Abstract
Objective
To determine whether cognitive impairment and brain injury as measured by proton magnetic resonance spectroscopy (MRS) persist in the setting of highly active antiretroviral therapy (HAART).
Design
This study is an observational cohort study.
Methods
MRS was performed in 268 patients: HIV-negative controls (N=28), HIV-positive neuroasymptomatic (NA) subjects (N=124), and subjects with AIDS Dementia Complex (ADC; N=50) on stable ART with a mean duration of infection of 12 years and CD4 of 309 cells/mm3. Four metabolites were measured over creatine (Cr): N-acetyl aspartate (NAA), marker of neuronal integrity; Choline (Cho), myoinositol (MI), markers of inflammation, and glutamate and glutamine (Glx) in the basal ganglia (BG), frontal white matter (FWM) and mid-frontal Cortex (MFC). Analyses included ANOVA, ANCOVA, linear and nonparametric regression models.
Results
Cognitive impairment was found in 48% of HIV infected subjects. Both HIV positive groups showed significant increases in MI/Cr or Cho/Cr in all brain regions when compared to controls; a significant decrease in Glx/Cr in the FWM was observed in the NA group; only ADC subjects showed a significant reduction in NAA/ Cr although a significant trend for decreasing NAA/Cr in the BG was found across the groups. Effects related to aging and duration of infection but not central nervous system penetration effectiveness (CPE) were observed.
Conclusions
Brain inflammatory changes remain ubiquitous among HIV-infected subjects whereas neuronal injury occurs predominantly in those with cognitive impairment. Together these findings indicate that despite the widespread use of HAART, HIV-associated cognitive impairment and brain injury persist in the setting of chronic and stable disease.
Keywords: Neuroimaging, HIV dementia, Magnetic Resonance Spectroscopy, antiretroviral therapies
Background
HIV infection is well known to cause neurological damage resulting in cognitive and behavioral impairments that comprise the AIDS dementia complex (ADC), also referred to as HIV-associated neurocognitive disorder (HAND) [1–3]. The introduction of highly active antiretroviral therapy (HAART) or combined antiretroviral therapy (cART) has resulted in marked improvement in survival with a substantial increase in the number of asymptomatic infected patients with improved immunological status [3,4]. Despite the reported systemic and cognitive benefits, the effects of HAART on neurological function have remained uncertain, and specifically, whether HIV-infected patients who are otherwise asymptomatic can develop brain pathology and cognitive impairment in the setting of chronic and stable disease [5–8]. Preliminary data suggest that HIV may continue to affect the brain even in the presence of HAART [9–14]. Coupled with increased survival and an aging patient population, the potential persistence of brain pathology associated with HIV infection could result in an increase in the prevalence of impairment in the chronically infected and treated population.
Proton magnetic resonance spectroscopy (1H-MRS) provides a sensitive and noninvasive in vivo method to detect inflammatory and neuronal changes in the brain [15–29]. Such changes are reflected in levels of specific cerebral metabolites, including N -acetyl aspartate (NAA), choline (Cho), myoinositol (MI), glutamate and glutamine (Glx). Prior to the routine use of cART, a number of MRS-derived abnormalities were describing including reduced levels in the ratio of NAA to total creatine (NAA/Cr), a marker of neuronal metabolism, particularly among patients with moderate to severe ADC [15–17; 21–25] while elevations in the Cho compounds (and Cho/Cr), considered markers of cell membrane damage and MI/Cr, a glial cell marker, were reported at all stages of HIV [14–25, 29]. Earlier work by these authors identified increases in MI/Cr in the frontal white matter as the primary abnormality among neurologically asymptomatid (NA) subjects while those with cognitive impairment showed more diffuse inflammatory changes with evidence of neuronal injury [22]. In a subsequent study based on a factor analysis, basal ganglia and neuronal factors were identified as critical determinants of ADC [25, 27], consistent with the ex vivo findings [30–32].
To date there have been no studies that have demonstrated the pattern or extent of brain injury in chronically infected patients on cART, particularly among NA subjects and more specifically whether the previously described patterns of brain pathology have changed in response to such treatment. The HIV Neuroimaging Consortium (HIVNC) was formed to examine this issue in a prospective multicenter study of a large cohort of 300 chronically HIV-infected subjects on stable cART. Participants undergo comprehensive neurocognitive, biomarker and imaging assessments that include both volumetric imaging and magnetic resonance spectroscopy across seven centers. We hypothesized that HIV-infected patients with a history of chronic and stable disease on cART would continue to show patterns of brain injury similar to those observed in the pre-HAART era. Such findings would suggest that HIV infection ether through direct or indirect mechanisms remains active in the brain in the setting of chronic and stable disease and would pose a continued challenge in the management of these patients. In this report we described the findings from the multicenter MRS study. We have recently described evidence of volumetric loss based on structural imaging analysis in a subset of these patients [33].
