Abstract
The cardiovascular system is one of the most characteristic and important targets for developmental toxicity by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in fish larvae. However, knowledge of the mechanism of TCDD-induced edema after heterodimerization of aryl hydrocarbon receptor type 2 (AHR2) and AHR nuclear translocator type 1 (ARNT1) is still limited. In the present study, microscopic analysis with a high-speed camera revealed that TCDD increased the size of a small cavity between the heart and body wall in early eleutheroembryos, a toxic effect that we designate as precardiac edema. A concentration-response curve for precardiac edema at 2 days post fertilization (dpf) showed close similarity to that for conventional pericardial edema at 3 dpf. Precardiac edema caused by TCDD was reduced by morpholino knockdown of AHR2 and ARNT1, as well as by an antioxidant (ascorbic acid). A selective inhibitor of cyclooxygenase type 2 (COX2), NS398, also markedly inhibited TCDD-induced precardiac edema. A thromboxane receptor (TP) antagonist, ICI-192,605 almost abolished TCDD-induced precardiac edema and this effect was cancelled by U46619, a TP agonist, which was not influential in the action of TCDD by itself. Knockdown of COX2b and thromboxane A synthase 1 (TBXS), but not COX2a, strongly reduced TCDD-induced precardiac edema. Knockdown of COX2b was without effect on mesencephalic circulation failure caused by TCDD. The edema by TCDD was also inhibited by knockdown of c-mpl, a thrombopoietin receptor necessary for thromobocyte production. Finally, induction of COX2b, but not COX2a, by TCDD was seen in eleutheroembryos at 3 dpf. These results suggest a role of the COX2b-thromboxane pathway in precardiac edema formation following TCDD exposure in developing zebrafish.
Keywords: cyclooxygenase, Danio rerio, developmental toxicology, edema, TCDD, thromboxane
1. Introduction
Polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and dioxin-like polychlorinated biphenyls are persistent, bioaccumulative, and toxic contaminants widely distributed in the environment. These chemicals cause congenital malformations such as cleft palate and hydronephrosis in mice, alterations in development and function of reproductive tract in rats, and heart anomaly and circulation failure with edema in chicks (Peterson et al., 1993). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a typical exogenous ligand for the aryl hydrocarbon receptor (AHR), which translocates to the nucleus and dimerizes with an aryl hydrocarbon receptor nuclear translocator (ARNT) (Whitlock, 1999). Based on studies with targeted disruption in mice, it is evident that various toxicological endpoints of TCDD are dependent on AHR (Fernandez-Salguero et al., 1996; Mimura et al., 1997) and ARNT (Tomita et al., 2000).
Fish embryos are one of the most sensitive organisms to TCDD toxicity (Goldstone and Stegeman, 2006), showing a reduction in peripheral circulation, edema, craniofacial malformation, and growth retardation resulting in mortality (Walker and Peterson, 1994; Henry et al., 1997; Teraoka et al., 2002). Among these, the cardiovascular system is one of the most characteristic and important targets of developmental toxicity caused by TCDD and polycyclic aromatic hydrocarbons (Guiney et al., 1997; Cantrell et al., 1996; 1998; Willett et al., 2001). Zebrafish Danio rerio have been extensively studied as a model fish for environmental toxicology (Henry et al., 1997; Teraoka et al., 2003a; Hill et al., 2005; Stegeman et al., 2010). Several isoforms of AHR and ARNT have been identified in zebrafish, as in other fish species (Karchner et al., 2005). Gene knockdown with morpholino antisense oligonucleotides has revealed that zebrafish AHR2 is responsible for circulation failure in the mesencephalic vein (Dong et al., 2004) and pericardial edema (Prasch et al., 2003; Teraoka et al., 2003b). Involvement of AHR2 was also confirmed by the experiments with AHR2 null fish (Goodale et al., 2012). Studies with ARNT1 null mutant fish have shown that the AHR2/ARNT1 signaling pathway is important for TCDD-induced circulation failure in developing zebrafish (Antkiewicz et al., 2006; Prasch et al., 2004; 2006; Teraoka et al., 2010). However, mechanism(s) underlying TCDD-induced circulation failure downstream of the interaction of AHR2/ARNT1 with xenobiotic response elements (XREs) of target genes is largely unclear.
