Abstract
Objective
To compare growth patterns in the first year of life between children born to perinatally HIV-infected (PHIV) vs. non-perinatally HIV-infected (NPHIV) women in the U.S.
Design
Retrospective cohort study of HIV-infected pregnant women who received care and delivered a liveborn at two urban tertiary centers from January 2004 - March 2012.
Methods
We collected data via chart review on demographics, behavioral risk factors, HIV clinical markers, combination antiretroviral therapy (cART), mode of HIV acquisition, pregnancy outcomes, and infant anthropometrics on study subjects. Mixed effects models were used to assess the association between maternal mode of HIV acquisition and weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length (WLZ) z-scores.
Results
Of 152 pregnancies evaluated, 32 and 120 infants were born to 25 PHIV and 99 NPHIV women respectively. Infants of PHIV women exhibited lower mean WAZ and LAZ throughout the first year of life in unadjusted analyses. After adjusting for potential confounders, the relationship between PHIV & LAZ persisted (β=−0.54, p=0.026). Small for gestational age for each birth anthropometric parameter [birth length, birth weight, and both birth length and weight] was associated with decreased LAZ (β=−0.48, p=0.007), WAZ (β=−0.99, p<0.001) and WLZ (β=−0.36, p=0.027) respectively. A delivery HIV RNA level <400 copies/mL was associated with increased WAZ & WLZ (β=0.43, p=0.015; β=0.38, p=0.021).
Conclusions
Infants of PHIV women may remain at persistently decreased lengths throughout the first year of life. Further studies aimed at understanding intrauterine and environmental factors in PHIV women are warranted.
Introduction
With the success of combination antiretroviral therapy (cART), an increasing number of women infected with HIV since birth are reaching their reproductive years. [1-3] As these long-term survivors are achieving pregnancies, maternal immunological alterations associated with chronic HIV infection and inflammation, [4, 5] as well as maternal behavioral, [6, 7] neurocognitive, [8] and metabolic complications [9] from long-term HIV and antiretroviral (ARV) exposure present a growing concern for a potential impact on the developing fetus/infant. To date, limited information exists on neonatal outcomes of infants born to perinatally HIV-infected (PHIV) pregnant women [10-15]. In contrast to non-perinatally HIV-infected (NPHIV) pregnant women, PHIV women have been observed to have poorer immunologic status throughout pregnancy,[16-18] lower rates of viral suppression at delivery,[13, 14, 17] and higher rates of low birth weight (LBW) (<2500 g) [13] and small-for-gestational age (SGA)[10] outcomes in their infants. Given the recognized association of compromised growth during infancy with adverse long-term health outcomes [19-21] our analysis focuses on a comparison of growth between offspring of PHIV and NPHIV women during the first year of life.
Methods
Study Population
Mount Sinai Hospital (MSH) and Johns Hopkins University (JHU) provide comprehensive care to HIV-infected pregnant women and their offspring in an urban tertiary care environment. This analysis includes all HIV-infected pregnant women/children who received obstetrical care and pediatric follow-up at these institutions from January 2004- March 2012. Pregnancies with multiple gestations [22] or ending in spontaneous/therapeutic abortions or intra-uterine fetal demise (IUFD) were excluded from primary analysis. In addition, infants <28 weeks gestational age (GA), with documented HIV infection, deceased before 1 year, or not followed at the clinics above so as to result in > 3 anthropometric measurements each separated by > 6 weeks in the first year of life were also excluded. The majority of data was obtained at birth, 1-2 weeks, 4 weeks, and 2, 4, 6, 9 and 12 months according to well child visit schedules. The study was approved by the Institutional Review Boards of MSH and JHU.
Primary Outcome
We evaluated growth in the first year of life using routinely collected weight and length measurements. Weight-for-age (WAZ), length-for-age (LAZ), and weight-for-length (WLZ) z-scores were calculated based on World Health Organization (WHO) child growth standards.[23]
Predictor Measurements
Our main exposure of interest was the documented mode of maternal HIV acquisition; women were categorized as PHIV or non-PHIV (NPHIV) according to medical records or patient report. Other potential confounders for infant growth including maternal age, pre-pregnancy body mass index (BMI), race [24], and substance use during pregnancy were determined by medical record review. In addition we controlled for all parameters of infant SGA [SGA by birth weight (BW), SGA by birth length (BL) and SGA by BW and BL for each of the mixed models (WAZ, LAZ and WFLZ respectively) as these are known to be associated with subsequent growth.[25] Substance use was defined as any tobacco, alcohol, or illicit drug use. We collected CD4 cell counts including pregnancy nadirs, HIV RNA levels (using <400 copies/mL to define viral suppression,) and cART recorded throughout pregnancy and at delivery. Second-line cART regimens were defined as containing enfuvirtide, etravirine, darunavir, or raltegravir.
