = 499 | ||
| 16:0 | 1648.6 ± 487.9 | 1620.0 ± 440.5 | 0.37 |
| 18:0 | 483.8 ± 121.9 | 492.9 ± 126.0 | 0.30 |
| 16:1 c9 | 129.7 ± 67.8 | 135.2 ± 66.8 | 0.01* |
| 18:1 c9 | 1332.7 ± 454.5 | 1275.0± 390.8 | 0.28 |
| 18:1 c11 | 131.2 ± 61.0 | 127.8 ± 58.6 | 0.41 |
| 18:2 c9c12 | 2174.6 ± 599.0 | 2272.06 ± 634.5 | 0.03* |
| 18:3 c6c9c12 | 26.0 ± 15.1 | 21.9 ± 12.7 | 0.01* |
| 18:3 c9c12c15 | 55.1 ± 27.5 | 53.9 ± 23.5 | 0.86 |
| 20:3 c8c11c14 | 78.4 ± 32.1 | 71.6 ± 29.0 | 0.19 |
| 20:4 c5c8c11c14 | 403.9 ± 125.1 | 385.7 ± 114.9 | 0.27 |
| 20:5 c5c8c11c14c17 | 39.5 ± 25.1 | 40.6 ± 30.1 | 0.27 |
| 22:5 c7c10c13c16c19 | 25.3 ± 11.1 | 23.0 ± 9.1 | ˂ 0.01* |
| 22:6 c4c7c10c13c16c19 | 81.0 ± 31.8 | 93.6 ± 38.8 | ˂ 0.01* |
Data represented as Mean±SD.
*A p-value < 0.05 was considered statistically significant.
Linear regression models were adjusted for age, BMI, physical activity, % Energy from dietary fat, and ethnicity. Abbreviation: FA, fatty acids.
Table 6. Concentration (μmol/L) of select FA in Caucasians, East Asians and South Asians.
| FA | Caucasians | East Asians | South Asians | p- value |
|---|---|---|---|---|
| n = 287 | n = 353 | n = 107 | ||
| 16:0 | 1654.5 ± 527.1 | 1646 ± 420.7 | 1558.1 ± 426.5 | 0.13 |
| 18:0 | 488.1 ± 136.1 | 498.1 ± 123.6 | 473.7 ± 105.3 | 0.62 |
| 16:1 c9 | 142.7 ± 79.5a | 133.0 ± 59.6a | 113.1 ± 56.6b | ˂ 0.01* |
| 18:1 c9 | 1322.5 ± 466.1a | 1288.1 ± 399.4a, b | 1205.5 ± 393.2b | 0.01* |
| 18:1 c11 | 131.1 ± 66.0 | 132.0 ± 53.1 | 117.6 ± 59.1 | 0.18 |
| 18:2 c9c12 | 2144.7 ± 638.8 | 2352.0 ± 630.0 | 2145.0 ± 543.3 | 0.34 |
| 18:3 c6c9c12 | 25.9 ± 13.9a | 19.9 ± 13.7b | 28.2 ± 13.3a | 0.02* |
| 18:3 c9c12c15 | 51.9 ± 25.2 | 56.5 ± 25.2 | 57.7 ± 27.1 | 0.20 |
| 20:3 c8c11c14 | 82.4 ± 34.7a | 65.9 ± 25.5b | 76.0 ± 27.8a | 0.03* |
| 20:4 c5c8c11c14 | 401.8 ± 129.4 | 376.6 ± 108.4 | 412.6 ± 111.6 | 0.25 |
| 20:5 c5c8c11c14c17 | 39.2 ± 27.0 | 43.2 ± 31.8 | 36.1 ± 18.6 | 0.55 |
| 22:5 c7c10c13c16c19 | 24.5 ± 11.5 | 23.6 ± 8.9 | 23.9 ± 10.1 | 0.72 |
| 22:6 c4c7c10c13c16c19 | 78.2 ± 35.1 | 103.6 ± 36.3 | 73.9 ± 31.0 | 0.79 |
Data represented as Mean±SD. Different letters (a/b) denote values that are significantly different between groups.
*A p-value < 0.05 was considered statistically significant.