TCDD reduced numbers of cardiac muscle cells at very early stages occurring before pericardial edema (Antkiewicz et al., 2005). Recent studies reported that TCDD blocked development of the epicardium, which is crucial to heart development, through inhibition of proepicardium formation in developing zebrafish (Plavicki et al., 2013). The same studies further clarified the role of Sox9b in epicardial formation and pericardial edema using Sox9b-morphant and -null mutant, suggesting partial involvement of Sox-9b in TCDD-induced edema (Hofsteen et al., 2013). Thus, mechanisms of the circulation failure, particularly pericardial edema, caused by TCDD are only beginning to be understood in developing zebrafish as well as other fish.
We reported elsewhere that TCDD decreased blood flow in the mesencephalic vein as well as in the prosencephalic artery in early zebrafish eleutheroembryos before a significant increase in pericardial edema (Dong et al., 2002; 2004; Teraoka et al., 2009; 2010; Kubota et al., 2011). Mesencephalic circulation failure was blocked by antioxidants and general cytochrome P450 (CYP) inhibitors (Dong et al., 2002; 2004).
Recently, we reported the involvement of type 2 cyclooxygenase (COX2)–thromboxane pathway in TCDD-induced mesencephalic circulation failure in developing zebrafish (Teraoka et al., 2009; Kubota et al., 2011). Cyclooxygenases (COXs), also known as prostaglandin (PG) endoperoxide G/H synthases, catalyze the rate-limiting step of the production of various kinds of PGs. PGH2 was converted into thromboxane via thromboxane synthase to interact with thromboxane receptor (TP). Zebrafish have two isozymes of cyclooxygenase type 2, COX2a and COX2b, in addition to COX1 (Grosser et al., 2002; Ishikawa et al, 2007). TCDD-induced mesencephalic circulation failure was markedly inhibited by selective inhibitors of COX2 and COX2a knockdown (Teraoka et al., 2009). The inhibitory effect of TCDD on blood flow was prevented also by selective TP antagonists and knockdown of thromboxane A synthase 1 (TBXS, also known as CYP5A). Additionally, a TP agonist mimicked the effect of TCDD to cause mesencephalic circulation failure, and this effect was inhibited by TP antagonists. These results suggest the prostaglandin synthesis pathway involving COX2a-TBXS-TP plays important roles in TCDD-induced mesencephalic circulation failure in developing zebrafish. Involvement of COX2 in pericardial edema by TCDD was also suggested in developing medaka (Dong et al., 2010).
Efforts to elucidate mechanisms of pericardial edema formation by TCDD have been hampered by lower reproducibility of conventional analysis with lateral still images at 72 hours post fertilization (hpf) or later, with some exceptions where there has been knockdown of AHR2 or ARNT1 (Teraoka et al., 2003b; Prasch et al., 2003; 2004; Antkiewicz et al., 2006). This is partly because heart volume is very large in the pericardial cavity, resulting in a small rate of change in pericardial area difficult to measure by conventional analysis. A recent study reported that COX2-thromboxane pathway plays an essential role in valve formation in heart of developing zebrafish around 60 hpf (Scherz et al., 2008), which makes the situation complex for studying involvement of this pathway in pericardial edema formation by TCDD at 3 dpf or later.
In the present study using microscopic analysis with a high-speed camera we found that TCDD increased the area of the small cavity between the heart and the body wall of 55 hpf eleutheroembryos, an effect which we designate as precardiac edema. We assess involvement of prostanoid signaling in TCDD-induced edema formation by analysis of effects on the precardiac area.
