Abstract
The reprogramming of somatic cells into induced pluripotent stem cells (iPSCs) upon overexpression of OCT4, KLF4, SOX2, and c-MYC (OKSM) provides a powerful system to interrogate basic mechanisms of cell fate change. However, iPSC formation with standard methods is protracted and inefficient, resulting in heterogeneous cell populations. Here we show that exposure of OKSM-expressing cells to both ascorbic acid and a GSK3-beta inhibitor (termed “AGi”) facilitates more synchronous and rapid iPSC formation from a variety of mouse cell types. AGi treatment restored the ability of refractory cell populations to yield iPSC colonies, and it attenuated the activation of developmental regulators commonly observed during the reprogramming process. Moreover, AGi supplementation gave rise to chimera-competent iPSCs after as little as 48 hours of OKSM expression. Our results offer a simple modification to the reprogramming protocol, facilitating iPSC induction at unparalleled efficiencies and enabling dissection of the underlying mechanisms in more homogeneous cell populations.
INTRODUCTION
The generation of induced pluripotent stem cells (iPSCs)1–3 with defined transcription factors such as POU5F1 (hereafter referred to as OCT4), KLF4, SOX2 and c-MYC (OKSM) usually takes weeks to months and gives rise to iPSC colonies at frequencies of less than 5%4 with a few notable exceptions5,6. Different approaches have been developed to overcome the low efficiency and slow kinetics of iPSC formation, which constitute major bottlenecks for the mechanistic dissection of the reprogramming process7–9. For example, surface markers have been employed to prospectively identify and isolate those rare cells that are poised to become iPSCs10,11. While this approach led to the first characterization of defined intermediate stages of cellular reprogramming10, it typically requires time-consuming and costly cell isolation procedures that yield small cell numbers of variable purity. Another approach is based on the manipulation of additional genes to enhance overall reprogramming efficiencies. For instance, loss of the methyl-binding protein MBD3 was shown to endow every somatic cell with induced pluripotency after only 6–8 days of OKSM expression12. Similarly, transient activation of the myeloid transcription factor C/EBPα in B cells was reported to generate OCT4-GFP+ cells with a short latency and at high efficiency13. Of note, the enhancing effect of C/EBPα on induced pluripotency appears to be limited to the B cell lineage and MBD3 suppression was recently suggested to oppose cellular reprogramming14. Moreover, both approaches require the introduction of additional transgenes into cells, which is cumbersome and potentially hazardous.
We therefore set out to test whether efficient and synchronous iPSC formation could be induced from OKSM-expressing somatic cells without further genetic manipulation. We screened for combinations of commonly available cell culture supplements that could improve the speed and efficiency of iPSC formation by using optimized OKSM transgenes, fluorescent reporter systems and clonal reprogramming assays. This effort led to the identification of small molecules that acted synergistically and enabled near-homogeneous iPSC formation from somatic cell types, thus providing a straightforward and affordable approach to study this remarkable cell fate transition in bulk cultures.
RESULTS
A transgenic system to track induced pluripotency
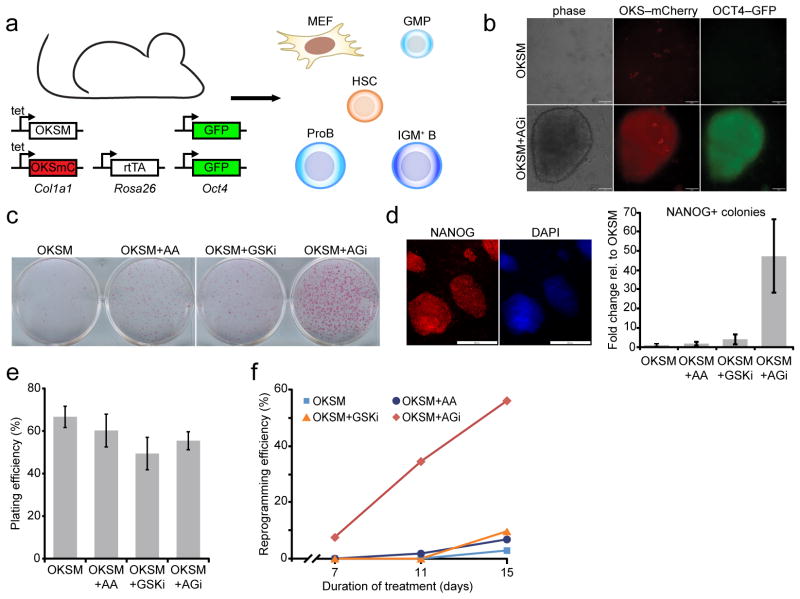
Studying the process of cellular reprogramming with classical tools has been hampered by the inability to monitor exogenous OKSM expression patterns in somatic cells. We therefore generated a transgenic reprogramming system in mice that allowed us to simultaneously induce and track high-level OKSM expression in any target cells (Fig. 1a). To this end, mice homozygous for the doxycycline-inducible, polycistronic tetOP-OKSM construct in the Col1a1 locus15 were crossed to mice homozygous for a cassette containing the coding regions for Oct4, Klf4, Sox2 and an IRES-mCherry reporter in the Col1a1 locus (tetOP-OKSmC)(data not shown) and the M2rtTA allele in the Rosa26 locus (R26-M2rtTA)15. Doxycycline treatment of murine embryonic fibroblasts (MEFs) isolated from this cross induced strong and homogeneous expression of reprogramming factors, as determined by microscopy for mCherry (Fig. 1b), and consistently gave rise to iPSCs from different cell types under conventional culture conditions (Fig. 1c–f, Fig. 2a). Cells carrying the tetOP-OKSM, tetOP-OKSmC and R26-M2rtTA alleles were used for all subsequent experiments unless noted otherwise.
Figure 1.
Ascorbic acid and GSK3-beta inhibitor (“AGi”) act synergistically on reprogramming. (a) Schematic of inducible, secondary reprogramming system. (b) Top panel: OKSM-expressing GMPs (as indicated by mCherry fluorescence) that remain OCT4-GFP negative after 8 days of doxycycline treatment. Bottom panel: a nascent iPSC colony after 8 days of treatment with doxycycline+AGi, showing OCT4-GFP and mCherry fluorescence (scale bar is 50 μm). (c) Alkaline phosphatase staining of doxycyline-independent, MEF-derived iPSC colonies, documenting individual and synergistic effects of ascorbic acid (AA) and GSK3-beta inhibitor (GSKi) on iPSC formation. Cells were subjected to reprogramming for 9 days, at which point doxycycline and supplements were withdrawn for an additional 3 days. (d) Representative staining of NANOG-positive iPSC colonies generated with AGi (scale bar is 200 μm). A quantitative representation of reprogramming efficiency based on transgene-independent NANOG-positive clones for the indicated conditions (n=3 biological replicates, error bars represent standard deviation for three independent experiments). (e) Plating efficiency for clonal reprogramming analyses using MEFs. Values represent the mean for three independent time points and error bars represent standard deviation. (f) Clonal analysis of reprogramming efficiency for single MEFs expressing OKSM under the indicated conditions. OKSM, Oct4, Klf4, Sox2, c-Myc; mC, mCherry; MEF, murine embryonic fibroblast; GMP, granulocyte/macrophage progenitor; HSC, hematopoietic stem cell.