Linear regression models were adjusted for age, BMI, physical activity, energy intake from fat, and sex. Abbreviation: FA, fatty acids.
Comparison of FA concentrations between Caucasians, East Asians and South Asians revealed that South Asians had significantly lower concentrations of palmitoleic acid and oleic acid while East Asians had lower concentrations of GLA and dihomo-γ-linolenic (DGLA) acid (Table 6). The low levels of GLA and DGLA in East Asians are consistent with findings from the Multi-Ethnic Study of Atherosclerosis [25]. Differences in circulating FA concentrations between ethnicities can be a result of genetic variations [14].
Differences in circulating FA concentrations identified in this study give insight into sex- and ethnicity-specific susceptibility to health outcomes. Studies have revealed significant associations between various circulating FA and risk of chronic disease. For instance plasma levels of GLA and DGLA have been shown to be positively associated, whereas levels of LA, EPA and DHA are inversely associated, with biomarkers of inflammation [25,26]. Plasma levels of DGLA are also associated with depression and insulin resistance [27,28]. Levels of palmitoleic acid are positively associated, while levels of LA are inversely associated, with ischemic stroke and insulin resistance [28,29]. On the other hand the reason for the strikingly small number of FA that are different between sexes or ethnicities could be attributed to diet. Although our study cohort consisted of ethnically diverse males and females, participants were young Canadians and many were students that were more likely to share similar dietary habits. We also acknowledge that although this is a randomly sampled free living population, the dietary habits and FA profile of participants in this study, all being from Toronto, cannot be generalized to other regions of Canada and the world. The young age and healthy status of our study participants may also explain the weak positive correlations, albeit significant, between FA and LDL, triglycerides and total cholesterol reported in Tables 7, 8, and 9, respectively. Correlations between FA and BMI, HOMA-IR, glucose, insulin, HDL, free fatty acids were also investigated; however, R2 values equal or higher than 0.09 were not found (data not shown). The weak correlations are reflective of the healthy status of our study cohort. Nonetheless, the correlations shown exemplify the potential use of FA as biomarkers of health. As such, future studies will include participants with wider age range to capture metabolic changes in aging populations. Determining concentrations of FA in aging and unhealthy individuals will allow for the identification of correlations of FA with biomarkers of health, which will aid in establishing FA reference ranges.
Table 7. Correlation between select plasma FA and LDL-cholesterol.
| FA | R2 | p- value |
|---|---|---|
| 14:0 | 0.09 | ˂ 0.0001* |
| 15:0 | 0.12 | ˂ 0.0001* |
| 16:0 | 0.20 | ˂ 0.0001* |
| 16:1 c9 | 0.09 | ˂ 0.0001* |
| 18:0 | 0.18 | ˂ 0.0001* |
| 18:1 c9 | 0.15 | ˂ 0.0001* |
| 18:1 c11 | 0.09 | ˂ 0.0001* |
| 18:2 c9c12 | 0.20 | ˂ 0.0001* |
| 18:3 c9c12c15 | 0.10 | ˂ 0.0001* |
| 20:2 c11c14 | 0.09 | ˂ 0.0001* |
| 20:3 c8c11c14 | 0.14 | ˂ 0.0001* |
| 20:4 c5c8c11c14 | 0.19 | ˂ 0.0001* |
| 22:5 c7c10c13c16c19 | 0.12 | ˂ 0.0001* |
| 22:6 c4c7c10c13c16c19 | 0.09 | ˂ 0.0001* |
Linear regression models were adjusted for age, BMI, physical activity, energy intake from fat, sex and ethnicity. All correlations identified below are positive. R2 corresponds to the coefficient of determinations. Only R2 with a value of 0.09 or more were reported.
*A p-value < 0.05 was considered statistically significant. N = 826. Abbreviation: FA, fatty acids.