Methods
Clinical Assessments
Patients were enrolled a longitudinal study of brain injury and neurocognitive functioning at the following sites: UCSD, UCLA, Harbor-UCLA, Stanford, Colorado, Pittsburgh, and Rochester. Inclusion criteria included: Nadir CD4 count < 200 cells/mm3; stable antiretroviral (ARV) regimen with any FDA-approved therapy (for at least 12 consecutive weeks prior to study screening); hemoglobin > 9.0 gm/dL; serum creatinine ≤ 3 × upper limit of normal (ULN); AST (SGOT), ALT (SGPT), and alkaline phosphatase ≤ 3 × ULN. Exclusion criteria included severe premorbid or comorbid psychiatric disorders, confounding neurologic disorders such as chronic seizures, stroke, head trauma resulting in loss of consciousness> 30 minutes, multiple sclerosis, brain infection (except for HIV), or brain neoplasms, including CNS lymphoma; active alcohol and drug abuse or related medical complications within 6 months of study; diabetes mellitus with a fasting glucose > 140. Assessments include magnetic resonance imaging and spectroscopy, a neurological and neuropsychological assessments, plasma and CSF assays for HIV RNA. Cognitive status was assessed using the ADC Staging as previously described [2] and patients were assessed on both clinical and neuropsychological tests and rated as no impairment, stage 0; subclinical impairment, 0.5; mild, stage 1; moderate, stage 2; or severe impairment, stage 3. Neuropsychological impairment was defined as performance of at least 1.0 standard deviation below normative values on two or more neuropsychological tests or at least 2.0 standard deviations below normative values on one or more tests [20]. Subsequent to the inception of this study, Antinori and colleagues published a new classification in which ADC stage 0.5 and 1 would correspond to mild cognitive disorder or mild neurocognitive disorder (MND) and ADC stage 2 or greater would correspond to HIV associated Dementia or HAD. As there were no differences in MRS metabolites between ADC stage 0 and 0.5, these groups were combined for analysis [3]. Institutional review boards at each site approved the protocol. All participants gave written informed consent for the protocol. All the authors vouch for the accuracy and completeness of the data reported.
MRS methods
Single-voxel 1H spectra were acquired using a customized version of the GE PRESS sequence. Voxels 6 cc in volume were prescribed in 3 regions: midline frontal gray matter, right (or left) mid-frontal centrum semiovale (white matter), and right (or left) basal ganglia (deep gray matter). The basal ganglia and white matter are typically involved in HIV infection of the brain [30–32], while the frontal gray matter was chosen as an indicator of the extent of cortical involvement. Field homogeneity and water suppression were adjusted using automated algorithms from GE. Water suppressed spectra were collected with TE/TR = 35/3000 ms, bandwidth = 2500 Hz, 128 averages, NEX=8To control for a possible instrument phantoms containing fixed concentrations of the metabolites, were obtained at the time of the subject evaluation. The time domain spectral data were transferred via file transfer protocol (FTP) to an FTP server at the central MRS processing site, John A. Burns School of Medicine, University of Hawaii. The metabolite ratios NAA/Cr, Cho/Cr, MI/Cr, and Glx (=Glu+Gln)/Cr were determined using the LC Model spectral analysis software and an unsuppressed water FID at TE=35 ms for eddy-current correction. This automated processing method yields standard deviations of metabolite ratios < 15% [24].
Statistical Methods
A linear regression model was fitted to the phantom measurements to obtain site-specific estimates, which were then used as adjustments to the MRS metabolite ratios. Baseline demographic and clinical covariates were assessed for group differences by the Kruskal-Wallis test for continuous data, and the Mantel-Haenszel test for categorical data. Analysis of Covariance (ANCOVA) was performed adjusting for the effects of age and sex. To assess for the possible confounding effects of race (control 96% white compared to 74% white in the HIV infected group), a sensitivity analyses including only subjects with a self-reported white race was done. Differences were qualitatively the same as for the whole sample and the effect sizes were consistent with the overall comparisons. Within each brain region and metabolite ratio, the Tukey procedure was used to adjust for multiple comparisons. For the 12 comparisons performed across the 3 brain regions and 4 metabolite ratios, the Bonferroni adjustment was done at the 0.0042 (=0.05/12) level of significance. A trend analysis was performed to assess incremental increases or decreases in the metabolite ratios across the three HIV-positive groups by applying a linear regression model to the ordinal subject group data ranked according to progressive ADC stage. Analyses of the effects of age on the metabolite ratios were done using nonparametric additive regression models. All analyses were carried out in R-2.9.2 (R Core Development System: http://www.r-project.org).
The central nervous system penetration effectiveness score (CPE), a measure of the relative effectiveness of ARV regimens to cross the blood brain barrier, was derived as previously described from an algorithm that considers the effectiveness of each agent in the antiretroviral regimen to cross the blood brain barrier as follows : 0=lowest penetration, 0.5=intermediate penetration, 1=highest penetration) [34]. Regimens of ARV drugs were also categorized into groups based on the number and mechanism of action of the component ARV drugs: (1) nonnucleoside reverse transcriptase inhibitor (NNRTI)–based regimens included at least 1 NNRTI with at least 2 other ARVs and no protease inhibitors; (2) protease inhibitor–based regimens included at least 1 protease inhibitor with at least 2 other ARVs and no NNRTIs; (3) other regimens containing any number of drugs.
Results
Subject characteristics
Summary statistics are provided for subjects having at least one out of nine metabolite ratios available and at least one neurological evaluation. As gender and age were significantly different between HIV-negative controls and HIV-infected subjects (Table 1) all subsequent analyses were adjusted for these two factors in the linear regression models. As a group, HIV infected subjects showed a median age of 47 years with a median duration of HIV infection of 12 years, including 59% infected for more than 10 years and 9% for over 20 years (Table 2). The median nadir CD4 count was 34 cells/mm3, but the baseline count was 309 cells/mm3, and the median CPE score was 1.5, comparable to scores previously reported [27]. Nearly 52% of subjects were cognitively normal and 48% were cognitive impaired, including 28% with sub-clinical impairment and 20% with definite cognitive impairment as defined by ADC stages 1–3 [2].
Table 1.