Figure 2.
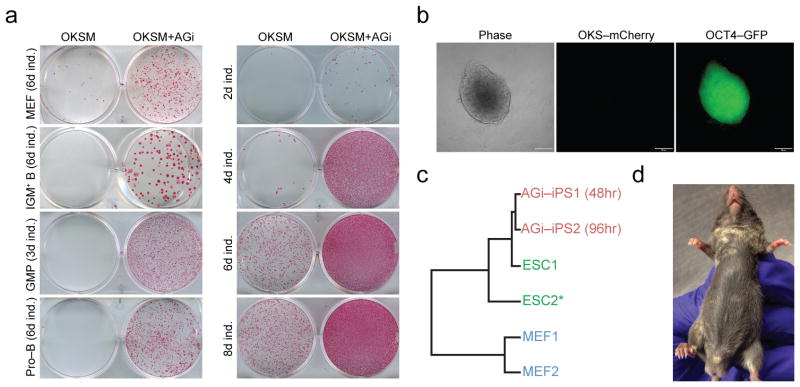
AGi enhances and accelerates iPSC formation across different cell types. (a) Left panel: effect of AGi on reprogramming potential of different cell types. Doxycycline was withdrawn at the indicated time points (ind., induction) and colonies were assessed for alkaline phosphatase (AP) staining 3 days after doxycycline withdrawal. Right panel: Time course analysis for reprogramming potential of GMPs. Transgene-independent iPSC colonies were obtained from GMPs after 2 days of doxycycline induction in the presence of AGi, followed by 6 days of doxycycline-independent growth. (b) Day 2 iPSCs express OCT4-GFP but no longer express OKSM (mCherry negative; scale bar is 100 um) following doxycycline withdrawal. (c) Cluster analysis based upon global gene expression analysis of the indicated samples. Note that expression data from the ESC2 line was previously published43. (d) Chimeric mouse showing donor-derived agouti coat color contribution from iPSCs generated in (b).
Ascorbate and CHIR-99021 synergize during reprogramming
Extracellular cues enhance the formation of iPSCs by influencing signaling pathways, oxidative state, or epigenetic modifications7,16,17. We therefore tested 16 common cell culture supplements, individually or in combination, for their effect on iPSC generation (see methods section for a full list)18,19. This effort identified ascorbic acid (AA) and the GSK3-beta inhibitor, CHIR-99021, as the most effective treatments to boost iPSC generation from MEFs (Fig. 1c,d). Both compounds reportedly enhance iPSC formation in different cellular contexts20–22. Remarkably, however, combined AA supplementation and GSK3-beta inhibition (termed “AGi”) had a strong synergistic effect on the derivation of iPSCs, enhancing reprogramming by at least an order of magnitude compared to individual treatments (Fig. 1c,d); the synergistic effect of AGi was determined by measuring the number of OKSM transgene-independent, NANOG-positive colonies (Fig. 1d and Supplementary Fig. 1). To corroborate these findings with an independent reprogramming assay, we sorted single MEFs (genotype: Col1a1-tetOP-OKSM; R26-M2rtTA) into 96-well plates and measured iPSC formation efficiencies at the clonal level. Specifically, reprogramming was induced for 7, 11, or 15 days, at which time doxycycline was removed and cells were cultured for an additional four days to ensure transgene-independent growth. Plating efficiencies were comparable among different culture conditions (Fig. 1e). Authentic iPSC colonies were identified by immunostaining with a NANOG-specific antibody, yielding approximately 3% reprogramming efficiency for doxycycline alone, 7% for AA, 10% for CHIR-99021 and 56% for AGi by day 15 of treatment, thus confirming the synergistic effect of AGi (Fig. 1f). Importantly, AGi supplementation increased iPSC colony formation from other cell types including granulocyte/macrophage progenitors (GMPs), proB cells and IgM+ B cells (Fig. 2a). Of note, exposure of OKSM-expressing cells to AGi for prolonged periods mitigated the stimulatory effect, most likely due to overcrowding and subsequent cell death. It will therefore be important to determine the optimal duration of AGi treatment for each cell type. Together, these data show that AGi supplementation strongly enhances the derivation of iPSCs from a variety of somatic cell types.
Stable iPSCs produced after 48 hours of OKSM expression
AGi exposure reduced the minimal requirement for exogenous factor expression from 4 days to just 2 days when GMPs were used as starting cells (Fig. 2a); we chose GMPs because they are more susceptible to iPSC formation than more differentiated cell types5,23. iPSC lines, generated after only 2 days of OKSM expression in the presence of AGi, activated an endogenous Oct4-GFP reporter (Fig. 2b) and could be stably propagated for over 50 passages. AGi treatment had no discernible effect on cell cycle, cell proliferation or apoptosis in the context of OKSM or OKS expression in the first few days of reprogramming, thus excluding the possibility that the enhancement of reprogramming was due to increased cell division or cell loss (Supplementary Fig. 2).
To molecularly characterize colonies generated by OKSM expression with AA, CHIR-99021, or both compounds, we picked ten colonies from each condition and found that every clone exhibited endogenous NANOG expression 7 days after doxycycline withdrawal, suggesting proper activation of the core pluripotency network (Supplementary Fig. 3). Microarray analysis confirmed that iPSC lines, obtained after only two days of OKSM expression, exhibited global gene expression patterns that were highly similar to mouse embryonic stem cells (ESCs) (Fig. 2c) and showed activation of key pluripotency genes (Suppl. Fig. 4). Functionally, iPSC lines produced after 2 days of OKSM expression in the presence of AGi contributed to chimeric mice when injected into blastocysts (Fig. 2d). Thus, AGi treatment of reprogramming cultures accelerates iPSC formation, yielding stable and faithfully reprogrammed iPSC colonies after as little as 48 hours of OKSM expression (Fig. 2).