Table 8. Correlations between select plasma FA and triglycerides.
| FA | R2 | p- value |
|---|---|---|
| 14:0 | 0.36 | ˂ 0.0001* |
| 14:1 | 0.15 | ˂ 0.0001* |
| 15:0 | 0.16 | ˂ 0.0001* |
| 16:0 | 0.31 | ˂ 0.0001* |
| 16:1 t9 | 0.14 | ˂ 0.0001* |
| 16:1 c9 | 0.29 | ˂ 0.0001* |
| 18:0 | 0.14 | ˂ 0.0001* |
| 18:1 t11 | 0.09 | ˂ 0.0001* |
| 18:1 c9 | 0.40 | ˂ 0.0001* |
| 18:2 c9t12 | 0.11 | ˂ 0.0001* |
| 18:2 c9c12 | 0.10 | ˂ 0.0001* |
| 18:3 c6c9c12 | 0.17 | ˂ 0.0001* |
| 20:1 c11 | 0.11 | ˂ 0.0001* |
| 18:3 c9c12c15 | 0.26 | ˂ 0.0001* |
| 18:2 c9t11 CLA | 0.24 | ˂ 0.0001* |
| 21:0 | 0.10 | ˂ 0.0001* |
| 20:2 c11c14 | 0.13 | ˂ 0.0001* |
| 20:3 c8c11c14 | 0.16 | ˂ 0.0001* |
| 22:2 c13c16 | 0.14 | ˂ 0.0001* |
| 22:5 c7c10c13c16c19 | 0.10 | ˂ 0.0001* |
Linear regression models were adjusted for age, BMI, physical activity, energy intake from fat, sex and ethnicity. All correlations identified are positive. R2 corresponds to the coefficient of determinations. Only R2 with a value of 0.09 or more were reported.
*A p-value < 0.05 was considered statistically significant. N = 826. Abbreviation: FA, fatty acids.
Table 9. Correlations between select plasma FA and total cholesterol.
| FA | R2 | p- value |
|---|---|---|
| 14:0 | 0.14 | ˂ 0.0001* |
| 15:0 | 0.14 | ˂ 0.0001* |
| 16:0 | 0.26 | ˂ 0.0001* |
| 16:1 t9 | 0.09 | ˂ 0.0001* |
| 16:1 c9 | 0.15 | ˂ 0.0001* |
| 18:0 | 0.25 | ˂ 0.0001* |
| 18:1 c9 | 0.20 | ˂ 0.0001* |
| 18:2 c9c12 | 0.29 | ˂ 0.0001* |
| 18:3 c6c9c12 | 0.09 | ˂ 0.0001* |
| 18:3 c9c12c15 | 0.11 | ˂ 0.0001* |
| 18:2 c9t11 CLA | 0.10 | ˂ 0.0001* |
| 20:2 c11c14 | 0.12 | ˂ 0.0001* |
| 20:3 c8c11c14 | 0.15 | ˂ 0.0001* |
| 20:4 c5c8c11c14 | 0.18 | ˂ 0.0001* |
| 22:2 c13c16 | 0.09 | ˂ 0.0001* |
| 22:5 c7c10c13c16c19 | 0.13 | ˂ 0.0001* |
| 22:6 c4c7c10c13c16c19 | 0.14 | ˂ 0.0001* |
Linear regression models were adjusted for age, BMI, physical activity, energy intake from fat, sex and ethnicity. All correlations identified were positive. R2 corresponds to the coefficient of determinations. Only R2 with a value of 0.09 or more were reported.
*A p-value < 0.05 was considered statistically significant. N = 826. Abbreviation: FA, fatty acids.
Generally, epidemiological studies investigating the link between FA intake and disease often depend on food frequency questionnaires (FFQ) for the estimation of exposure to different types of FA. The limitations of detailed dietary intake records are well documented and these include dependence on participants recall and bias [30]. In addition FFQ do not reflect the inter-individual differences in metabolism, absorption and genetic variations leading to different concentrations of circulating FA. Correlation studies between food intake and circulating FA levels in US women revealed that circulating levels of saturated and monounsaturated FA did not reflect intake, possibly as a result of endogenous FA synthesis [31]. Taken together exposure to FA should be determined objectively by measuring blood or tissue levels of FA as opposed to dietary levels. Measurement of plasma as an aggregate of both dietary and de novo synthesis of FA may be more appropriate for assessing linkages to biomarkers. A relevant example is the measurement of blood cholesterol, the net contribution of dietary and de novo synthesis, for ascertaining cardiovascular disease risk.