Demographic characteristics of the subject population.
| ADC stage
|
||||||
|---|---|---|---|---|---|---|
| HIV-negative controls N=28 |
0 N=124 |
0.5 N=66 |
1+ N=50 |
Total N=268 |
P-value | |
|
| ||||||
| Male (%) | 10 (36%) | 103 (83%) | 56 (85%) | 45 (90%) | 214 (80%) | <0.001 |
|
| ||||||
| Age (years) | 0.001 | |||||
| Median (IQR) | 53.0 (44.5–61.2) |
44.5 (40.0–52.0) |
48.5 (43.2–53.0) |
47.0 (43.0–57.0) |
47.0 (42.0–53.2) |
|
|
| ||||||
| Race/ethnicity (n=266) | 0.0096 | |||||
| White | 25 | 93 | 41 | 37 | 196 | |
| Non-white | 1 | 31 | 25 | 13 | 70 | |
|
| ||||||
| Education level (n=266) | 0.264 | |||||
| High School or less | 11 | 46 | 34 | 19 | 110 | |
| Some College or more | 15 | 78 | 32 | 31 | 156 | |
Table 2.
HIV-related characteristics assessed at baseline on all HIV-positive subjects
| ADC stage
|
|||||
|---|---|---|---|---|---|
| 0 N=124 |
0.5 N=66 |
1+ N=50 |
Total N=240 |
P-value | |
|
| |||||
| IV drug use, n (%) | 18 (15) | 21 (32) | 20 (40) | 59 (25) | 0.0001 |
|
| |||||
| HIV infection (years) | 0.093 | ||||
| Median (IQR) | 12.0 (7.0–17.2) | 11.0 (7.0–16.0) | 13.5 (10.0–17.0) | 12.0 (7.0–17.0) | |
|
| |||||
| CD4 count (cells/mm3) | 0.447 | ||||
| Median(IQR) | 312 (188–466) | 330 (215–510) | 269 (130–455) | 309 (187–471) | |
|
| |||||
| Nadir CD4 (cells/mm3) | 0.943 | ||||
| Median(IQR) | 31 (12–82) | 49 (19–83) | 22 (7–90) | 34 (12–84) | |
|
| |||||
| Plasma viral load (n=235) | 0.453 | ||||
| Undetectable, n (%) | 96 (79) | 47 (72) | 34 (71) | 177 (75) | |
|
| |||||
| CSF viral load (n=96) | 0.208 | ||||
| Undetectable, n (%) | 24 (62) | 19 (73) | 25 (81) | 68 (71) | |
|
| |||||
| Known on ART at baseline, n (%) | 112 (90) | 58 (88) | 49 (98) | 219 (91) | 0.140 |
|
| |||||
| Anti-HIV drug class, n (%) | 0.019 | ||||
| ART unknown | 12 (10) | 8 (12) | 1 (2) | 21 (9) | |
| ART including PI | 66 (53) | 42 (64) | 34 (68) | 142 (59) | |
| ART without PI | 40 (32) | 13 (20) | 8 (16) | 61 (25) | |
| Other combination | 6 (5) | 3 (5) | 7 (14) | 16 (7) | |
|
| |||||
| CPE score (n=220) | 0.501 | ||||
| Median(IQR) | 1.5 (1.0–2.5) | 1.5 (1.0–2.5) | 1.5 (1.5–2.5) | 1.5 (1.0–2.5) | |
Comparison of MRS metabolite ratios between HIV-infected subjects and HIV-negative controls
Phantom evaluation was done to remove possible variability related to site differences in imaging acquisition. As the differences between the HIV-negative and HIV-infected subjects remained qualitatively the same, results from the unadjusted analyses are presented here. In the majority of cases, metabolite ratios from each of the three HIV-infected groups, including NA subjects, were significantly different from the HIV-negative