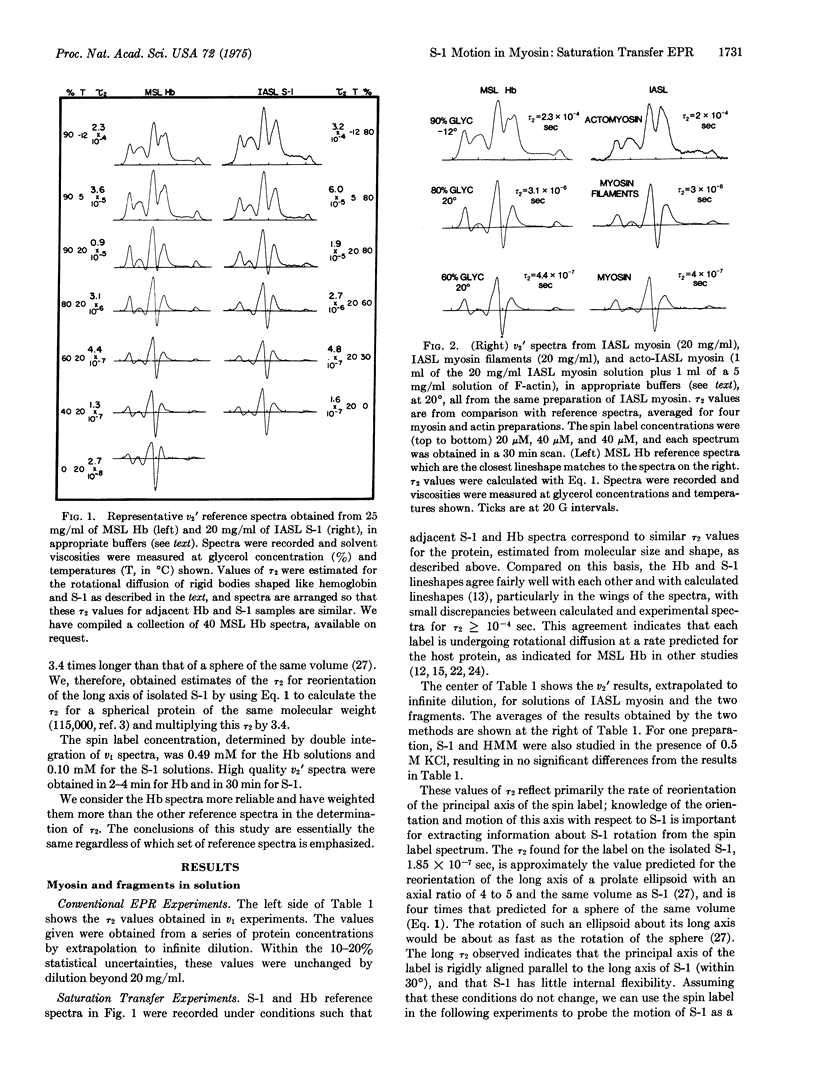
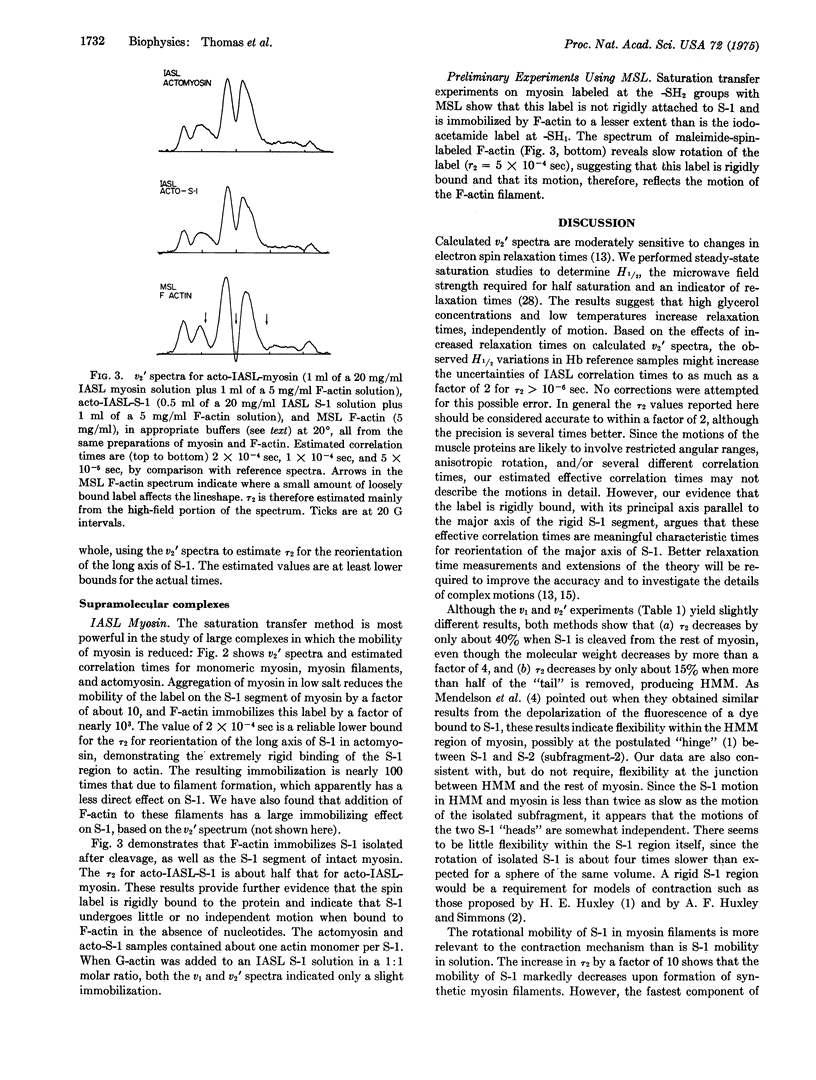
Abstract
Molecular dynamics in spin-labeled muscle proteins was studied with a recently developed electron paramagnetic resonance (EPR) technique, saturation transfer spectroscopy, which is uniquely sensitive to rotational motion in the range of 10(-7)-10(-3) sec. Rotational correlation time (tau2) were determined for a spin label analog of iodoacetamide bound to the subfragment-1 (S-1) region of myosin under a variety of conditions likely to shed light on the molecular mechanism of muscle contraction. Results show that (a) the spin labels are rigidly bound to the isolated S-1 (tau2 = 2 x 10(-7) sec) and can be used to estimate values of tau2 for the S-1 region of the myosin molecule; (b) in solutions of intact myosin, S-1 has considerable mobility relative to the rest of the myosin molecule, the value of tau2 for the S-1 segment of myosin being less than twice that for isolated S-1, while the molecular weights differ by a factor of 4 to 5; (c) in myosin filaments, tau2 increases by a factor of only about 10, suggesting motion of the S-1 regions independent of the backbone of the myosin filament, but slower than that in a single molecule; (d) addition of F-actin to solutions of myosin or S-1 increases tau2 by a factor of nearly 10(3), indicating strong immobilization of S-1 upon binding to actin. Saturation transfer spectroscopy promises to provide an extremely useful tool for the study of the motions of the crossbridges and thin filaments in reconstituted systems and in glycerinated muscle fibers.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burley R. W., Seidel J. C., Gergely J. The stoichiometry of the reaction of the spin labeling of F-actin and the effect of orientation of spin-labeled F-actin filaments. Arch Biochem Biophys. 1971 Oct;146(2):597–602. doi: 10.1016/0003-9861(71)90167-6. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Hyde J. S., Thomas D. D. New EPR methods for the study of very slow motion: application to spin-labeled hemoglobin. Ann N Y Acad Sci. 1973 Dec 31;222:680–692. doi: 10.1111/j.1749-6632.1973.tb15295.x. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. Studies on the formation and physical chemical properties of synthetic myosin filaments. Biochemistry. 