FA are commonly measured in adipose tissue, erythrocytes or plasma. In this study FA concentrations were determined in plasma total lipids. While FA levels in adipose tissue reflect intake in years [32] and levels in erythrocytes reflect intake in months [31], FA levels in plasma reflect intake in weeks [33] and; therefore, are more reflective of current dietary habits of subjects. Availability of adipose tissue limits its use in epidemiological studies and similarly excludes its appropriateness for rapid and frequent determination of endogenous FA levels [33]. We recognize that both plasma and red blood cells are commonly used for assessing circulating levels of fatty acids. A recent report by Skeaff et al have challenged the notion that plasma only reflects current intake by showing that plasma fatty acids levels correlates with intake for up to 2 weeks [34]. Studies have also shown that red blood cell fatty acid levels do not correlate with dietary saturated and monounsaturated fatty acids because of the contribution of de novo synthesis to their circulating levels. The study by Patel et al showed stronger associations between disease risk and plasma FA compared to erythrocyte fatty acids [35]. Measuring FA levels in total plasma lipids is more applicable to large populations due to simplicity of the analytical methodology [36]. Furthermore, plasma is composed of all major circulating lipid species including triglycerides, phospholipids, cholesterol-esters and free fatty acids [37]. Therefore, plasma provides a highly accessible source of lipids and provides a high level overview of changes in metabolism of all these lipid species as a potential indicator of health.
In conclusion the present study provides knowledge regarding a broad panel of circulating FA. The generalizability of this study requires further replication in other populations but these data are the first step in establishing FA reference ranges which is a vital gap in elucidating the role of individual FA in chronic disease. Further research is warranted, building upon the present results, to examine how very high or low circulating FA concentrations relate to different chronic diseases.
Supporting Information
(XLSX)
Acknowledgments
The authors would like to thank Lyn Hillyer for technical support.
Data Availability
All relevant data are within the Supporting Information file.
Funding Statement
This research was funded by grants from the Advanced Food and Materials Network (AES), the Public Health Agency of Canada (DMM and AB), and the Canada Foundation for Innovation with matching funds from the Ontario Research Fund (DWLM and DMM) and Natural Sciences and Engineering Research Council of Canada (DWLM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kris-Etherton PM (1999) AHA Science Advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation 100: 1253–1258. [DOI] [PubMed] [Google Scholar]
- 2. Simopoulos AP (1999) Essential fatty acids in health and chronic disease. Am J Clin Nutr 70: 560S–569S. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (2006) Trans fatty acids and cardiovascular disease. N Engl J Med 354: 1601–1613. [DOI] [PubMed] [Google Scholar]
- 4. Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 233: 674–688. 10.3181/0711-MR-311 [DOI] [PubMed] [Google Scholar]
- 5. Lavie CJ, Milani RV, Mehra MR, Ventura HO (2009) Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol 54: 585–594. 10.1016/j.jacc.2009.02.084 [DOI] [PubMed] [Google Scholar]
- 6. Wilk JB, Tsai MY, Hanson NQ, Gaziano JM, Djousse L (2012) Plasma and dietary omega-3 fatty acids, fish intake, and heart failure risk in the Physicians’ Health Study. Am J Clin Nutr 96: 882–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang YJ, Lee SH, Hong SJ, Chung BC (1999) Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem 32: 405–409. [DOI] [PubMed] [Google Scholar]