controls (Figure 1, Tables 3 and 4). Notably, MI/Cr in all three regions of the brain was significantly higher in all HIV-infected groups compared to the HIV-negative group whereas Cho/Cr was significantly higher in the basal ganglia and mid-frontal cortex. Subjects with sub-clinical impairment (ADC stage 0.5) generally showed the same pattern as the NA group. In contrast, NAA/Cr in the basal ganglia and frontal white matter was significantly reduced only in subjects with cognitive impairment (ADC stage ≥1) when compared to HIV-negative controls. Glx/Cr ratio in the frontal white matter was lower in all HIV groups but reached statistical significance only in the NA group.
Figure 1.
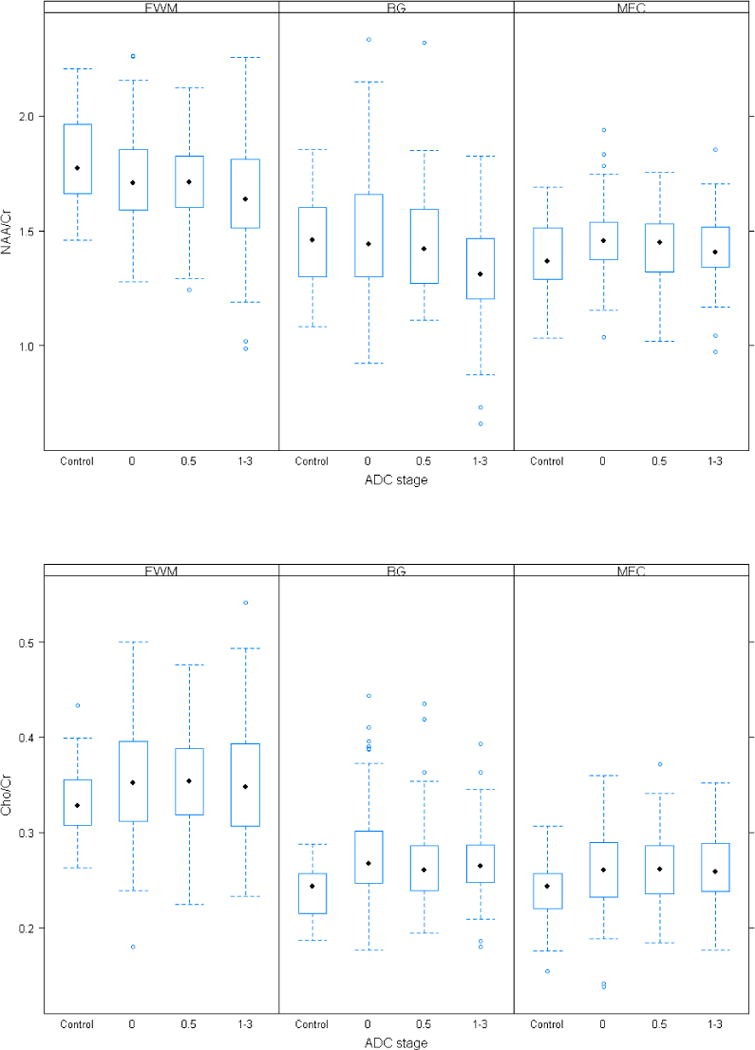
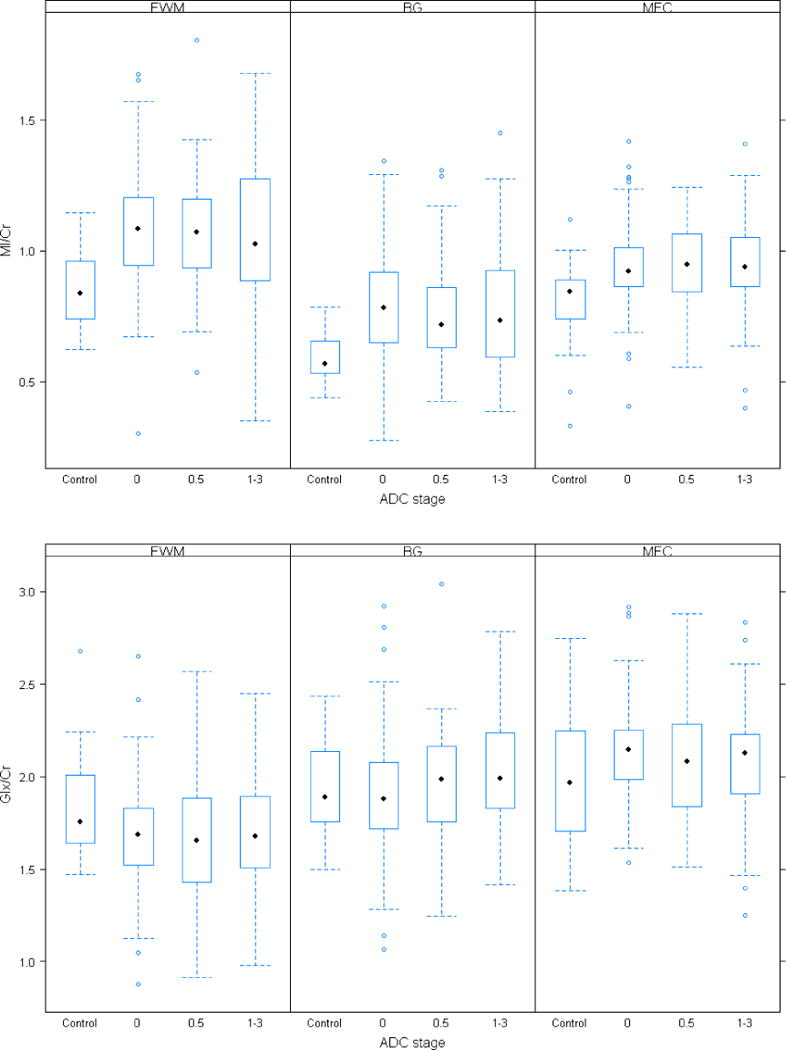
Boxplots of MRS metabolites are shown for the control and the three patient subgroups (NA, ADC stage 0.5 and ADC stage > 1) and for three regions of interest (BG, FWM, MFC). Black dots depict the median metabolite level and the box is the 1st quartile (lower end) and the 3rd quartile (upper end) of the metabolite level distribution.
Table 3.
Metabolite ratios across groups. 1=NEG vs. NA, 2=NEG vs. ADC 0.5, 3=NEG vs. ADC 1–3. Values listed are only those that are statistically significant at either 0.05 or the 0.05/12 level. Comparisons with p-values < 0.05 are listed in regular type and the Bonferroni adjusted (i.e., p-value < 0.0042) are in bold type.
| ADC stage | |||||
|---|---|---|---|---|---|
|
|
|||||
| HIV-negative controls N=28 Mean (SD) |
0 N=124 Mean (SD) |
0.5 N=66 Mean (SD) |
1+ N=50 Mean (SD) |
p-value | |
|
| |||||
| Basal Ganglia | |||||
| NAA/Cr | 1.45 (0.19) | 1.48 (0.25) | 1.46 (0.24) | 1.32 (0.25) | 3, * |
| Cho/Cr | 0.24 (0.03) | 0.28 (0.05) | 0.27 (0.05) | 0.27 (0.04) | 1, 2, 3 |
| MI/Cr | 0.59 (0.10) | 0.80 (0.19) | 0.75 (0.18) | 0.77 (0.24) | 1, 2, 3 |
| Glx/Cr | 1.92 (0.37) | 1.93 (0.26) | 1.96 (0.29) | 2.02 (0.35) | |
|
| |||||
| Frontal White Matter | |||||
| NAA/Cr | 1.80 (0.20) | 1.72 (0.20) | 1.71 (0.18) | 1.65 (0.26) | 3, * |
| Cho/Cr | 0.33 (0.04) | 0.35 (0.06) | 0.35 (0.05) | 0.35 (0.07) | |
| MI/Cr | 0.85 (0.14) | 1.09 (0.21) | 1.07 (0.20) | 1.09 (0.31) | 1, 2, 3 |
| Glx/Cr | 1.84 (0.29) | 1.69 (0.29) | 1.69 (0.34) | 1.68 (0.33) | 1 |
|
| |||||
| Mid-frontal cortex | |||||
| NAA/Cr | 1.39 (0.17) | 1.46 (0.14) | 1.44 (0.15) | 1.41 (0.16) | 1, * |
| Cho/Cr | 0.24 (0.04) | 0.26 (0.04) | 0.26 (0.04) | 0.26 (0.04) | 1, 2, 3 |
| MI/Cr | 0.80 (0.16) | 0.94 (0.15) | 0.94 (0.15) | 0.94 (0.19) | 1, 2, 3 |
| Glx/Cr | 2.01 (0.37) | 2.14 (0.26) | 2.07 (0.29) | 2.08 (0.35) | |
is used for the trend analysis within the HIV-positive group
Table 4.