1966 Nov;5(11):3474–3487. doi: 10.1021/bi00875a013. [DOI] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., McFarland B. G. Physics and chemistry of spin labels. Q Rev Biophys. 1970 Feb;3(1):91–136. doi: 10.1017/s003358350000442x. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Nauss K. M., Kitagawa S., Gergely J. Pyrophosphate binding to and adenosine triphosphatase activity of myosin and its proteolytic fragments. Implications for the substructure of myosin. J Biol Chem. 1969 Feb 25;244(4):755–765. [PubMed] [Google Scholar]
- Ohnishi S., Boeyens J. C., McConnell H. M. Spin-labeled hemoglobin crystals. Proc Natl Acad Sci U S A. 1966 Sep;56(3):809–813. doi: 10.1073/pnas.56.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J. C., Chopek M., Gergely J. Effect of nucleotides and pyrophosphate on spin labels bound to S1 thiol groups of myosin. Biochemistry. 1970 Aug 4;9(16):3265–3272. doi: 10.1021/bi00818a021. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Gergely J. Electron spin resonance of myosin spin labeled at the S1 thiol groups during hydrolysis of adenosine triphosphate. Arch Biochem Biophys. 1973 Oct;158(2):853–863. doi: 10.1016/0003-9861(73)90581-x. [DOI] [PubMed] [Google Scholar]
- Seidel J. C., Gergely J. The use of spin labels in the study of muscle proteins. Ann N Y Acad Sci. 1973 Dec 31;222:574–587. doi: 10.1111/j.1749-6632.1973.tb15288.x. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. The effects of actin on the electron spin resonance of spin-labeled myosin. Arch Biochem Biophys. 1973 Aug;157(2):588–596. doi: 10.1016/0003-9861(73)90678-4. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. The effects of nucleotides and Mg 2+ on the electron spin resonance spectra of myosin spin labeled at the S 2 thiol groups. Arch Biochem Biophys. 1972 Oct;152(2):839–848. doi: 10.1016/0003-9861(72)90280-9. [DOI] [PubMed] [Google Scholar]
- Smigel M. D., Dalton L. R., Hyde J. S., Dalton L. A. Investigation of very slowly tumbling spin labels by nonlinear spin response techniques: theory and experiment for stationary electron electron double resonance. Proc Natl Acad Sci U S A. 1974 May;71(5):1925–1929. doi: 10.1073/pnas.71.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa T. EPR spectral observations on the binding of ATP and F-actin to spin-labeled myosin. Biochem Biophys Res Commun. 1971 Jul 16;44(2):471–476. doi: 10.1016/0006-291x(71)90625-5. [DOI] [PubMed] [Google Scholar]