- 8. Baum SJ (2013) A Survey of Internists and Cardiologists: Are Discoveries in Fatty Acids Truly being translated into Clinical Practice? Prostaglandins Leukot Essent Fatty Acids 88: 3–4. 10.1016/j.plefa.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 9. Yaemsiri S, Sen S, Tinker LF, Robinson WR, Evans RW, et al. (2013) Serum fatty acids and incidence of ischemic stroke among postmenopausal women. Stroke 44: 2710–2717. 10.1161/STROKEAHA.111.000834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fontaine-Bisson B, Wolever TM, Connelly PW, Corey PN, El-Sohemy A (2009) NF-kappaB-94Ins/Del ATTG polymorphism modifies the association between dietary polyunsaturated fatty acids and HDL-cholesterol in two distinct populations. Atherosclerosis 204: 465–470. 10.1016/j.atherosclerosis.2008.10.037 [DOI] [PubMed] [Google Scholar]
- 11. Matthews DR, Connolly AA, Holman RR, Turner RC (1985) Physiology of insulin secretion: problems of quantity and timing. Neth J Med 28 Suppl 1: 20–24. [PubMed] [Google Scholar]
- 12. Lee IM, Paffenbarger RS Jr. (1998) Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke 29: 2049–2054. [DOI] [PubMed] [Google Scholar]
- 13. Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509. [PubMed] [Google Scholar]
- 14. Merino DM, Johnston H, Clarke S, Roke K, Nielsen D, et al. (2011) Polymorphisms in FADS1 and FADS2 alter desaturase activity in young Caucasian and Asian adults. Mol Genet Metab 103: 171–178. 10.1016/j.ymgme.2011.02.012 [DOI] [PubMed] [Google Scholar]
- 15. Monteiro J, Li FJ, Maclennan M, Rabalski A, Moghadasian MH, et al. (2012) Menhaden oil, but not safflower or soybean oil, aids in restoring the polyunsaturated fatty acid profile in the novel delta-6-desaturase null mouse. Lipids Health Dis 11: 60 10.1186/1476-511X-11-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sera RK, McBride JH, Higgins SA, Rodgerson DO (1994) Evaluation of reference ranges for fatty acids in serum. J Clin Lab Anal 8: 81–85. [DOI] [PubMed] [Google Scholar]
- 17. Schwertner HA, Mosser EL (1993) Comparison of lipid fatty acids on a concentration basis vs weight percentage basis in patients with and without coronary artery disease or diabetes. Clin Chem 39: 659–663. [PubMed] [Google Scholar]
- 18. Bradbury KE, Skeaff CM, Crowe FL, Green TJ, Hodson L (2011) Serum fatty acid reference ranges: percentiles from a New Zealand national nutrition survey. Nutrients 3: 152–163. 10.3390/nu3010152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reeves VB, Matusik EJ Jr., Kelsay JL (1984) Variations in plasma fatty acid concentrations during a one-year self-selected dietary intake study. Am J Clin Nutr 40: 1345–1351. [DOI] [PubMed] [Google Scholar]
- 20. Garneau V, Rudkowska I, Paradis AM, Godin G, Julien P, et al. (2012) Omega-3 fatty acids status in human subjects estimated using a food frequency questionnaire and plasma phospholipids levels. Nutr J 11: 46 10.1186/1475-2891-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pawlosky RJ, Hibbeln JR, Lin Y, Goodson S, Riggs P, et al. (2003) Effects of beef- and fish-based diets on the kinetics of n-3 fatty acid metabolism in human subjects. Am J Clin Nutr 77: 565–572. [DOI] [PubMed] [Google Scholar]
- 22. Pawlosky R, Hibbeln J, Lin Y, Salem N Jr. (2003) n-3 fatty acid metabolism in women. Br J Nutr 90: 993–994. [DOI] [PubMed] [Google Scholar]
- 23. Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL (2004) Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr 80: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, et al. (2009) Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet 5: e1000338 10.1371/journal.pgen.1000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steffen BT, Steffen LM, Tracy R, Siscovick D, Jacobs D, et al. (2012) Ethnicity, plasma phospholipid fatty acid composition and inflammatory/endothelial activation biomarkers in the Multi-Ethnic Study of Atherosclerosis (MESA). Eur J Clin Nutr 66: 600–605. 10.1038/ejcn.2011.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roke K, Ralston JC, Abdelmagid S, Nielsen DE, Badawi A, et al. (2013) Variation in the FADS1/2 gene cluster alters plasma n-6 PUFA and is weakly associated with hsCRP levels in healthy young adults. Prostaglandins Leukot Essent Fatty Acids. 10.1016/j.plefa.2013.12.006 [DOI] [PubMed] [Google Scholar]