Summary of the metabolite ratio differences amongst 4 groups: HIV−, NA, Subclinical and ADC. Comparisons with p-values < 0.05 are listed in regular type and the Bonferroni adjusted (i.e., p-value < 0.0042) are in bold type.
| NA vs HIV negative | ||
| ↑ NAA/Cr | MFC | |
| ↑ Cho/Cr | BG, MFC | |
| ↑ MI/Cr | BG, FWM, MFC | |
| ↓ Glx/Cr | FWM | |
| Subclinical vs. HIV negative | ||
| ↑ Cho/Cr | BG, MFC | |
| ↑ MI/Cr | BG, FWM, MFC | |
| ADC vs HIV negative | ||
| ↓ NAA/Cr | BG, FWM | |
| ↑ Cho/Cr | BG, MFC | |
| ↑ MI/Cr | BG, FWM, MFC | |
| ADC vs NA | ||
| ↓ NAA/Cr | BG | |
| Trend analysis within HIV+ group | ||
| ↓ NAA/Cr | BG, FWM, MFC |
Comparison among HIV-infected groups
We then compared the metabolite ratios among the HIV-infected subjects with varying levels of cognitive impairment in the three regions of interest. Levels for Cho/Cr and MI/Cr did not differ among the three groups. In contrast, NAA/Cr levels in the BG were significantly lower in the advanced ADC group compared to the NA group.
To further assess how metabolite levels change with respect to cognitive impairment, a trend analysis was performed in the HIV positive group (Table 3). A prominent negative trend in the NAA/Cr was found in the BG (p=0.001) with the neuroasymptomatic group showing the highest NAA/Cr levels, while levels progressively decreased with ADC stage. Weaker negative trends were observed in the frontal white matter and mid-frontal cortex, but did not reach statistical significance after correction for multiple comparisons. Consistent with the pairwise comparisons, no trends were observed with Cho/Cr or MI/Cr.
Risk Factors Associated with MRS Abnormalities
Given the characteristics of the cohort and recent reports suggesting that age and treatment may play a role in certain cognitive and systemic complications [14], we postulated that age, duration of HIV infection and the lack of CNS penetrating ARV agents would correlate with greater inflammation (higher Cho/Cr and MI/Cr) and neuronal injury (lower NAA/Cr). Significant age-related effects were noted for Glx/Cr, MI/Cr and NAA/ Cr, but these results varied depending on the metabolite (Figure 2). All groups, including HIV-negative controls, showed similar declines in NAA/Cr in the MFC with increasing age (p-value < 0.0001). In contrast, a significant HIV-by-age interaction (p-value = 0.0004) was observed with respect to MI/Cr in the FWM which increased with age in the NA and ADC stage 0.5 groups but unexpectedly decreased with age among subjects with ADC ≥1 while these levels remained relatively unchanged in controls. A significant HIV-by-age interaction effect (p-value = 0.0026) was also observed for Glx/Cr in the FWM across all HIV-positive groups, independent of cognitive status. Further analysis showed that an HIV-positive subject at age 30 years would have the same value as HIV-negative subject at age 56 years. A significant interaction with duration of HIV infection was found for MI/Cr levels in the basal ganglia such that subjects with subclinical neurological disease (ADC 0.5) displayed decreased MI/Cr levels with duration; those with ADC stage 1 or greater displayed an inverted “U” relationship, i.e. increased levels which then decreased with duration while NA subjects showed an increased in MI/Cr (p-value=0.002). Duration was also associated with decreasing NAA/Cr levels in the MFC regardless of cognitive status (p-value=0.0065). In contrast, there was no relationship between the CPE rank and any of the metabolite ratios.
Figure 2.
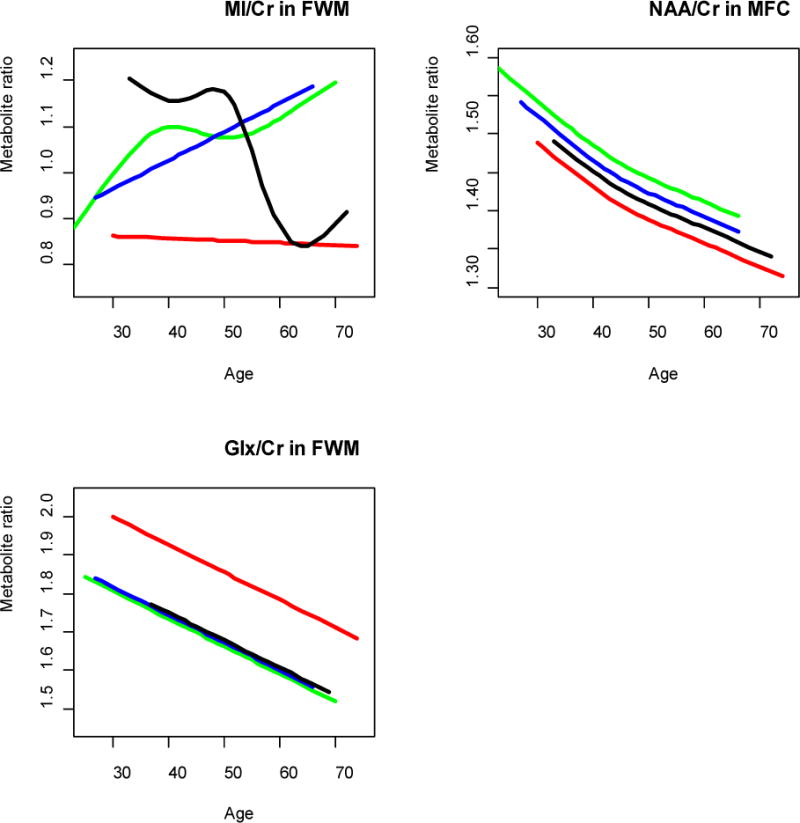
The interaction effects of age and group membership (NA, ADC) were studied using semi-parametric and linear models and the model which best fit the data was selected (see Methods). The colors of the curves indicate different groups: HIV− (red), NA (green), sub-clinical (blue) and ADC (black).