2. Materials and Methods
2.1. Chemicals
TCDD was obtained from Cambridge Isotope Laboratories (98% purity; Andover, MA). 4-(Z)-6-(2-o-Chlorophenyl-4-o-hydroxyphenyl-1, 3-dioxan- cis-5-yl) Hexenoic acid (ICI-192,605), N-[2-(cyclohexyloxy)-4- nitrophenyl] methanesulfonamide (NS398), 9,11-dideoxy-9α,11α- methanoepoxy prosta-5Z-13E-dien-1-oic acid (U46619) were from Cayman Chemical (Ann Arbor, MI). Other chemicals were obtained from Kanto Chemical (Japan).
2.2. Zebrafish and TCDD treatment
Fertilized eggs were obtained from natural mating of adult zebrafish (Long-fin) in our laboratory according to the Zebrafish Book (Westerfield, 1995). Adult fish and embryos were maintained at 28.5 °C with a lighting schedule of 14 hr light and 10 hr dark. Eggs were collected within 1 hr of spawning, rinsed, and placed into a clean petri dish. At 24 hr post fertilization (hpf), newly fertilized eggs were exposed to either the vehicle, dimethyl sulfoxide (DMSO, usually 0.1%) or nominal concentration of waterborne TCDD of 0.3, 0.5, 1.0 or 2.0 parts per billion (ppb; 1 ppb ≈ 3.1 nM) dissolved in 0.1% DMSO in 3 ml of Zebrafish Ringer (ZR) solution (38.7 mM NaCl, 1.0 mM KCl, 1.7 mM HEPES-NaOH pH 7.2, 2.4 mM CaCl2) in 3.5 cm petri dishes (Asahi Glass, Funabashi, Japan) for the duration of the experiment (10 embryos/dish), as described elsewhere (Teraoka et al., 2009). Other chemicals were included in ZR solution from 24 to 48 hpf in the presence or absence of TCDD.
2.3. Gene knockdown with morpholino antisense oligos
Morpholino antisense oligonucleotides (MOs) against translation of AHR2 (AHR2-MO) and ARNT1 (ARNT1-MO) were synthesized by Gene Tools (Philomath, OR), as described previously (Teraoka et al., 2009). MOs against splicing of cyclooxygenase 2a (COX2a), cyclooxygenase 2b (COX2b), thromboxane A synthase 1 (TBXS-MO) and c-mpl (mpl-MO) were also used according to the previous study (Lin et al., 2006; Teraoka et al., 2009; Yeh et al., 2009). Standard morpholino (STD-MO), which was recommended by Gene Tools as a standard negative control, was used as universal control for injection in all morpholino studies unless otherwise indicated. Each MO was injected into the yolk of embryos at one to four cell stages with a fine glass needle connected to an automatic injector (IM-300: Narishige, Japan). Approximately 2 nL of 50 μM MOs in Ca2+-free Zebrafish Ringer solution were injected.