Statistical Analysis
Characteristics of PHIV and NPHIV women at first pregnancy were compared using Wilcoxon test, Chi-square, or Fisher’s exact test as appropriate. Loess plots [26] were fit to compare infant WAZ, LAZ, and WLZ over time between groups. Mixed effects models were used to assess the association between maternal mode of HIV acquisition and each of the three primary growth outcomes. Models included random effects for intercept, slope, and the mother (to account for repeat pregnancies) with an unstructured covariance matrix. Additional analyses were conducted to compare baseline characteristics of infants with incomplete growth data due to lack of clinical follow-up and those with complete data. We did not impute missing data because using our exclusion criteria for those lost to follow-up, <5% had missing (at random) data. Statistical analyses were performed using SAS® 9.3 (SAS Institute, Cary, NC.)
Results
Between January 2004 and March 2012, 170 pregnant women (38 PHIV, 132 NPHIV) received care at the institutions above, resulting in 223 pregnancies. After excluding 18 spontaneous/induced abortions, two pregnancies with IUFD, three twin gestations, five extreme premature, two HIV-infected and three deceased infants, and 38 infants not followed at MSH or JHU, 152 pregnancies remained for analysis: 32 and 120 infants born to 25 PHIV and 99 NPHIV women respectively. Baseline characteristics of women at first pregnancy and of all infants are shown. (Table 1) PHIV women were younger (p<0.001), had lower gravidity (p<0.001), had lower CD4 nadirs during pregnancy (p=0.004), were less likely to demonstrate viral suppression at delivery (p=0.036), and more likely to be on second-line cART (p=0.005) than the NPHIV women. Similar rates of PI-based cART were seen in PHIV and NPHIV women (92 vs. 91%). Substance use patterns differed between groups as no PHIV women reported heroin, cocaine or alcohol use during pregnancy, but these differences did not reach statistical significance. Two PHIV and five NPHIV women were HIV/Hepatitis C co-infected. Three NPHIV women were positive for hepatitis B surface antigen. Infants born to PHIV women were less likely to be female (p=0.03) and had lower mean BWs (p=0.04) as well as birth WAZ (p=0.032). Median birth GA, rates of prematurity and SGA BW [27] were similar between groups. Comparison of the study sample infants to those who were not followed by MSH or JHU (n=38) did not reveal any differences in maternal characteristics (reported substance abuse, baseline CD4, and delivery HIV RNA level) or infant outcomes [GA, BW, and birth length (BL)].
Table 1. Linear Mixed Effects Model of Maternal/Infant Factors Associated with Z-scores of Weight, Length, and Weight-for-Length in HIV-Exposed Uninfected Infants.
| Weight (WAZ) | Length (LAZ) | Weight-for-Length (WFLZ | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Coefficient | p value | Coefficient | p value | Coefficient | p value | |