TOP LEFT PANEL: MI/Cr ratio in Frontal White Matter as a function of age, HIV status, ADC stage and their interactions. MI/Cr ratio in the HIV− group is consistently the lowest at all ages, the NA and subclinical groups exhibit increasing MI/Cr ratio as a function of age, while the ADC group shows a decreasing MI/Cr ratio trend as a function of age.
TOP RIGHT PANEL: NAA/Cr ratio in mid-frontal cortex as a function of age, HIV status and ADC stage. All groups show a decreasing NAA/Cr trends as a function of age.
BOTTOM LEFT PANEL: Glx/Cr ratio in frontal white matter as a function of age, HIV status, ADC stage and their interactions. All groups show a decreasing Glx/Cr trend as a function of age with the HIV− group consistently higher than the HIV+ groups.
Discussion
This study aimed to determine whether patterns of brain injury as measured by MRS have changed in response to cART in a chronically infected and aging cohort of 268 subjects. The cohort was unique and different from those previously described due to its size (N= 240), age (40% older than 50 years), and duration of infection (60% were infected more than 10 years and nearly 10% more than 20 years), reflecting the established benefits of ART on survival. Several studies have reported that despite these benefits, subjects are facing a number of complications related to the effects of aging, chronic immune activation or treatment, including lipodystrophies, metabolic disorder and cardiovascular disease [35, 36]. More recently, there is some evidence suggesting that chronically infected yet stable subjects may be developing cognitive impairment [37, 38]. Therefore it is noteworthy that nearly 50% of our subjects showed some degree of cognitive impairment ranging from subclinical impairment in 30% to frank impairment in the remaining subjects. Further prominent increases in Cho/Cr and MI/Cr, markers of inflammation, were found in all HIV-positive groups, including neurologically asymptomatic subjects, when compared to HIV negative controls while a significant decrease in NAA/Cr, indicative of neuronal injury, was detected only among ADC patients. Combined, the current results indicate that despite effective and lasting ARV treatments, cognitive and metabolite impairments, indicative of inflammation and neuronal injury, persist in the setting of chronic and stable HIV infection. Furthermore, the pattern of MRS findings has remained remarkably consistent with those observed prior to the widespread use of cART [23–25]. The results therefore provide compelling evidence that the early treatment benefits following the introduction of cART either do not persist or do not affect brain injury to the extent anticipated and should renew concern about the possibility of resurgence in HIV-associated brain pathology and cognitive impairment in subjects who are otherwise stable on ARV treatment.
Brain Inflammation in HIV
Neuropathological studies have consistently demonstrated that HIV is associated with gliosis and mononuclear cell infiltration with a particular predilection for the basal ganglia and white matter pathways [30–32]. Significant elevations in Cho and MI reflective of these inflammatory responses have been previously observed in the same regions but less often in the cortical gray matter [16–20]. The ubiquitous presence of an inflammatory response as shown in this study continues to support prevailing models of HIV neuropathogenesis, suggesting that chronic inflammation, possibly as a result of chronic immune activation, plays a critical role in HIV-associated brain injury [31, 32, 43, 44]. The lack of further increases in inflammation with worsening cognitive impairnent suggests that a threshold level of inflammation is reached during early stages of infection. Alternatively, this finding could in part reflect the effects of decreases in inflammation with either duration of infecton or aging among ADC subjects (see below). Prominent involvement of the mid-frontal cortex suggests that the pattern of brain injury may be evolving from a predominantly subcortical disorder as previously described to one which, in the setting of chronic disease and cART, now prominently includes the cortex, consistent with our own recent findings of volumetric loss and ventricular enlargement in these patients [33]. Future studies will need to address the effects of this evolving pattern of cortical injury on cognitive performance.
Neuronal Injury in HIV
Significant neuronal loss, as well as damage to the synaptic dendritic tree, is a well described feature in brains of HIV-infected patients [40–42]. It is of interest then that in the current study, reduced levels in NAA/Cr, reflecting these events, were found among patients with cognitive impairment (ADC stage ≥1). Further, the trend analysis showed decreasing levels, predominantly in the basal ganglia, suggesting that neuronal injury may also be present at subclinical stages, possibly in subjects who may be at risk for cognitive impairment. When combined with the results from the Cho/Cr and MI/Cr analysis, these findings support a two-stage model of brain injury, which suggests that inflammation during the subclinical stages of HIV infection is followed by decreases in neuronal function, which eventually lead to cognitive impairment in susceptible individuals. These studies also support the notion that a decrease in NAA/Cr may represent a critical event in HIV neuropathogenesis and provide a sensitive and useful in vivo biomarker for cognitive impairment in the setting of chronic and stable disease (29).