2.4. Measurement of edema
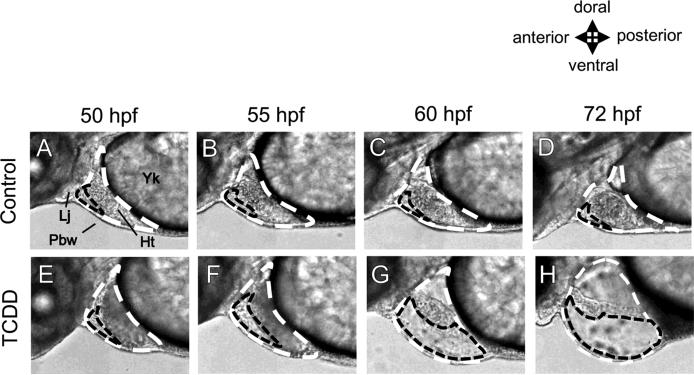
For pericardial edema (Former method) (Teraoka et al., 2003b), lateral images of eleutheroembryos, which were immobilized in 3% carboxymethyl cellulose/Zebrafish Ringer solution in a hand-made plastic bath, were obtained with a digital camera connected to an inverted microscope (DP70-IX71, Olympus). Temperature of the suspension solution was maintained at 28.5°C with a PDMI-2 Micro-Incubator (Harvard Apparatus, Holliston, MA). The pericardial space was circled on a Photoshop image at the same magnification and the area was obtained in pixels (Fig. 1) (Teraoka et al., 2003b). For precardiac edema (New method), lateral images were obtained using a high-speed camera (250 images/sec) (LRH1601BL, Digimo, Tokyo, Japan) connected to an inverted microscope. The area of the small cavity between the heart and body wall at maximal diastole was quantified in pixels (Fig. 1). Precardiac edema in each treatment group was expressed as a percentage of the area in non-injected controls, to normalize edema among separate experiments.
FIG. 1.
Precardiac cavity in comparison with pericardial cavity revealed by high-speed camera. Embryos were treated with either DMSO (Control) or 2 ppb TCDD, beginning at 24 hours post fertilization (hpf) until observation. All images are lateral views of developing zebrafish (50-72 hpf) with a focus on pericardial region. The pericardial cavity (white dashed line) around heart (Ht) is surrounded by the pectoral body wall (Pbw), lower jaw (Lj) and yolk (Yk) as indicated in panel A. Otherwise, a smaller cavity surrounded by the front edge of the heart, pectoral body wall and lower jaw can be recognized (black dashed line). For evaluation of pericardial edema (former method), pericardial cavity (white dashed line) was circled on photoshop image and the area was determined in pixels. For precardiac edema (new method), small cavity between heart and body wall in maximal diastole (black dashed line) was quantified in pixels. Upper panels (A-D): control, lower panels (E-H): 2 ppb TCDD.
2.5. Mesencephalic blood flow
Blood flow in the mesencephalic vein was evaluated by time-lapse recording as described previously, except the use of a digital-video camera (LRH1601BL, Digimo) (Teraoka et al., 2002; Kubota et al., 2011). Eleutheroembryos were suspended in 200 μl of 3% carboxymethyl cellulose/Zebrafish Ringer solution in a plastic bath mounted on the stage of an inverted microscope.
2.6. Real-time qRT-PCR
Real-time quantitative RT-PCR (qPCR) analysis was carried out to examine expression levels of COX2b as well as COX2a, as described previously (Teraoka et al., 2009). Total RNA was extracted from zebrafish embryos with Trizol (Invitrogen, Carlsbad, CA), and cDNA was synthesized from total RNA with Omniscript (Qiagen, Germany) and oligo (dT) primer. qRT-PCR analysis was performed in Real-Time PCR Detector (Chromo4: Bio-Rad, Hercules, CA) using Thunderbird qPCR mix containing SYBR Green (Toyobo, Osaka, Japan). The same primers as reported in Jönsson et al. (2012) were used; COX2b (GenBank ID: NM_001025504.2): F-GGCTCATCCTTATTGGTGAGACTAT and R-TCGGGATCAAACTTGAGCTTAAAATA (5' to 3' sequences), and COX2a (GenBank ID: NM_153657.1): F-ACTACCCCTGAGCTTCTCACA and R-GATGCTGTTGATGATATCCCAGATTG.
2.7. Statistics
Results are presented as mean±SEM. Significance of differences between vehicle control and TCDD-exposed groups was determined by one-way ANOVA followed by Tukey-Kramer test (p < 0.05). One-way ANOVA followed by Dunnett's test was also used for multiple comparisons with control in a line graph.