| Maternal HIV Acquisition | ||||||
| PHIV | −0.29 | 0.137 | −0.54 | 0.026 | 0.09 | 0.597 |
| NPHIV | 0 | 0 | 0 | |||
| Maternal age, per year | −0.02 | 0.090 | −0.01 | 0.611 | −0.01 | 0.391 |
| Pre-pregnancy BMI of mother | 0.00 | 0.913 | 0.00 | 0.678 | 0.00 | 0.622 |
| Nadir CD4 cells in pregnancy | ||||||
| ≤ 200 cells/mm3 | 0.16 | 0.42 | 0.44 | 0.085 | −0.29 | 0.114 |
| > 200 cells/mm3 | 0 | 0 | 0 | |||
| HIV RNA level at delivery | ||||||
| < 400 copies/mL | 0.43 | 0.015 | 0.17 | 0.443 | 0.38 | 0.021 |
| ≥ 400 copies/mL | 0 | 0 | 0 | |||
|
Second line cART use in
pregnancy |
||||||
| Not on second line cART | 0.03 | 0.912 | 0.24 | 0.483 | −0.31 | 0.216 |
| On second line cART | 0 | 0 | 0 | |||
| Infant SGA for BW | --- | --- | ---- | ---- | ||
| SGA for BW | −0.99 | <0.001 | ||||
| Not SGA for BW | 0 | |||||
| Infant SGA for BL | --- | --- | ---- | ---- | ||
| SGA for BL | −0.48 | 0.007 | ||||
| Not SGA for BL | 0 | |||||
| Infant SGA for BW and BL | --- | --- | --- | --- | ||
| SGA for BW and BL | −0.36 | 0.027 | ||||
| Not SGA for BW and BL | 0 | |||||
WAZ=Weight for Age Z score, LAZ=Length for Age Z score, WFLZ=Weight for Length Z score, PHIV=Perinatally HIV-infected, NPHIV=Non-Perinatally HIV-infected, BMI=Body Mass Index, cART=Combination Antiretroviral Therapy, SGA=Small for Gestational Age, BW=Birth Weight, BL=Birth Length
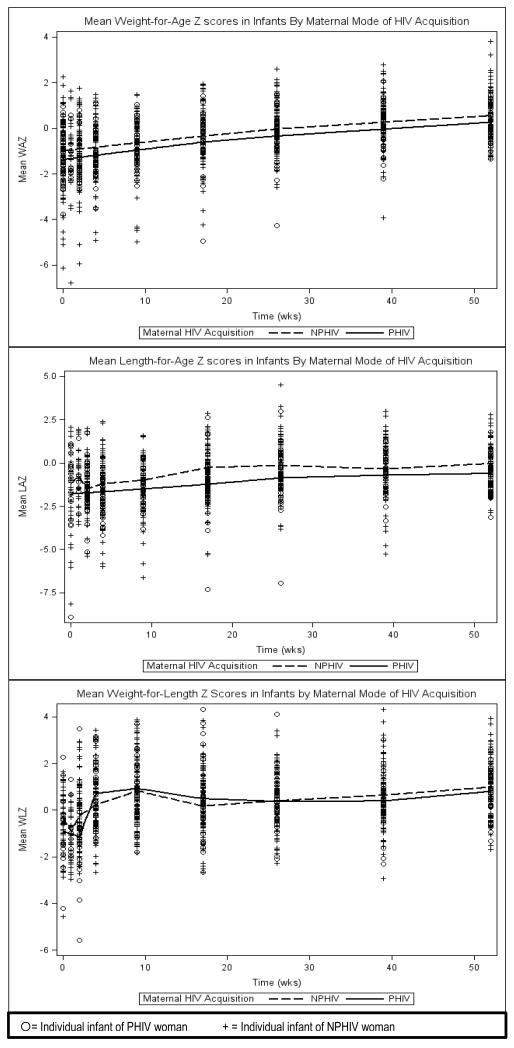
Overall, by 12 months, 9.2% (14/152) of infants remained underweight (WAZ < −2), and 13.2% (20/152) remained short statured (LAZ < −2). Infants of PHIV women exhibited lower mean WAZ and LAZ throughout the first year of life in unadjusted analyses. (Figure 1) After adjusting for maternal age, pre-pregnancy BMI, nadir CD4 cell count during pregnancy, HIV RNA level at delivery, use of second-line cART, and infant SGA at birth, the relationship between PHIV & LAZ persisted (β=−0.54, p=0.026). SGA for each birth anthropometric parameter [BL, BW, and BW and BL] was associated with decreased LAZ (β=−0.48, p=0.007), WAZ (β=−0.99, p<0.001) and WLZ (β=−0.36, p=0.027) respectively. A delivery HIV RNA level <400 copies/mL was associated with increased WAZ & WLZ (β=0.43, p=0.015; β=0.38, p=0.021). We did evaluate tenofovir use instead of second-line cART use in multivariate modeling, but found no effect of tenofovir on our outcomes; nor did the effect of PHIV on LAZ change (data not shown.)
Figure 1. Loess Plots of Mean Weight for Age (WAZ), Length for Age (LAZ), and Weight for Length (WLZ) Z-scores by Maternal Mode of HIV Acquisition.
Discussion
In this study of HIV-infected pregnant women, we found that HIV-exposed uninfected (HEU) infants born to PHIV women exhibited decreased WAZ and LAZ throughout the first year of life compared to HEU infants born to NPHIV women in unadjusted analysis. HEU infants born to PHIV women were found to be at risk for decreased length in the first year, while maternal viral suppression was protective for growth (weight and length), even after adjusting for confounders.