AGi exposure triggers rapid and synchronous reprogramming
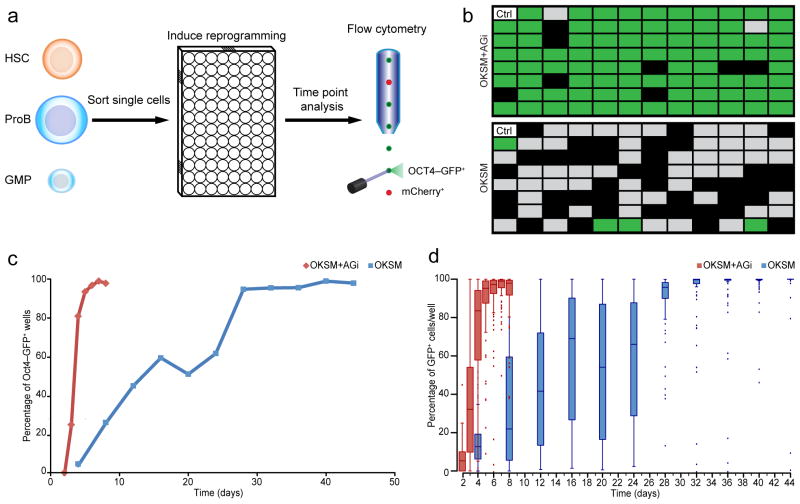
To accurately quantify reprogramming efficiency and kinetics, we sorted single blood progenitor cells carrying the tetOP-OKSM/tetOP-OKSmC alleles as well as an Oct4-GFP knock-in reporter15 by fluorescence-activated cell sorting (FACS) into individual wells of 96-well plates (Fig. 3a). We then evaluated OCT4-GFP activation at discrete time points (Fig. 3b,c). For GMPs, wells containing 53% or more OCT4-GFP+ cells at any given time point were considered positive hits for reprogrammed iPSCs. We determined this cutoff empirically to eliminate false-positive “iPSC calls” that were prevalent at earlier time points due to low cell numbers and autofluorescent feeders (see methods section). By applying these stringent criteria, we noticed a marked difference in reprogramming efficiency with and without AGi at day 6 of induction (Fig. 3b). Examination of reprogramming efficiencies for cells treated with doxycycline alone revealed that roughly 20% of GMPs became OCT4-GFP+ by day 8, ~50% by day 20 and close to 100% by day 30 (Fig. 3c). These reprogramming efficiencies were two-fold higher than previously observed by our lab when comparing similar time points (25% at day 15)5, which is likely due to the superior reprogramming system used here15. Remarkably, exposure of replicate plates to AGi yielded over 20% OCT4-GFP+ clones by day 3, 80% by day 4 and over 95% by day 5 (Fig. 3c). These results indicate that AGi treatment facilitates more synchronous iPSC formation from myeloid progenitors when using a clonal reprogramming assay.
Figure 3.
Clonal analysis of GMP reprogramming indicates synchronous reprogramming with AGi. (a) Schematic for clonal reprogramming assay in 96-well plates. (b) Representative example from three technical replicates of clonal reprogramming analysis of GMPs at day 6 of OKSM expression in control (doxycycline alone) vs. AGi (doxycycline plus AGi) setting. Green fields represent wells of a 96 well-plate that contained 53% or more OCT4-GFP+ cells and were thus regarded iPSCs (see also text and methods section); gray fields were GFP-negative or contained less than 53% OCT4-GFP+ cells; black fields represent wells in which fewer than 10 cells were detected. (c) Time course analysis of clonal iPSC formation in the presence or absence of AGi. (d) Examination of intra-well heterogeneity of OCT4-GFP expression in GMPs during the course of reprogramming. Box and whisker plots were used in which the band in the middle of the box represents the median; the top and bottom part of the box represent the first and third quartiles. The whiskers represent the lowest and highest value within 1.5-fold of the inter-quartile ranges.
Clonal cell populations might activate OCT4-GFP either homogeneously or in a subset of cells within each well, which is not taken into account when measuring iPSC formation efficiency based on a binary system used here and in previous studies12,24. We therefore measured the fraction of GMPs that activated OCT4-GFP within individual wells at different time points of reprogramming. Consistent with the synchronous emergence of clonal iPSC cultures, the majority of cells within individual wells exhibited more homogeneous OCT4-GFP activation (0–10% at day 2, 60–90% at day 4 and 90–100% by day 5) in the presence of AGi whereas untreated cultures (doxycycline alone) remained highly heterogeneous for OCT4-GFP expression until day 30 (Fig. 3d). We confirmed this observation for other cell types, including hematopoietic stem cells (HSCs) and proB cells (Supplementary Fig. 5a,b).
It was previously reported that AID knockout cells express pluripotency factors earlier in the reprogramming process although these cells often fail to form stable iPSC colonies25. To ensure that OCT4-GFP activation indeed coincided with the formation of stable iPSC colonies, we withdrew doxycycline (i.e., discontinued transgene expression) and AGi from GMPs at various time points and maintained the cells for at least four days in ESC media before scoring for iPSC colonies (Supplementary Fig. 5c). Consistent with our FACS results, clonal, transgene-independent colonies formed rapidly and efficiently in samples treated with AGi (Supplementary Fig. 5d). We conclude that AGi exposure induces near-homogeneous activation of a key pluripotency gene and concomitant formation of stable, transgene-independent iPSCs in clonal progenitor cell populations expressing OKSM.