3. Results
3.1. Precardiac edema caused by TCDD
Zebrafish eleutheroembryos/larvae do not have a pericardium membrane, which in mammals covers the whole heart. Instead, the pericardial cavity is surrounded by the pectoral body wall, lower jaw and yolk (Fig. 1, white dashed line). TCDD treatment increased the size of the pericardial cavity, which is referred to as pericardial edema (Teraoka et al., 2003b). However, the rate of change in the pericardial area was small and usually became statistically significant only at 72 hpf or later, except in some studies, in which there were significant effects at 60 hpf (Teraoka et al., 2003b). It is obvious that heart volume occupies a majority of the pericardial cavity (Fig. 1).
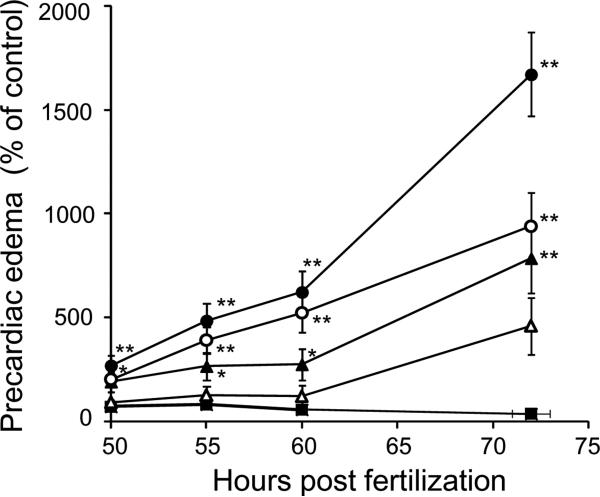
We found a smaller cavity surrounded by the front edge of the heart, body wall and lower jaw, which we designate as the precardiac cavity (Fig. 1). The area of the precardiac cavity in control embryos (lateral view) was very small and decreased in size during development (Fig. 2). As the control value was very small, the rate of increase in the precardiac cavity in fish exposed to TCDD was much greater than that of the pericardial cavity, and thus significant differences from control values were obtained as early as 50 hpf (Fig. 2; Teraoka et al., 2003b). Effects of TCDD on precardiac edema (Fig. 2) and on pericardial edema (Teraoka et al., 2003b) showed a similar concentration-response relationship. We therefore used precardiac edema as an index of edema around the heart in the following studies.
FIG. 2.
Time course of precardiac edema formation induced by TCDD. Precardiac area was indicated as percentage of control in developing zebrafish exposed to graded concentration of TCDD (0.3 ppb, open triangles; 0.5 ppb, filled triangles; 1 ppb, open circles; 2 ppb, filled circles) and vehicle (0.1% DMSO, filled square), at various time points (50-72 hpf). Results are expressed as mean ± SEM (N = 15-23). Asterisk indicates statistical significance compared to control at each stage (*p < 0.05, ** p < 0.01).
3.2. Effects of AHR2-KD, ARNT1-KD and antioxidant on TCDD-induced precardiac edema
It has been shown that knockdown of AHR2 translation with antisense morpholino oligonucleotides (AHR2-KD) can rescue pericardial edema caused by TCDD (Prasch et al., 2003; Teraoka et al., 2003b). Here, we examined whether AHR2 has a role also in precardiac edema induced by TCDD. As shown in Table 1, AHR2-KD markedly inhibited precardiac edema by 1 ppb TCDD, while 4mis-AHR2-MO did not alter the TCDD response significantly. Similarly, morpholino knockdown of ARNT1 translation (ARNT1-KD) significantly inhibited precardiac edema induced by 1 ppb TCDD (Table 1). None of these knockdowns affected basal levels of precardiac area. Thus, TCDD-induced precardiac edema also involves the AHR2/ARNT1 pathway.
Table 1.