We previously described the association between maternal PHIV and SGA birth outcomes [10] and expand upon this analysis to assess infant growth in this larger study population. To our knowledge, no studies to date have evaluated growth in HEU infants born to PHIV vs. NPHIV women, though several have evaluated differences in maternal factors [14, 17, 18, 28, 29] and pregnancy outcomes [12-17, 30, 31]. Evidence shows that PHIV pregnant women are more immunosuppressed than their NPHIV counterparts. Several studies reported similarly low CD4 cell counts both before (mean initial CD4 cell count 144-314 cells/mm3) [13, 17, 18] and during the pregnancy (136-394 cells/mm3) [14, 15, 17, 18, 30, 32] in PHIV women as seen in our cohort. Our finding of decreased rates of maternal virologic suppression at delivery in PHIV women is also consistent with other reports. [13-15, 17, 18] Not unlike our results, a number of studies have reported increased rates of LBW [13, 15] and similar median BWs (2667-2688g) of children born to PHIV women. [14, 17] The overall rate of preterm birth in our cohort (22.4%) was comparable to rates found in the U.S. among HIV-infected pregnant women (18-22%) [33, 34] as well as reported rates in PHIV women (13.3 – 36%) [12-14, 16, 17].
While infant growth is subject to much confounding, we did find that being born SGA is a predictor for decreased growth in the first year of life, consistent with current literature.[35-37] The protective effect of maternal viral suppression at delivery on infant WAZ and WLZ throughout the first year of life may simply represent the fact that women who adhere to their cART during pregnancy are more likely to also attend to their own health as well as the health care of their infants. It is not entirely clear why infants born to PHIV women had decreased LAZ compared to those born to NPHIV throughout the first year of life, even after controlling for known risk factors. Maternal antenatal factors such as anthropometrics, CD4 nadir during pregnancy, and cART were not associated with poor postnatal growth, suggesting perhaps that other elements of the prenatal environment in PHIV women may affect intrauterine and early postnatal growth. The protracted state of impaired immunologic function in PHIV women may alter the prenatal environment of their offspring such that fetal programming in this unique in utero milieu affects metabolic pathways and postnatal growth and development. [38] Alternatively, external factors such as differences in early postpartum maternal socioeconomic status and nutritional security may be the reason for our findings. We were unable to thoroughly assess these given the retrospective nature of our study. However, the PHIV and NPHIV women were from the same clinical catchment areas where similar racial and socioeconomic backgrounds exist.
The long term implications for poor early postnatal gains in length, particularly in HEU infants born to PHIV remain unclear. However, studies in HIV-unexposed infants have demonstrated that poor early postnatal growth is associated with short adult stature, [19] diminished cognitive abilities, [20, 21, 39] and a host of diminished socioeconomic gains including lower educational attainment, employment, and wages. [39-41] Intrauterine growth and that attained by 12 months has been shown to be the critical period during which interventions can be made to avoid long term complications.[19, 39, 41]
In addition to our inability to properly assess maternal socioeconomic status mentioned above, our study is also limited by the small sample size. Since we determined the mode of HIV acquisition in some via patient report, there is the possibility of misclassification bias. Our study lacked power to assess effects of specific ARVs administered in utero on postnatal infant growth. Lastly, we were not able to assess diet or feeding habits in the first months of life.
In conclusion, because infants of PHIV women may remain at persistently decreased lengths in the first year of life, careful monitoring of these infants is essential. Further studies aimed at understanding both intrauterine and external environmental factors in PHIV women are warranted.
Supplementary Material
Acknowledgements
We thank all patients and staff at MSH and JHU obstetric and pediatric HIV clinics.
JJ received salary support from the National Institute of Child Health and Human Development 1K23HD070760-01A1 during the preparation of this manuscript. AA received salary support from the National Institutes of Allergy and Infectious Diseases 1K23AI084549 during the preparation of this manuscript.