AGi rescues reprogramming potential of refractory cells
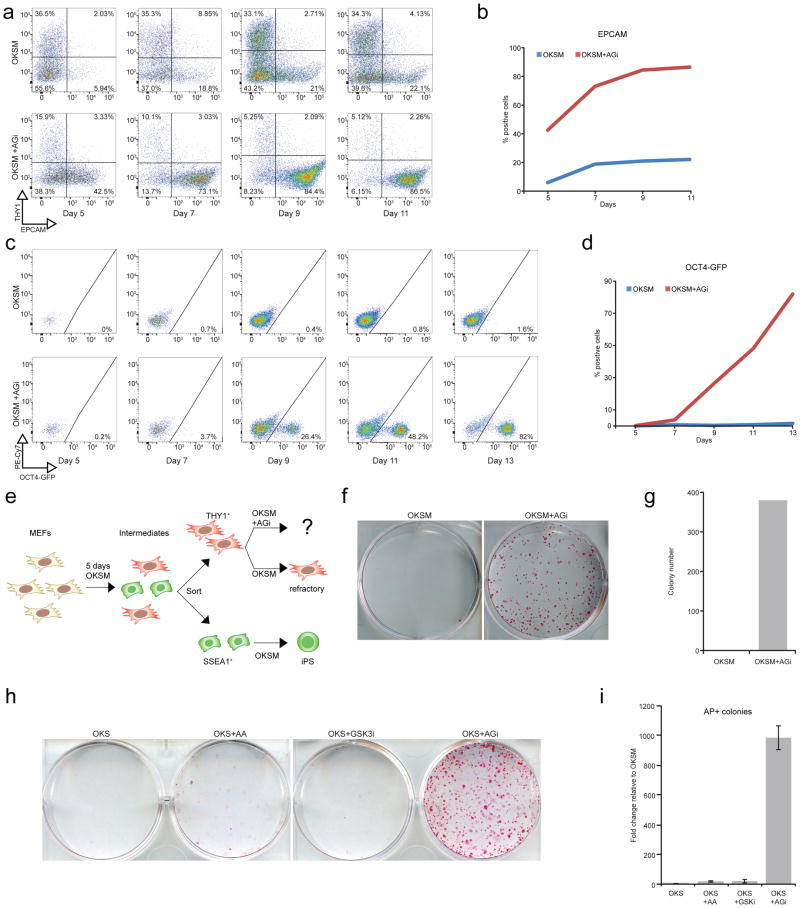
To gain mechanistic insights into how AGi treatment may influence the reprogramming process, we analyzed intermediate time points during iPSC formation by FACS using previously described surface markers and a reporter allele10,11. MEFs undergoing successful reprogramming initially downregulate the fibroblast marker THY1, followed by successive activation of the intermediate marker EPCAM, and subsequent expression of OCT4-GFP. In agreement with previous studies, we detected downregulation of THY1 in two thirds of the population and only a small fraction of intermediates expressing either EPCAM (22%) or OCT4-GFP (0.8%) by day 11 of reprogramming (Fig. 4a–d). In contrast, exposure of cells to AGi for the same duration triggered these phenotypic changes in a much larger fraction of cells (92% THY1−, 87% EPCAM+, 48% OCT4-GFP+) (Fig. 4a–d). These results thus suggested that AGi supplementation reduces a high fraction of OKSM-expressing cells that typically fail to form iPSCs. To directly test this hypothesis, we induced OKSM expression in MEFs for 5 days and sorted intermediates based on THY1 positivity and SSEA1 negativity, which identify cells that have become refractory to iPSC induction after day 3 (Fig. 4e)10,11. While THY1+ cells exposed to regular culture conditions failed to yield iPSCs, addition of AGi restored their potential to produce doxycycline-independent iPSCs (Fig. 4f,g, Supplementary Fig. 6). Thus, AGi’s effect on reprogramming is at least in part explained by its ability to prevent cells from arresting at intermediate stages of iPSC induction.
Figure 4.
AGi rescues the reprogramming defect of refractory cells. (a) FACS analysis of reprogramming intermediates in bulk MEF populations at days 5, 7, 9, 11 of OKSM or OKSM+AGi expression. Note the more homogeneous shift of intermediates from THY1+ to THY1-/EPCAM+ cells. (b) Graph summarizing the percentages of EPCAM+ cells depicted in (a). (c) FACS analysis for OCT4-GFP expression in bulk MEF populations at days 5, 7, 9, 11, and 13 of OKSM or OKSM+AGi expression. PE-Cy7 was used as an autofluorescent control with emission filter 750 nm. (d) Graph summarizing the percentages of OCT4-GFP+ cells shown in (c). (e) Schematic outlining the attempt to restore reprogramming potential in THY1+ refractory cells sorted at day 5 of OKSM expression. (f) AP staining for refractory, THY1+ intermediates following continued OKSM expression (doxycycline exposure) under the indicated conditions. Doxycylcine was removed for an additional 4 days prior to analysis to ensure transgene independence. (g) Quantification for the results shown in (f). Replicate analyses and individual treatment with ascorbic acid and GSK3-beta inhibitor are shown in Supplementary Figure 6. (h) AP staining for cells expressing OKS under the indicated conditions. Doxycylcine was removed for an additional 4 days prior to analysis to ensure transgene independence. (i) Quantification for the results shown in (h)(n=3 technical replicates).
We next examined AGi’s effect on iPSC formation in the absence of exogenous c-MYC expression, which strongly impairs cellular reprogramming26,27. To this end, we derived MEFs carrying the Col1a1-OKSmC and R26-M2rtTA alleles in a heterozygous configuration; exposure of these cells to doxycycline gave rise to extremely few, if any, iPSC colonies. Remarkably, AGi treatment increased iPSC formation nearly 1000-fold compared to OKS expression alone (Fig. 4h,i). The combination of ascorbic acid and GSK3-beta inhibitor was again synergistic since either compound alone only marginally increased the reprogramming capacity of these cells at the tested time point. We conclude that AGi supplementation provides robustness to the reprogramming process under suboptimal conditions.
AGi modulates somatic, transient and pluripotency genes
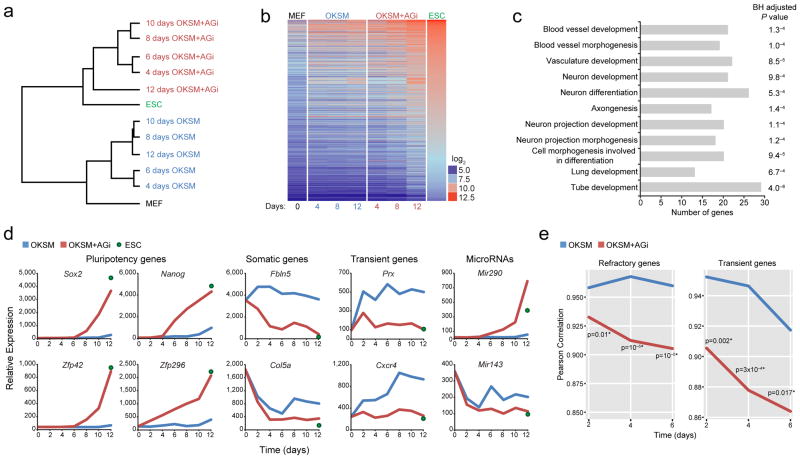
Successful reprogramming requires extinction of the somatic program and activation of key pluripotency factors7. Furthermore, cell populations expressing OKSM were reported to transiently upregulate developmental genes10,28; however, the relevance of these changes remains unclear. In an attempt to dissect the molecular consequences of AGi treatment, we performed expression profiling of reprogrammable MEFs exposed to doxycycline for 4, 6, 8, 10, 12 days in the presence or absence of AGi. Unsupervised clustering of the entire transcriptomes of these samples showed that bulk MEF cultures exposed to doxycycline and AGi were more similar to established iPSCs, whereas MEFs receiving only doxycycline for the same period were more similar to the starting MEF population (Fig. 5a,b). Notably, reprogrammable MEFs exposed to doxycycline and AGi for only 48 hours were already distinguishable from MEFs exposed to doxycycline alone (Supplementary Fig. 7a). MEF-related transcripts (e.g. Col5a, Fbln5, miR-143) were downregulated more rapidly in AGi-treated cells while key pluripotency transcripts including Nanog, Sox2, Zfp42, Zfp296, and miR-290-295 were upregulated much earlier in AGi-exposed cells than in control cells treated with doxycycline alone (Fig. 5d). Moreover, 48 hours of OKSM expression elicited more rapid downregulation of genes associated with FGF signaling, which reportedly blocks iPSC formation29,30 when AGi was present in the culture (Supplementary Fig. 7b). Accordingly, functional annotation analysis of downregulated genes in AGi-treated compared to untreated cells showed enrichment for categories related to differentiation and expression of somatic genes from different lineages (Fig. 5c).