- 27. Evans SJ, Kamali M, Prossin AR, Harrington GJ, Ellingrod VL, et al. (2012) Association of plasma omega-3 and omega-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res 46: 1435–1441. 10.1016/j.jpsychires.2012.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurotani K, Sato M, Ejima Y, Nanri A, Yi S, et al. (2012) High levels of stearic acid, palmitoleic acid, and dihomo-gamma-linolenic acid and low levels of linoleic acid in serum cholesterol ester are associated with high insulin resistance. Nutr Res 32: 669–675. 10.1016/j.nutres.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Yamagishi K, Folsom AR, Steffen LM (2013) Plasma Fatty Acid Composition and Incident Ischemic Stroke in Middle-Aged Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis 36: 38–46. 10.1159/000351205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cantwell MM (2000) Assessment of individual fatty acid intake. Proc Nutr Soc 59: 187–191. [DOI] [PubMed] [Google Scholar]
- 31. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB (2007) Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr 86: 74–81. [DOI] [PubMed] [Google Scholar]
- 32. Dayton S, Hashimoto S, Dixon W, Pearce ML (1966) Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J Lipid Res 7: 103–111. [PubMed] [Google Scholar]
- 33. Baylin A, Campos H (2006) The use of fatty acid biomarkers to reflect dietary intake. Curr Opin Lipidol 17: 22–27. [DOI] [PubMed] [Google Scholar]
- 34. Skeaff CM, Hodson L, McKenzie JE (2006) Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr 136: 565–569. [DOI] [PubMed] [Google Scholar]
- 35. Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, et al. (2010) Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 92: 1214–1222. 10.3945/ajcn.2010.29182 [DOI] [PubMed] [Google Scholar]
- 36. Hodson L, McQuaid SE, Karpe F, Frayn KN, Fielding BA (2009) Differences in partitioning of meal fatty acids into blood lipid fractions: a comparison of linoleate, oleate, and palmitate. Am J Physiol Endocrinol Metab 296: E64–E71. 10.1152/ajpendo.90730.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glaser C, Demmelmair H, Koletzko B (2010) High-throughput analysis of total plasma fatty acid composition with direct in situ transesterification. PLoS One 5: e12045 10.1371/journal.pone.0012045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein CJ, Havranek TG, Revenis ME, Hassanali Z, Scavo LM (2013) Plasma fatty acids in premature infants with hyperbilirubinemia: before-and-after nutrition support with fish oil emulsion. Nutr Clin Pract 28: 87–94. 10.1177/0884533612469989 [DOI] [PubMed] [Google Scholar]
- 39. Sauerwald UC, Fink MM, Demmelmair H, Schoenaich PV, Rauh-Pfeiffer AA, et al. (2012) Effect of different levels of docosahexaenoic acid supply on fatty acid status and linoleic and alpha-linolenic acid conversion in preterm infants. J Pediatr Gastroenterol Nutr 54: 353–363. 10.1097/MPG.0b013e31823c3bfd [DOI] [PubMed] [Google Scholar]
- 40. El-Ansary AK, Bacha AG, Al-Ayahdi LY (2011) Plasma fatty acids as diagnostic markers in autistic patients from Saudi Arabia. Lipids Health Dis 10: 62 10.1186/1476-511X-10-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Neggers YH, Kim EK, Song JM, Chung EJ, Um YS, et al. (2009) Mental retardation is associated with plasma omega-3 fatty acid levels and the omega-3/omega-6 ratio in children. Asia Pac J Clin Nutr 18: 22–28. [PubMed] [Google Scholar]
- 42. Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N (2012) Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med 9: e1001255 10.1371/journal.pmed.1001255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cunnane SC, Schneider JA, Tangney C, Tremblay-Mercier J, Fortier M, et al. (2012) Plasma and brain fatty acid profiles in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 29: 691–697. 10.3233/JAD-2012-110629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meldrum SJ, D’Vaz N, Casadio Y, Dunstan JA, Niels Krogsgaard-Larsen N, et al. (2012) Determinants of DHA levels in early infancy: differential effects of breast milk and direct fish oil supplementation. Prostaglandins Leukot Essent Fatty Acids 86: 233–239. 