Neurotoxicity in HIV
Glutamate and glutamine levels were assessed via the Glx peak on MRS. Glial cells and neurons are believed to be the primary sources of this metabolite, thus disturbances could reflect dysfunction in one or both elements [28]. Past studies have suggested that increases in glutamate resulting from glial cell dysfunction may lead to neuronal injury [43, 44]. The neurotoxic hypothesis of HIV-associated brain injury would predict that increases in the Glx peak would parallel decreases in NAA and a decline in cognitive function. However, in contrast to other metabolites, no significant differences in this index were found other than a decrease in the frontal white matter in the neuroasymptomatic group. The basis for this finding is unclear but could reflect damage to neuro-glial elements at earlier stages of infection that antedate decreases in NAA as previously described in the SIV- infected macaque [28].
Persistence and Risk Factors for HIV-associated Brain Injury
The reasons for the persistence of brain injury in the context of stable disease and treatment are unclear. Increases in MI and decreases in NAA have been described in ARV-naive subjects during the early stages of primary HIV infection [22]. Whether such patterns of injury persist in certain individuals or recur following treatment and whether such changes could result in chronic neurological damage over time will need to be addressed in prospective future studies such as those underway in the context of the HIVNC.
There have been no prior studies describing factors that may contribute to in vivo patterns of HIV-associated brain injury. We therefore conducted a preliminary analysis of this issue. Age had a profound effect on certain metabolites, notably MI/Cr and Glx/Cr. Interestingly, GLx/Cr levels approached those observed in older healthy individuals suggesting that HIV infection may be accelerating age-related processes in the brain similar to its effects on the cardiovascular system [35, 36]. The observed decreases in MI/Cr in the FWM with age and in the BG with increasing duration of disease may reflect age related disturbances in glial cell metabolism or the effects of chronic “burnt out” brain disease. The effects of chronic ARV treatment, chronic immune activation and comorbidities as well as their interactions also need to be considered. It is noteworthy no relationship between CPE rank and metabolite levels was found suggesting that the use of CNS penetrating agents may have little effect on brain injury associated with HIV infection and may not be as important as effective control of plasma HIV RNA levels. It remains to be determined whether the long term administration of certain antiretroviral agents can result in neurological injury [45, 46].
The current findings indicate that despite the systemic benefits of cART, the brains of HIV-infected individuals continue to show damage in the setting of chronic and stable disease. Ongoing monitoring of neurologic function, even additional therapeutic intervention, may thus be warranted in this population.
Acknowledgments
Yuen T. So, M.D., Ph.D., Janetta Matesan, Scott Letendre, M.D., Thomas Ernst, Ph.D., David Tate, M.D., Mark Brown, Ph.D., Deborah McMahon, M.D., Lisa Siqueiros, Shirley Paulose, Michelle Gaugh, Christine Tripoli, Sally Canmann, Kristin Brousseau, M.D., Laetitia Thompson, Ph.D., Miguel Valdes- Sueiras, Angela Grbic, Edward Lozano and Lisa Gualtieri, National Neurological AIDS Band (NAAB) and UCSD CNTN (California NeuroAIDS Tissue Network)
Sources: NS36524, NS38841, RR025780, U01MH083506, R01NS036524, AI069424, MH083550
Footnotes
Conflicts of Interest: None
Data presented at the Conference on Retroviruses and Opportunistic Infections (CROI) 2009
References
- 1.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19(6):517–24. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 2.Price RW, Brew BJ. The AIDS dementia complex. J Infect Dis. 1988;158(5):1079–83. doi: 10.1093/infdis/158.5.1079. [DOI] [PubMed] [Google Scholar]
- 3.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 5.Ledergerber B, Egger M, Opravil M, Telenti A, Hirschel B, Battegay M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353(9156):863–8. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 6.Ferrando S, van Gorp W, McElhiney M, Goggin K, Sewell M, Rabkin J. Highly active antiretroviral treatment in HIV infection: benefits for neuropsychological function. AIDS. 1998;12(8):F65–70. doi: 10.1097/00002030-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- 8.Tozzi V, Balestra P, Lorenzini P, Bellagamba R, Galgani S, Corpolongo A, et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: Results from an urban observational cohort. J Neurovirol. 2005;11(3):265–273. doi: 10.1080/13550280590952790. [DOI] [PubMed] [Google Scholar]
- 9.Robertson KR, Robertson WT, Ford S, Watson D, Fiscus S, Harp AG, et al. Highly active antiretroviral therapy improves neurocognitive functioning. J Acq Immun Def Synd. 2004;36(1):562–6. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–1253. doi: 10.1097/00002030-199907090-00015. [DOI] [PubMed] [Google Scholar]
- 11.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45(2):174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 12.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–7. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 13.Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 14.Valcour VG, Sacktor NC, Paul RH, Watters MR, Selnes OA, Shiramizu BT, et al. Insulin resistance is associated with cognition among HIV-1-infected patients: the Hawaii Aging With HIV cohort. J Acquir Immune Defic Syndr. 2006;43:405–410. doi: 10.1097/01.qai.0000243119.67529.f5. [DOI] [PubMed] [Google Scholar]
- 15.Neuenburg JK, Brodt HR, Herndier BG, Bickel M, Bacchetti P, Price RW, et al. HIV-related neuropathology, 1985 to 1999: rising prevalence of HIV encephalopathy in the era of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(2):171–7. doi: 10.1097/00126334-200210010-00007. [DOI] [PubMed] [Google Scholar]
- 16.Tracey I, Carr CA, Guimaraes AR, Worth JL, Navia BA, Gonzalez RG. Brain choline-containing compounds are elevated in HIV-positive patients before the onset of AIDS dementia complex: A proton magnetic resonance spectroscopic study. Neurology. 1996;46(3):783–8. doi: 10.1212/wnl.46.3.783. [DOI] [PubMed] [Google Scholar]