Figure 5.
Effect of AGi on gene expression patterns in reprogramming intermediates. (a) Unsupervised clustering of global gene expression analysis of indicated samples. “4–12 days OKSM” represent reprogrammable MEFs exposed for 4–12 days to doxycycline in the absence or presence of AGi. (b) A heat map showing expression levels for genes that were greater than three-fold higher in ESCs relative to MEFs. (c) DAVID functional analysis of genes whose expression is downregulated at least 1.5 fold in response to AGi treatment in MEFs expressing OKSM/OKSM+AGi. Note the prevalence of developmental regulators. A Benjamini-Hochberg (BH) adjusted p-value is presented. (d) Examples of somatic, pluripotency, microRNA, and transient developmental genes that change expression in response to doxycycline/AGi treatment. (e) Expression patterns of gene sets associated with a refractory phenotype (left panel) or transient upregulation during reprogramming (right panel) were compared with expression patterns obtained after OKSM expression in the presence or absence of AGi. Statistical significance is indicated with asterisks (One-tailed Fischer test).
We further noticed that AGi exposure abolished the transient upregulation of differentiation-associated genes such as Prx and Cxcr4, suggesting that these molecular changes normally resist iPSC formation in the absence of AGi (Fig. 5d). Loss of this transient expression pattern was confirmed at a global level by comparing our results with a previous study that catalogued all transient genes in defined intermediates of reprogramming10 (Fig. 5e, right panel). To exclude that the selected gene expression time points may have missed transient upregulation of these genes in the presence of AGi, we performed qRT-PCR analysis at 12, 24, 36, and 48 hours after the induction of reprogramming. Consistent with the microarray results, Prx and Cxcr4 expression remained constant at early time points in AGi-treated samples while it spiked in controls (OKSM expression alone) (Supplementary Fig. 8). Lastly, we observed that gene expression patterns of THY1+ refractory cells showed a higher degree of correlation with cells expressing OKSM than with cells expressing OKSM in the presence of AGi (Fig. 5e, left panel). This result is in agreement with our earlier finding that AGi treatment rescued the reprogramming potential of THY1+ refractory cells. Collectively, this unbiased genome-wide data provide a partial molecular explanation for our observation that reprogramming occurs more synchronously and rapidly in the presence of AGi.
DISCUSSION
In this study, we provided molecular and functional evidence that exposure of cells to two commonly used tissue culture supplements, ascorbic acid and CHIR-99021, induces more homogeneous reprogramming of different somatic cells into iPSCs at efficiencies and kinetics that have so far only been achieved with genetic manipulation of additional genes12,13. While AGi strongly enhanced and accelerated iPSC induction across diverse cell types, differentiated cells such as MEFs required more time to activate OCT4-GFP and acquire stable pluripotency compared to somatic progenitor cells such as GMPs. This observation confirms the previous notion that less differentiated cells are more amenable to iPSC formation than more differentiated cells5, and it further suggests that mature cells have to overcome additional reprogramming barriers that should be identifiable. Mechanistically, ascorbic acid and GSK3-beta inhibition synergize during reprogramming by preventing the generation of refractory cells, blocking the transient activation of developmental regulators and facilitating the early activation of key pluripotency genes necessary to achieve a self-sustaining pluripotent state. It remains to be determined which downstream effector(s) of ascorbic acid and CHIR-99021 mediate the enhancing effect of AGi on reprogramming. Based on the observation that ascorbic acid functions as a cofactor for histone demethylases and Tet enzymes implicated in stem cell biology20,31,32, we surmise that it contributes to the observed reprogramming phenotype by facilitating epigenetic activation of key pluripotency genes during iPSC generation. In agreement with this idea, we recently showed that ascorbic acid can functionally compensate for the absence of Nanog33, and it prevents aberrant silencing of the Dlk1-Dio3 imprinted cluster during reprogramming34. CHIR-99021, on the other hand, may synergize with ascorbic acid through its destabilizing effect of TCF3, a known repressor of key pluripotency genes35. In addition, CHIR-99021 may counteract inappropriate activation of developmental regulators; this notion is consistent with a recent study by Plath and colleagues, who showed attenuated expression of neural genes in nascent iPSCs depleted for TCF336.
From a practical point of view, AGi-mediated reprogramming should enable molecular studies of the reprogramming process in bulk cultures, which has not been feasible so far because of the extreme heterogeneity of iPSC intermediates. Similarly, AGi treatment offers a simple strategy to generate iPSCs under conditions that impede or resist reprogramming. We have demonstrated this principle by generating iPSCs from THY1+ cells, OKS-expressing fibroblasts lacking the c-MYC transgene and IgM+ lymphocytes, which are notoriously difficult to reprogram. We expect this finding to extend to several other cell types that are hard to reprogram and conditions in which omission of the c-MYC oncogene is warranted. For example, we anticipate that AGi treatment will streamline iPSC generation using integration-free systems that have been hampered by extremely low efficiencies37–39. The strong effect of AGi on mouse iPSC formation further raises the question of whether this treatment enhances human reprogramming. Preliminary data from our lab suggest that AGi does not impact human induced pluripotency (data not shown). However, given the well-established differences in signaling requirements between mouse and human pluripotent stem cells4, it may be feasible to take a rational approach and identify combinations of small molecules that enhance human iPSC generation in a similar fashion to AGi in mouse cells. Likewise, it will be informative to test whether AGi treatment is beneficial in other settings of cell fate change such as transdifferentiation40,41. Notably, the transdifferentiation of human fibroblasts to induced neurons is strongly enhanced by CHIR-99021 exposure alone42. It is therefore conceivable that this and other direct lineage conversion approaches might equally benefit from AGi supplementation.