10.1016/j.plefa.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 45. Miller MR, Seifert J, Szabo NJ, Clare-Salzler M, Rewers M, et al. (2010) Erythrocyte membrane fatty acid content in infants consuming formulas supplemented with docosahexaenoic acid (DHA) and arachidonic acid (ARA): an observational study. Matern Child Nutr 6: 338–346. 10.1111/j.1740-8709.2009.00230.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sabel KG, Lundqvist-Persson C, Bona E, Petzold M, Strandvik B (2009) Fatty acid patterns early after premature birth, simultaneously analysed in mothers’ food, breast milk and serum phospholipids of mothers and infants. Lipids Health Dis 8: 20 10.1186/1476-511X-8-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chien KL, Chao CL, Kuo CH, Lin HJ, Liu PH, et al. (2011) Plasma fatty acids and the risk of metabolic syndrome in ethnic Chinese adults in Taiwan. Lipids Health Dis 10: 33 10.1186/1476-511X-10-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Steer CD, Hibbeln JR, Golding J, Davey SG (2012) Polyunsaturated fatty acid levels in blood during pregnancy, at birth and at 7 years: their associations with two common FADS2 polymorphisms. Hum Mol Genet 21: 1504–1512. 10.1093/hmg/ddr588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhou YE, Egeland GM, Meltzer SJ, Kubow S (2009) The association of desaturase 9 and plasma fatty acid composition with insulin resistance-associated factors in female adolescents. Metabolism 58: 158–166. 10.1016/j.metabol.2008.09.008 [DOI] [PubMed] [Google Scholar]
- 50. Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, et al. (2010) Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res 51: 2325–2333. 10.1194/jlr.M006205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gallo S, Egeland G, Meltzer S, Legault L, Kubow S (2010) Plasma fatty acids and desaturase activity are associated with circulating adiponectin in healthy adolescent girls. J Clin Endocrinol Metab 95: 2410–2417. 10.1210/jc.2009-1975 [DOI] [PubMed] [Google Scholar]
- 52. Wheeler SJ, Poston L, Thomas JE, Seed PT, Baker PN, et al. (2011) Maternal plasma fatty acid composition and pregnancy outcome in adolescents. Br J Nutr 105: 601–610. 10.1017/S0007114510004083 [DOI] [PubMed] [Google Scholar]
- 53. Ottestad I, Vogt G, Retterstol K, Myhrstad MC, Haugen JE, et al. (2012) Oxidised fish oil does not influence established markers of oxidative stress in healthy human subjects: a randomised controlled trial. Br J Nutr 108: 315–326. 10.1017/S0007114511005484 [DOI] [PubMed] [Google Scholar]
- 54. Glew RH, Chuang LT, Berry T, Okolie H, Crossey MJ, et al. (2010) Lipid profiles and trans fatty acids in serum phospholipids of semi-nomadic Fulani in northern Nigeria. J Health Popul Nutr 28: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chorell E, Svensson MB, Moritz T, Antti H (2012) Physical fitness level is reflected by alterations in the human plasma metabolome. Mol Biosyst 8: 1187–1196. 10.1039/c2mb05428k [DOI] [PubMed] [Google Scholar]
- 56. Telle-Hansen VH, Larsen LN, Hostmark AT, Molin M, Dahl L, et al. (2012) Daily intake of cod or salmon for 2 weeks decreases the 18:1n-9/18:0 ratio and serum triacylglycerols in healthy subjects. Lipids 47: 151–160. 10.1007/s11745-011-3637-y [DOI] [PubMed] [Google Scholar]
- 57. Schuchardt JP, Schneider I, Meyer H, Neubronner J, von SC, et al. (2011) Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—a comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis 10: 145 10.1186/1476-511X-10-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mathias RA, Sergeant S, Ruczinski I, Torgerson DG, Hugenschmidt CE, et al. (2011) The impact of FADS genetic variants on omega6 polyunsaturated fatty acid metabolism in African Americans. BMC Genet 12: 50 10.1186/1471-2156-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buydens-Branch, Branchey M, Hibbeln JR (2011) Higher n-3 fatty acids are associated with more intense fenfluramine-induced ACTH and cortisol responses among cocaine-abusing men. Psychiatry Res 188: 422–427. 10.1016/j.psychres.2011.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim JY, Park JY, Kim OY, Ham BM, Kim HJ, et al. (2010) Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS). J Proteome Res 9: 4368–4375. 10.1021/pr100101p [DOI] [PubMed] [Google Scholar]