Effects of antioxidants and morpholino knockdown against AHR2 signaling molecules on precardial area in zebrafish at 55 hpf
| Control | Treatment | TCDD | Treatment+TCDD | |
|---|---|---|---|---|
| 4mis-AHR2-MO | 100.0±16.0 (20)a | 105.9±17.4 (20)a | 590.7±95.6 (20)b | 621.7±100.6 (20)b |
| AHR2-MO | ND | 106.0±17.0 (20)a | ND | 109.51±17.6 (20)a |
| STD-MO | 100.0±19.3 (10)a | 81.9±10.7 (10)a | 816.1±145.1 (9)b | 108.6±8.8 (10)b |
| ARNT1-MO | ND | 97.3±12.1 (10)a | ND | 89.3±11.6 (10)a |
| Ascorbic acid | 100.0±8.6 (32)a | 101.0±7.6 (32)a | 688.0±90.4 (32)b | 369.3±51.3 (32)c |
Embryos were injected with morpholino antisense oligos against AHR2 and ARNT1 at one to four cell stages (Treatment). The embryos were exposed to 1 ppb TCDD (TCDD) or vehicle only (DMSO: Control), from 24 hpf to 48 hpf. Additionally, in a separate experiment, embryos were treated with antioxidant (10 mM ascorbic acid) (Treatment) in the absence or presence of TCDD (1 ppb). Precardial area was evaluated as an index of edema. Results as percent of control are expressed as mean ± SEM. Numbers in parenthesis indicate the number of individuals examined per group. Significance of differences in the precardial edema was determined by one-way ANOVA followed by Tukey-Kramer test (p < 0.05). Values with different letters are significantly different. ND: not determined
We previously reported that antioxidants, such as N-acetylcysteine and ascorbic acid, inhibited TCDD-induced mesencephalic circulation failure (Dong et al., 2002). In contrast, the effects of antioxidants on TCDD-induced pericardial edema were not clearly observed. In the current study, we found that 10 mM ascorbic acid significantly inhibited precardiac edema by 1 ppb TCDD without effect on the basal level (Table 1).
3.3. Effects of COX2 inhibition on TCDD-induced precardiac edema
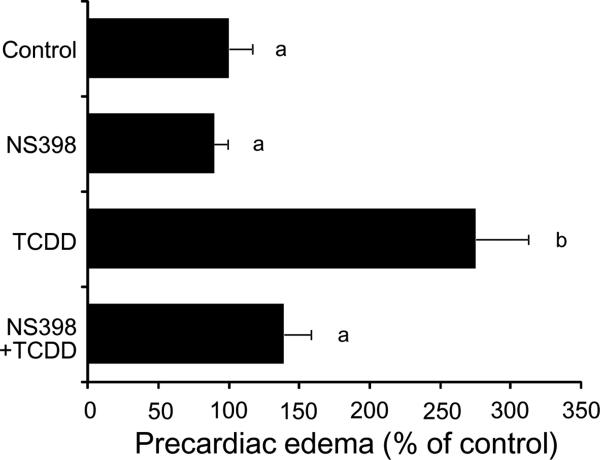
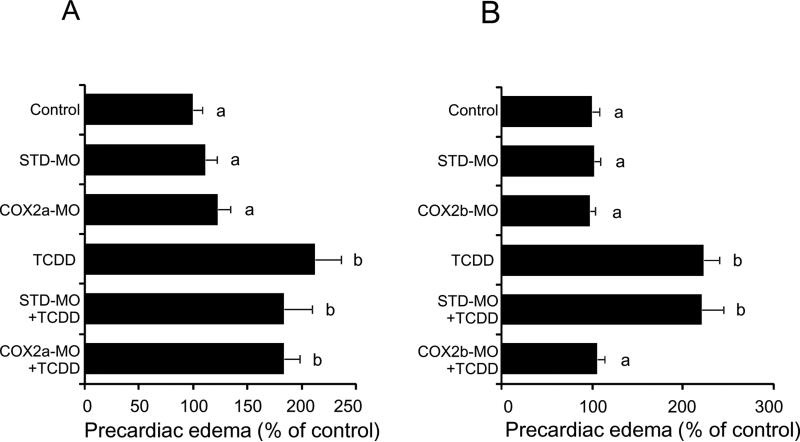
The cyclooxygenase inhibitor NS398 selective for COX2 rather than COX1, at 24 μM almost completely blocked precardiac edema caused by 1 ppb TCDD at 55 hpf, while it did not affect the basal level (Fig. 3). The same responses were also obtained for conventional pericardial edema by TCDD in some cases (data not shown). Knockdown of COX2a by splice inhibition with a morpholino antisense oligonucleotide failed to rescue precardiac edema caused by 0.5 ppb TCDD (Fig. 4A). On the other hand, precardiac edema induced by the same dose of TCDD (0.5 ppb) was almost abolished by knockdown of COX2b (Fig. 4B).