ONLINE METHODS
Derivation of reprogrammable cells
Reprogrammable cells were derived from mice carrying a polycistronic cassette containing Oct4, Sox2, Klf4, and c-Myc (OKSM)15 or Oct4, Sox2, Klf4, and mCherry (OKSmC) in the Col1a1 locus. Where indicated, EGFP was knocked into one allele of the Oct4 locus under control of the endogenous Oct4 promoter5. Images in Figure 1b–c and Figure 2a–b are representative of at least 5 experiments, except for IGM+ B cell images, which represent 3 technical replicates. All of the mice carried a ROSA26-M2rtTA allele, completing the inducible system. Murine embryonic fibroblast (MEF) cultures were established from E13.5 mouse embryos from reprogrammable mice as previously described15. For blood lineages, bone marrow was first extracted from the femur and tibia of reprogrammable mice. The raw bone marrow was dissociated by pipetting and filtered through a 40 μm cell strainer (BD). Bone marrow was pelleted and resuspended in 2 ml of FACS buffer (4% fetal bovine serum in PBS (Life Technologies). Then 8 ml of ACK buffer (150 mM NH4Cl, 10 mM KHCO3, 0.2 mM EDTA) was added, mixed, and incubated for 5 minutes at 23°C. Finally, 0.5 ml of FBS was added directly to the bottom of the mixture and the cells were again pelleted. Bone marrow was plated directly on feeders for experiments. Granulocyte-macrophage precursors (GMPs) were isolated using fluorescence activated cell sorting (FACS) on an Aria II sorter (BD) with the following antibodies: B220-PE-Cy5, Ter119-PE-Cy5, CD3e-PE-Cy5, Mac1-PE-Cy5, Gr1-PE-Cy5, Kit-APC780, Sca1-PE-Cy5, FcγR-FITC (all from eBiosciences). GMPs undergoing reprogramming were plated on feeders with 10 ng/ml IL3, 10 ng/ml IL6, and 20 ng/ml SCF (Prospec) for the first three days. Hematopoietic stem cells (HSCs) were isolated using FACS with the following antibodies: B220-PE-Cy5, Ter119-PE-Cy5, CD3e-PE-Cy5, Mac1-PE-Cy5, Gr1-PE-Cy5, Kit-APC780, Sca1-PE-Cy5, CD48-FITC, CD150-PE (all eBiosciences). HSCs undergoing reprogramming were maintained on 10 ng/ml IL3, 10 ng/ml IL6, 10 ng/ml Flt3 ligand, 20 ng/ml TPO and 50 ng/ml SCF (Prospec) for the first 3 days. ProB cells were isolated using FACS with the following antibodies: CD43-FITC, B220-PE-Cy7, IgM-APC (all from eBiosciences). ProB cells were maintained in 10 ng/ml IL7, 10 ng/ml FLT3 ligand, and 25 ng/ml SCF (Prospec) for the first three days. IGM-positive B cells were isolated using FACS with the following antibodies: IgM-APC, IgD-PE (all eBiosciences). All cells were sorted onto irradiated feeder cells into either 6-well plates or as single cells in 96-well plates (Corning Costar). All procedures, including maintenance of animals, were performed according to a mouse protocol approved by the MGH Subcommittee on Research Animal Care.
Single cell analysis
Single cell analysis was performed using a MACSQuant analyzer (Miltenyi Biotec). Cells were dissociated using trypsin-EDTA (Life Technologies) and collected in FACS buffer prior to analysis. The red channel was used to detect mCherry expression as a reporter for the reprogramming cassette. The green channel was used to detect GFP expression under the control of the Oct4 promoter. FlowJo software was used to analyze all cytometry data. Feeder cells were excluded from analysis by gating out cells that exhibited auto-fluorescence. The false determination rate for this gating strategy was determined by running a plate with feeders alone. Only wells in which more than 10 cells were detected following the exclusion of feeders were included for analysis (False determination rate <1%). For calculating reprogramming efficiencies, we deemed a positive event as a well in which greater than 53% of cells expressed GFP. This threshold was experimentally determined for GMPs as follows: GMPs were sorted onto a 96-well plate and treated with AGi for 6 days. Figure 3b is representative of 3 independent experiments. At this time, cells were split and half were analyzed via flow cytometry for OCT4-GFP expression while the other half was re-plated in the absence of doxycycline. The latter cells were grown for another 10 days in ESC media alone and analyzed by FACS to determine which wells maintained OCT4-GFP expression. This experiment showed that wells exhibiting over 53% OCT4-GFP during the initial FACS analysis maintained GFP expression after 10 days of doxycycline withdrawal, indicating transgene-independent growth. Box plots for single cell reprogramming experiments were plotted using the statistical program R.
Alkaline phosphatase staining
Alkaline phosphatase kits (Vector Labs) were used according to the manufacturer’s recommendations to assess pluripotency. Prior to staining, doxycycline and cell culture supplements were removed for a minimum of 3–4 days to eliminate exogenous OKSM expression.
Quantification of alkaline phosphatase staining using imageJ software
ImageJ was used to process alkaline phosphatase (AP) images for colony counting44. A region of interest covering a given well was selected using the oval tool. The process, “find edges,” was then selected. The threshold tool was applied, with the “default thresholding method”, “red” as the threshold color, “HSB” as the color space, and “dark background” selected. “Analyze particles” quantified the number of AP-positive colonies and the area of those colonies using the following parameters: “Size (pixel^2)=25-infinity”; “Circularity=0–1”; “Exclude on edges” was applied; “Include holes” was applied. The settings were identical for all samples processed.
Cell proliferation, cell cycle, and apoptosis assays
Cell proliferation analysis was performed by counting cells on a hemocytometer on consecutive days. Cell cycle analysis was performed using 5-bromo-2′deoxyuridine (BrdU). Briefly, BrdU (Sigma) was added directly into culture medium at a final concentration of 10 μM for 30 minutes. Cells were then dissociated using trypsin-EDTA (Life Technologies) and washed twice in PBS (Life Technologies). The cells were resuspended in 100 μl of normal saline on ice. Ice-cold 70% EtOH was added dropwise to the cells, which were subsequently incubated for 30 minutes on ice. Then, an equal volume of 4N HCl was applied to the cells with incubation for 30 minutes at 23°C. The cells were centrifuged at 500 × g for 5 minutes and resuspended in 1 ml of 0.1 Na2B4O7, pH 8.5. Cells were again pelleted and resuspended in FACS buffer. Anti-BrdU-FITC was then applied for 30 min at 23°C before washing the cells and analyzing on a MACSQuant flow cytometer (Miltenyi Biotec). Annexin staining to assess apoptosis and necrosis was performed according the manufactuer’s recommendations (BD) and analyzed using a MACSQuant flow cytometer.
Cell culture
Mouse ESCs and iPSCs were cultured on irradiated feeders (GlobalStem) in KO-DMEM (Life Technologies) supplemented with 15% FBS (Life Technologies), 1% Glutamax (Life Technologies), 1% Non-essential amino acid (Life Technologies), 1% Penicillin-streptomycin (Life Technologies), 0.5% β-mercaptoethanol (Sigma-Aldrich), and 1000 U/ml LIF (“mESC media”). Mouse embryonic fibroblasts were grown in DMEM (Life Technologies) supplemented with 10% FBS, 1% Glutamax, 1% None-essential amino acid, 1% Penicillin-streptomycin, 0.1% β-mercaptoethanol. Reprogramming of MEFs and blood progenitors were performed in mESC media supplemented with 2 μg/ml doxycycline (Sigma). Ascorbic acid (Sigma) was diluted in water and prepared fresh every 10–14 days and added at a final concentration of 50 μg/ml. GSK3 inhibitor CHIR99021 (from Stemgent or Tocris) was administered at 3μM final concentration for all experiments. Testing for Mycoplasma was routinely performed.