REFERENCES
- 1.Dollfus C, Le Chenadec J, Faye A, Blanche S, Briand N, Rouzioux C, et al. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10) Clin Infect Dis. 2010;51:214–224. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
- 2.Abrams EJ, Weedon J, Bertolli J, Bornschlegel K, Cervia J, Mendez H, et al. New York City Pediatric Surveillance of Disease Consortium Aging cohort of perinatally human immunodeficiency virus-infected children in New York City. Pediatr Infect Dis J. 2001;20:511–517. doi: 10.1097/00006454-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kapogiannis BG, Soe MM, Nesheim SR, Abrams EJ, Carter RJ, Farley J, et al. Mortality Trends in the US Perinatal AIDS Collaborative Transmission Study (1986-2004) Clin Infect Dis. 2011;53:1024–1034. doi: 10.1093/cid/cir641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang S, Dunkley-Thompson J, Tang Y, Macklin EA, Steel-Duncan J, Singh-Minott I, et al. Deficiency of HIV-Gag-specific T cells in early childhood correlates with poor viral containment. J Immunol. 2008;181:8103–8111. doi: 10.4049/jimmunol.181.11.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 6.Mellins CA, Malee KM. Understanding the mental health of youth living with perinatal HIV infection: lessons learned and current challenges. J Int AIDS Soc. 2013;16:18593. doi: 10.7448/IAS.16.1.18593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenig LJ, Nesheim S, Abramowitz S. Adolescents with perinatally acquired HIV: emerging behavioral and health needs for long-term survivors. Curr Opin Obstet Gynecol. 2011 doi: 10.1097/GCO.0b013e32834a581b. [DOI] [PubMed] [Google Scholar]
- 8.Laughton B, Cornell M, Boivin M, Van Rie A. Neurodevelopment in perinatally HIV-infected children: a concern for adolescence. J Int AIDS Soc. 2013;16:18603. doi: 10.7448/IAS.16.1.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlow-Mosha L, Eckard AR, McComsey GA, Musoke PM. Metabolic complications and treatment of perinatally HIV-infected children and adolescents. J Int AIDS Soc. 2013;16:18600. doi: 10.7448/IAS.16.1.18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jao J, Sigel KM, Chen KT, Rodriguez-Caprio G, Posada R, Shust G, et al. Small for gestational age birth outcomes in pregnant women with perinatally acquired HIV. AIDS. 2012 doi: 10.1097/QAD.0b013e328351f6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crane S, Sullivan M, Feingold M, Kaufman GE. Successful pregnancy in an adolescent with perinatally acquired human immunodeficiency virus. Obstet Gynecol. 1998;92:711. doi: 10.1016/s0029-7844(98)00292-0. [DOI] [PubMed] [Google Scholar]
- 12.Kenny J, Williams B, Prime K, Tookey P, Foster C. Pregnancy outcomes in adolescents in the UK and Ireland growing up with HIV. HIV Med. 2011 doi: 10.1111/j.1468-1293.2011.00967.x. [DOI] [PubMed] [Google Scholar]
- 13.Williams SF, Keane-Tarchichi MH, Bettica L, Dieudonne A, Bardeguez AD. Pregnancy outcomes in young women with perinatally acquired human immunodeficiency virus-1. Am J Obstet Gynecol. 2009;200:149 e141–145. doi: 10.1016/j.ajog.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Thorne C, Townsend CL, Peckham CS, Newell ML, Tookey PA. Pregnancies in young women with vertically acquired HIV infection in Europe. AIDS. 2007;21:2552–2556. doi: 10.1097/QAD.0b013e3282f08b5f. [DOI] [PubMed] [Google Scholar]
- 15.Cruz ML, Cardoso CA, Joao EC, Gomes IM, Abreu TF, Oliveira RH, et al. Pregnancy in HIV vertically infected adolescents and young women: a new generation of HIV-exposed infants. AIDS. 2010;24:2727–2731. doi: 10.1097/QAD.0b013e32833e50d4. [DOI] [PubMed] [Google Scholar]
- 16.Agwu AL, Jang SS, Korthuis PT, Araneta MR, Gebo KA. Pregnancy incidence and outcomes in vertically and behaviorally HIV-infected youth. JAMA. 2011;305:468–470. doi: 10.1001/jama.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips UK, Rosenberg MG, Dobroszycki J, Katz M, Sansary J, Golatt MA, et al. Pregnancy in women with perinatally acquired HIV-infection: outcomes and challenges. AIDS Care. 2011:1–7. doi: 10.1080/09540121.2011.554643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munjal ID J, Fakioglu E, Rosenberg M, Wiznia AA, Katz M, Steiner A, Sansary J, Heo M, Abadi J. Impact of HIV-1 infection and pregnancy on maternal health: comparison between perinatally and behaviorally infected young women. Adolescent Health, Medicine and Therapeutics. 