- 17.Paley M, Cozzone PJ, Alonso J, Vion-Dury J, Confort-Gouny S, Wilkinson ID, et al. A multicenter proton magnetic resonance spectroscopy study of neurological complications of AIDS. AIDS Res Hum Retroviruses. 1996;12(3):213–22. doi: 10.1089/aid.1996.12.213. [DOI] [PubMed] [Google Scholar]
- 18.Salvan AM, Vion-Dury J, Confort-Gouny S, Nicoli F, Lamoureux S, Cozzone PJ. Brain proton magnetic resonance spectroscopy in HIV-related encephalopathy: identification of evolving metabolic patterns in relation to dementia and therapy. AIDS Res Hum Retroviruses. 1997;13(12):1055–66. doi: 10.1089/aid.1997.13.1055. [DOI] [PubMed] [Google Scholar]
- 19.Lopez-Villegas D, Lenkinski RE, Frank I. Biochemical changes in the frontal lobe of HIV-infected individuals detected by magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1997;94(18):9854–9. doi: 10.1073/pnas.94.18.9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52(5):995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- 21.Chang L, Ernst T, Lionido-Yee M, Walot I, Singer E. Cerebral metabolite abnormalities correlate with clinical severity of HIV-1 cognitive motor complex. Neurology. 1999;52(1):100–8. doi: 10.1212/wnl.52.1.100. [DOI] [PubMed] [Google Scholar]
- 22.Chang L, Ernst T, Witt MD, Ames N, Walot I, Jovicich J, et al. Persistent brain abnormalities in antiretroviral-naive HIV patients 3 months after HAART. Antivir Ther. 2003;8(1):17–26. [PubMed] [Google Scholar]
- 23.Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. Neuroimage. 2004;23(4):1336–47. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- 24.Lee PL, Yiannoutsos CT, Ernst T, Chang L, Marra CM, Jarvik JG, et al. A multi-center 1H MRS study of the AIDS dementia complex: validation and preliminary analysis. J Magn Reson Imaging. 2003;17(6):625–33. doi: 10.1002/jmri.10295. [DOI] [PubMed] [Google Scholar]
- 25.Yiannoutsos CT, Ernst T, Chang L, Lee PL, Richards T, Marra CM, et al. Regional patterns of brain metabolites in AIDS dementia complex. Neuroimage. 2004;23(3):928–35. doi: 10.1016/j.neuroimage.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Schifitto G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21(14):1877–86. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- 27.Yiannoutsos CT, Nakas CT, Navia BA. Assessing multiple-group diagnostic problems with multi-dimensional receiver operating characteristic surfaces: application to proton MR Spectroscopy (MRS) in HIV-related neurological injury. Neuroimage. 2008;40(1):248–55. doi: 10.1016/j.neuroimage.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lentz MR, Kim WK, Lee V, Bazner S, Halpern EF, Venna N, et al. Changes in MRS neuronal markers and T cell phenotypes observed during early HIV infection. Neurology. 2009;72(17):1465–72. doi: 10.1212/WNL.0b013e3181a2e90a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed MA, Lentz MR, Lee V, Halpern EF, Sacktor N, Selnes O, et al. Factor analysis of proton MR spectroscopic imaging data in HIV infection: metabolite-derived factors help identify infection and dementia. Radiology. 2010;254(2):577–86. doi: 10.1148/radiol.09081867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19(6):525–35. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 31.Rostasy K, Monti L, Yiannoutsos C, Kneissl M, Bell J, Kemper TL, et al. Human immunodeficiency virus infection, inducible nitric oxide synthase expression, and microglial activation: pathogenetic relationship to the acquired immunodeficiency syndrome dementia complex. Ann Neurol. 1999;46(2):207–16. [PubMed] [Google Scholar]
- 32.Navia BA, Rostasy K. The AIDS dementia complex: clinical and basic neuroscience with implications for novel molecular therapies. Neurotox Res. 2005;8(1–2):3–24. doi: 10.1007/BF03033817. [DOI] [PubMed] [Google Scholar]
- 33.Cohen RA, Harezlak J, Schifitto G, Hana G, Clark U, Gongvatana A, et al. Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. Journal of Neurovirology. 2010;16(1):25–32. doi: 10.3109/13550280903552420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, et al. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ances BM, Bhatt A, Vaida F, Rosario D, Alexander T, Marquie-Beck J, et al. Role of metabolic syndrome components in human immunodeficiency virus-associated stroke. J of Neurovirology. 2009;15:249–256. doi: 10.1080/13550280902962443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsue PY, Hunt PW, Schnell A, Kalapus SC, Hoh R, Ganz P, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–1067. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valcour VG, Shikuma CM, Shiramizu BT, Williams AE, Watters MR, Poff PW, et al. Diabetes, insulin resistance, and dementia among HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;38:31–36. doi: 10.1097/00126334-200501010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, et al. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. doi: 10.1097/QAD.0b013e32833336c8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiley CA, Masliah E, Morey M, Lemere C, DeTeresa R, Grafe M, et al. Neocortical damage during HIV infection. Ann Neurol. 1991;29(6):651–7. doi: 10.1002/ana.410290613. [DOI] [PubMed] [Google Scholar]
- 40.Masliah E, Achim CL, Ge N, DeTeresa R, Terry RD, Wiley CA. Spectrum of human immunodeficiency virus-associated neocortical damage. Ann Neurol. 1992;32(3):321–9. doi: 10.1002/ana.410320304. [DOI] [PubMed] [Google Scholar]
- 41.Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol. 1993;52(6):561–6. doi: 10.1097/00005072-199311000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. HIV Neurobehavioral Research Center. Ann Neurol. 1997;42(6):963–72. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- 43.Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci U S A. 1999;96(14):8212–6. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 45.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–66. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, et al. Brain mitochondrial injury in human immunodeficiency virus seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol. 2005;11(4):356–64. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]