Doxycycline withdrawal assays
To test the stability of iPSC colonies formed under different reprogramming conditions, cells were treated for various times with doxycycline in the presence or absence of cell culture supplements. The supplements and doxycycline were removed at the indicated times points, cells were then washed with ESC media, and ESC media was applied for at least 3 days prior to analysis. In the case of GMPs, ESC media contained the following cytokines: 10 ng/ml IL3, 10 ng/ml IL6, and 20 ng/ml SCF (Prospec).
Reprogramming of MEFs and blood progenitors
In a typical reprogramming experiment, 20,000 MEFs from a reprogrammable mouse were counted and seeded onto 6-well plates one day prior to induction. Doxycycline (dox) or Doxycycline+AGi (dox+AGi) were added fresh to the cells every other day. Depending on the experiment, usually 2–9 days for blood progenitors and 6–12 days for MEFs were used for iPSC induction, after which doxycycline and small molecules were removed and replaced with fresh mESC media. Of note, administration of AGi in mESC media containing serum replacement instead of FBS showed a marked reduction in the reprogramming efficiency in dox alone or dox+AGi treated cells. Importantly, cell passage highly affects the reprograming efficiency with AGi. Low-passage, highly proliferative reprogrammable MEFs showed a higher reprogramming efficiency compared to high-passage, less proliferative MEFs in the absence or presence of AGi.
Candidate chemical screen
To test which molecules reprogram somatic cells most efficiently into iPSCs, we tested several compounds that we pre-selected from the literature. The compounds were tested at different concentrations and in different combinations and included: EGF (Life Technologies), bFGF (R&D), Wnt3a (R&D), ascorbic acid (Sigma), GSK3 inhibitor (Stemgent or Tocris), LiCl (Sigma), MEK inhibitor (R&D), Forskolin (Sigma), ALK-4/5/7 inhibitor (Sigma) and RepSOX-616452 (Sigma), VPA (Sigma), 3-Deazaneplanocin (Cayman), TTNPB (Sigma), Tranylcypromine (BPS biosciences), JNK inhibitor (EMD) and BMP4 (Stemgent). Although some other different small molecule combinations facilitated reprogramming, the dual administration of ascorbic acid and GSK3i showed the strongest effect.
Blastocyst injections and generation of chimeric mice
Chimeric mice were generated via blastocyst injections as previously reported45. To induce superovulation, female B6D2F1/J mice (5–6 weeks old; Jackson Laboratory) were treated with pregnant mare serum (Sigma-Aldrich) by intraperitoneal injection. Human chorionic gonadatrophin (Sigma-Aldrich) was administered 48 hours later by intraperitoneal injection and the female mice were mated to B6D2F1/J males. Blastocysts were harvested three days later and injected with iPSCs that were expanded from a colony generated after 48 hours of transgene expression in the presence of AGi. The blastocysts were transferred to pseudo-pregnant Swiss Webster female mice (Jackson Laboratory; approximately 2 months of age) and allowed to developed to term.
Immunostaining
To ensure that iPSC colonies generated under different conditions expressed NANOG, we carried out immunofluorescence as follows. Cells were washed once with PBS (Life Technologies). The cells were then fixed in 4% formaldehyde for 20 minutes at 23°C and washed again in PBS. Triton X-100 was used at a concentration of 0.5% and applied to cells for 10 minutes at 23°C. The cells were washed three times in PBS. Blocking was performed in 10% goat serum with 0.2% Triton X-100 for 30 min at 23°C. Primary antibody (anti-mouse NANOG; Abcam; Ab80892) was added at a dilution of 1:400 in 1.5% goat serum at 23°C for one hour. The cells were washed three times in PBS. Secondary antibody (anti-rabbit, ALEXA 555; Life Technologies) was added at a dilution of 1:1000 in 1.5% goat serum at 23°C for one hour. The cells were washed three times in PBS and mounted in mounting media with DAPI (Vectashield; Vector Labs).
Reprogramming of refractory cells
Reprogrammable MEFs grown in mESC media were dox-treated for 5 days, then harvested and stained for various surface markers: Anti-mouse CD326 EPCAM (PE), anti-mouse SSEA1 APC-647 (Biolegend) and anti-mouse CD90 THY1.2 eFluor 450 (eBiosciences). THY1+/SSEA1-/EPCAM-/OCT4-GFP-cells (“refractory cells”) were sorted in equal numbers onto gelatin-coated 6-well plates containing mESC media containing doxycycline, doxycycline+ascorbic acid, doxycycline+GSK3i or doxycycline+AGi. Media and small molecules were replaced every other day, for a total of 7 days, after which dox and AGi were withdrawn and replaced with fresh mESC media. Dox-independent iPSCs were scored at least three days later by alkaline phosphatase staining kit (Vector Labs SK-5100).
Microarrays and RNA extraction
DNase-treated (Qiagen) total RNA was extracted using RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Hybridization to the GeneChip Mouse 2.0 ST arrays (Affymetrix) were done according to protocols in the Partners Center for Personalized Genetic Medicine. RMA was performed using Expression Console (Affymetrix). Hierarchical clustering was performed using Expander (EXPression Analyzer and DisplayER). Classification and annotations of selected gene groups was performed using the DAVID online functional annotation tool (http://david.abcc.ncifcrf.gov/). Heat maps were generated using the statistical software program R. The gene expression data has been deposited to Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) and given the accession number GSE57774. The gene expression data for ESC-2 was downloaded from the GEO study GSE5664643.