2013;4:51–58. doi: 10.2147/AHMT.S39885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stein AD, Wang M, Martorell R, Norris SA, Adair LS, Bas I, et al. Growth patterns in early childhood and final attained stature: data from five birth cohorts from low- and middle-income countries. Am J Hum Biol. 2010;22:353–359. doi: 10.1002/ajhb.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129:1555–1562. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- 21.Heinonen K, Raikkonen K, Pesonen AK, Kajantie E, Andersson S, Eriksson JG, et al. Prenatal and postnatal growth and cognitive abilities at 56 months of age: a longitudinal study of infants born at term. Pediatrics. 2008;121:e1325–1333. doi: 10.1542/peds.2007-1172. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri AP, Cremona V. The effect of parity on birthweight. J Obstet Gynaecol Br Commonw. 1970;77:145–147. doi: 10.1111/j.1471-0528.1970.tb03493.x. [DOI] [PubMed] [Google Scholar]
- 23.WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization; Geneva: 2006. [Google Scholar]
- 24.Alexander GR, Kogan M, Bader D, Carlo W, Allen M, Mor J. US birth weight/gestational age-specific neonatal mortality: 1995-1997 rates for whites, hispanics, and blacks. Pediatrics. 2003;111:e61–66. doi: 10.1542/peds.111.1.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albertsson-Wikland K, Karlberg J. Natural growth in children born SGA with and without catch up growth. Horm Res. 2003;59(Suppl 1):129. doi: 10.1159/000067839. [DOI] [PubMed] [Google Scholar]
- 26. [last accessed 12/13/09]; http://hivinsite.ucsf.edu/global?page=cr09-cm-00.
- 27.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125:e214–224. doi: 10.1542/peds.2009-0913. [DOI] [PubMed] [Google Scholar]
- 28.Koenig LJ, Espinoza L, Hodge K, Ruffo N. Young, seropositive, and pregnant: epidemiologic and psychosocial perspectives on pregnant adolescents with human immunodeficiency virus infection. Am J Obstet Gynecol. 2007;197:S123–131. doi: 10.1016/j.ajog.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Brogly SB, Watts DH, Ylitalo N, Franco EL, Seage GR, 3rd, Oleske J, et al. Reproductive health of adolescent girls perinatally infected with HIV. Am J Public Health. 2007;97:1047–1052. doi: 10.2105/AJPH.2005.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chibber R, Khurranna A. Birth outcomes in perinatally HIV-infected adolescents and young adults in Manipur, India: a new frontier. Arch Gynecol Obstet. 2005;271:127–131. doi: 10.1007/s00404-003-0564-z. [DOI] [PubMed] [Google Scholar]
- 31.Badell ML, Lindsay M. Thirty years later: pregnancies in females perinatally infected with human immunodeficiency virus-1. AIDS Res Treat. 2012;2012:418630. doi: 10.1155/2012/418630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.From the Center of Disease Control and Prevention Pregnancy in perinatally HIV-infected adolescents and young adults--Puerto Rico, 2002. JAMA. 2003;289:1496–1497. doi: 10.1001/jama.289.12.1496. [DOI] [PubMed] [Google Scholar]
- 33.Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989-2004. Pediatrics. 2007;119:e900–906. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 34.Haeri S, Shauer M, Dale M, Leslie J, Baker AM, Saddlemire S, et al. Obstetric and newborn infant outcomes in human immunodeficiency virus-infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol. 2009;201:315 e311–315. doi: 10.1016/j.ajog.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P. International Small for Gestational Age Advisory Board consensus development conference statement: management of short children born small for gestational age, April 24-October 1, 2001. Pediatrics. 2003;111:1253–1261. doi: 10.1542/peds.111.6.1253. [DOI] [PubMed] [Google Scholar]
- 36.Albertsson-Wikland K, Boguszewski M, Karlberg J. Children born small-for-gestational age: postnatal growth and hormonal status. Horm Res. 1998;49(Suppl 2):7–13. [PubMed] [Google Scholar]
- 37.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38:733–739. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Barker DJ. Rise and fall of Western diseases. Nature. 1989;338:371–372. doi: 10.1038/338371a0. [DOI] [PubMed] [Google Scholar]
- 39.Crookston BT, Schott W, Cueto S, Dearden KA, Engle P, Georgiadis A, et al. Postinfancy growth, schooling, and cognitive achievement: Young Lives. Am J Clin Nutr. 2013 doi: 10.3945/ajcn.113.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, et al. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98:1170–1178. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.