- 61. Park Y, Harris WS (2009) Dose-response of n-3 polyunsaturated fatty acids on lipid profile and tolerability in mildly hypertriglyceridemic subjects. J Med Food 12: 803–808. 10.1089/jmf.2008.1250 [DOI] [PubMed] [Google Scholar]
- 62. Kawashima A, Sugawara S, Okita M, Akahane T, Fukui K, et al. (2009) Plasma fatty acid composition, estimated desaturase activities, and intakes of energy and nutrient in Japanese men with abdominal obesity or metabolic syndrome. J Nutr Sci Vitaminol (Tokyo) 55: 400–406. [DOI] [PubMed] [Google Scholar]
- 63. Perez-Martinez P, gado-Lista J, Garcia-Rios A, Tierney AC, Gulseth HL, et al. (2012) Insulin receptor substrate-2 gene variants in subjects with metabolic syndrome: association with plasma monounsaturated and n-3 polyunsaturated fatty acid levels and insulin resistance. Mol Nutr Food Res 56: 309–315. 10.1002/mnfr.201100504 [DOI] [PubMed] [Google Scholar]
- 64. Lee S, Curb JD, Kadowaki T, Evans RW, Miura K, et al. (2012) Significant inverse associations of serum n-6 fatty acids with plasma plasminogen activator inhibitor-1. Br J Nutr 107: 567–572. 10.1017/S0007114511003199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Woods MN, Wanke CA, Ling PR, Hendricks KM, Tang AM, et al. (2009) Metabolic syndrome and serum fatty acid patterns in serum phospholipids in hypertriglyceridemic persons with human immunodeficiency virus. Am J Clin Nutr 89: 1180–1187. 10.3945/ajcn.2009.27444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rasic-Milutinovic Z, Popovic T, Perunicic-Pekovic G, Arsic A, Borozan S, et al. (2012) Lower serum paraoxonase-1 activity is related to linoleic and docosahexanoic fatty acids in type 2 diabetic patients. Arch Med Res 43: 75–82. 10.1016/j.arcmed.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 67. Kwak JH, Paik JK, Kim OY, Jang Y, Lee SH, et al. (2011) FADS gene polymorphisms in Koreans: association with omega6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery disease. Atherosclerosis 214: 94–100. 10.1016/j.atherosclerosis.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 68. Steffen BT, Steffen LM, Tracy R, Siscovick D, Hanson NQ, et al. (2012) Obesity modifies the association between plasma phospholipid polyunsaturated fatty acids and markers of inflammation: the Multi-Ethnic Study of Atherosclerosis. Int J Obes (Lond) 36: 797–804. 10.1038/ijo.2011.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Park Y, Park S, Yi H, Kim HY, Kang SJ, et al. (2009) Low level of n-3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans. Nutr Res 29: 825–830. 10.1016/j.nutres.2009.10.018 [DOI] [PubMed] [Google Scholar]
- 70. Sergeant S, Hugenschmidt CE, Rudock ME, Ziegler JT, Ivester P, et al. (2012) Differences in arachidonic acid levels and fatty acid desaturase (FADS) gene variants in African Americans and European Americans with diabetes or the metabolic syndrome. Br J Nutr 107: 547–555. 10.1017/S0007114511003230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tan ZS, Harris WS, Beiser AS, Au R, Himali JJ, et al. (2012) Red blood cell omega-3 fatty acid levels and markers of accelerated brain aging. Neurology 78: 658–664. 10.1212/WNL.0b013e318249f6a9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zulyniak MA, Ralston JC, Tucker AJ, MacKay KA, Hillyer LM, et al. (2012) Vaccenic acid in serum triglycerides is associated with markers of insulin resistance in men. Appl Physiol Nutr Metab 37: 1003–1007. 10.1139/h2012-081 [DOI] [PubMed] [Google Scholar]
- 73. Holub BJ, Wlodek M, Rowe W, Piekarski J (2009) Correlation of omega-3 levels in serum phospholipid from 2053 human blood samples with key fatty acid ratios. Nutr J 8: 58 10.1186/1475-2891-8-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the Supporting Information file.