Gene expression analysis: correlation study within refractory and transient gene sets
We performed a Pearson product-moment analysis to study the correlation between expression patterns from gene sets associated with a refractory/transient upregulation phenotype and OKSM expression with and without AGi. In statistics, Pearson correlation is a measure of the linear correlation (dependence) between 2 variables, where 1 indicates total positive correlation, 0 is no correlation and −1 is total negative correlation. The statistical significance of the difference between the two correlation coefficients, for a given time point, was calculated using a one-tailed Fisher test; highly significant differences between two correlation coefficients are indicated by a p-value < 0.05.
qRT-PCR analysis
Total RNA was extracted from one confluent well of a 6-well dish using the RNeasy Mini Kit with on-column DNAse digestion (Qiagen). cDNA was generated using the Superscript III First Strand Kit via the Oligo-dT method (Life Technologies). qRT-PCR was carried out using the Brilliant III SYBR QPCR Master Mix (Agilent Technologies) on a Lightcycler 480 (Roche). Relative expression was calculated using the ΔΔCt method with GAPDH as reference46. The following validated primers were used for qRT-PCR: Cxcr4_forward:GACTGGCATAGTCGGCAATG, Cxcr4_reverse: AGAAGGGGAGTGTGATGACAAA, Prx_forward: TCAGCGGCTTCAACGTAGC, Prx_reverse: TAGCTGCCGGTGAGTCCTC GAPDH _forward: AGGTCGGTGTGAACGGATTTG GAPDH_reverse: TGTAGACCATGTAGTTGAGGTCA
Surface marker analysis by flow cytometry
Cells were harvested using 0.25% trypsin and re-suspended in 1xPBS buffer containing 4% FBS (FACS buffer). The appropriate conjugated antibodies were added at similar ratios (1:200): Anti-mouse CD326 EpCAM (PE), and anti-mouse CD90 Thy1.2 eFluor 450 (eBiosciences). Cells were incubated on ice for 20 minutes after which they were washed twice with 1xPBS, resuspended in FACS buffer, filtered and then analyzed on MACSQuant flow cytometer (Miltenyi Biotec) machine. Analysis was performed using FlowJo software.
Supplementary Material
Acknowledgments
We thank members of the Hochedlinger lab for helpful suggestions and critical reading of the manuscript. We thank M. Borkent for providing study material and to A. Foudi and D. Kramer for scientific discussion. We thank L. Prickett, M. Weglarz, and K. Folz-Donahue at the MGH/HSCI flow cytometry core. O.B.N. was supported by a Gruss-Lipper postdoctoral fellowship. Support to K.H. was from the NIH (R01HD058013) and the Howard Hughes Medical Institute. J.B. is grateful for support from the Tosteson Fund for Medical Discovery Post-doctoral Fellowship by the MGH Executive Committee on Research and NIH (1F32HD078029-01A1).
Footnotes
ACCESSION NUMBERS
Microarray data has been deposited to Gene Expression Omnibus (GEO) and given the accession number GSE57774.
AUTHOR CONTRIBUTIONS
O.B.N., J.B. and K.H. conceived the experiments, interpreted results and wrote the manuscript. O.B.N. and J.B. conducted all iPSC experiments, performed statistical analyses and generated figures; C.V. assisted in experiments; E.A. produced transgenic OKSmC mice; J.B. and R.W. generated chimeric animals by blastocysts injections; O.B.N., I.P.M., and S.R. performed bioinformatics analysis of expression data.
COMPETING FINANCIAL INTERESTS
None of the authors have competing financial interests
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24:2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eminli S, et al. Differentiation stage determines potential of hematopoietic cells for reprogramming into induced pluripotent stem cells. Nat Genet. 2009;41:968–976. doi: 10.1038/ng.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009 doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apostolou E, Hochedlinger K. Chromatin dynamics during cellular reprogramming. Nature. 2013;502:462–471. doi: 10.1038/nature12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buganim Y, Faddah DA, Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet. 2013;14:427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papp B, Plath K. Epigenetics of reprogramming to induced pluripotency. Cell. 2013;152:1324–1343. doi: 10.1016/j.cell.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polo JM, et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell. 2012;151:1617–1632. doi: 10.1016/j.cell.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell stem cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rais Y, et al. Deterministic direct reprogramming of somatic cells to pluripotency. Nature. 2013;502:65–70. doi: 10.1038/nature12587. [DOI] [PubMed] [Google Scholar]
- 13.Di Stefano B, et al. C/EBPalpha poises B cells for rapid reprogramming into induced pluripotent stem cells. Nature. 2014;506:235–239. doi: 10.1038/nature12885. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos RL, et al. MBD3/NuRD facilitates induction of pluripotency in a context-dependent manner. Cell stem cell. 2014;15:102–110. doi: 10.1016/j.stem.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stadtfeld M, Maherali N, Borkent M, Hochedlinger K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010;7:53–55. doi: 10.1038/nmeth.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng B, Ng JH, Heng JC, Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell stem cell. 2009;4:301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Stadtfeld M, et al. Ascorbic acid prevents loss of Dlk1-Dio3 imprinting and facilitates generation of all-iPS cell mice from terminally differentiated B cells. Nat Genet. 2012;44:398–405. S391–392. doi: 10.1038/ng.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou P, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 19.Federation AJ, Bradner JE, Meissner A. The use of small molecules in somatic-cell reprogramming. Trends in cell biology. 2014;24:179–187. doi: 10.1016/j.tcb.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteban MA, et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell stem cell. 2010;6:71–79. doi: 10.1016/j.stem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Silva J, et al. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, et al. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo S, et al. Nonstochastic reprogramming from a privileged somatic cell state. Cell. 2014;156:649–662. doi: 10.1016/j.cell.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanna J, et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature. 2009;462:595–601. doi: 10.1038/nature08592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar R, et al. AID stabilizes stem-cell phenotype by removing epigenetic memory of pluripotency genes. Nature. 2013;500:89–92. doi: 10.1038/nature12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa M, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 27.Wernig M, Meissner A, Cassady JP, Jaenisch R. c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell stem cell. 2008;2:10–12. doi: 10.1016/j.stem.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 28.O’Malley J, et al. High-resolution analysis with novel cell-surface markers identifies routes to iPS cells. Nature. 2013;499:88–91. doi: 10.1038/nature12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichida JK, et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell stem cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maherali N, Hochedlinger K. Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Curr Biol. 2009;19:1718–1723. doi: 10.1016/j.cub.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaschke K, et al. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monfort A, Wutz A. Breathing-in epigenetic change with vitamin C. EMBO Rep. 2013;14:337–346. doi: 10.1038/embor.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarz BA, Bar-Nur O, Silva JC, Hochedlinger K. Nanog is dispensable for the generation of induced pluripotent stem cells. Curr Biol. 2014;24:347–350. doi: 10.1016/j.cub.2013.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stadtfeld M, et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wray J, et al. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nature cell biology. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell reports. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, et al. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324:797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 39.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ladewig J, et al. Small molecules enable highly efficient neuronal conversion of human fibroblasts. Nat Methods. 2012;9:575–578. doi: 10.1038/nmeth.1972. [DOI] [PubMed] [Google Scholar]
- 43.Ravens S, et al. MOF-associated complexes have overlapping and unique roles in regulating pluripotency in embryonic stem cells and during differentiation. eLife. 2014:e02104. doi: 10.7554/eLife.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneider CA, Rasband WS, Eliceiri KW. Nature methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eggan K, et al. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmittgen TD, Livak KJ. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.