FIG. 3.
Protection from TCDD-induced precardiac edema by COX2 inhibitor. Embryos, treated with a COX2 inhibitor (24 μM NS398), were exposed to TCDD (1 ppb) or vehicle alone (Control), beginning at 24 hpf. At 55 hpf, precardial cavity was determined as an index of TCDD-induced edema (precardiac edema). Results are expressed as percentage of control (mean ± SEM, N = 20). Significant differences in precardiac area between groups were determined by one-way ANOVA followed by Tukey-Kramer test (p < 0.05). Values with different letters are significantly different.
FIG 4.
Protection from TCDD-induced precardiac edema by knockdown of COX2b but not of COX2a. After injection of each of splicing-target type morpholino antisense oligonucleotides against COX2a (COX2a-MO) (A) and COX2b (COX2b-MO) (B), or standard morpholinos as negative control (STD-MO), embryos were exposed to vehicle or 0.5 ppb TCDD beginning at 24 hpf until the determination at 55 hpf. Other conditions are the same as given in the Fig. 3 legend. N=18-30 (A) and 18-20 (B). Values with different letters are significantly different (p < 0.05).
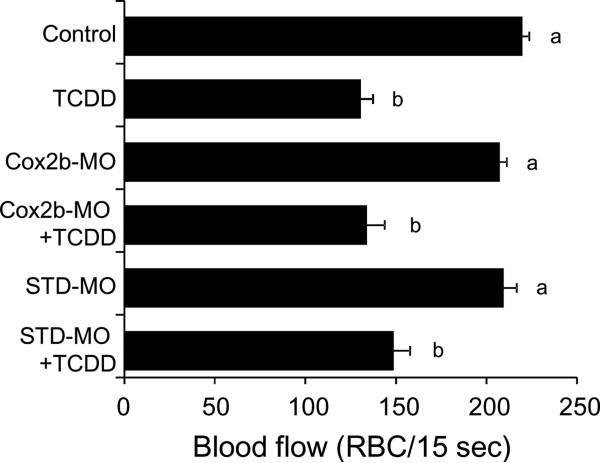
We previously found that COX2a is involved in the TCDD-induced reduction in mesencephalic vein blood flow (Teraoka et al., 2009). In the present study, we also examined the role of COX2b in the TCDD effect on blood flow. TCDD decreased blood flow in dorsal midbrain of 55 hpf eleutheroembryos, and this inhibitory effect of TCDD was not rescued by knockdown of COX2b (Fig. 5).
FIG. 5.
No effect of COX2b knockdown on TCDD-induced mesencephalic circulation failure by TCDD. After injection of each splicing-target type morpholino antisense oligonucleotides against COX2b (COX2b-MO) or standard morpholinos as negative control (STD-MO), embryos were exposed to vehicle or 0.5 ppb TCDD beginning at 24 hpf until the determination at 55 hpf. Other conditions are the same as given in the Fig. 3 legend. N=34-40. Values with different letters are significantly different (p < 0.05).
3.4. Effects of modulation of thromboxane receptor activity on TCDD-induced precardiac edema
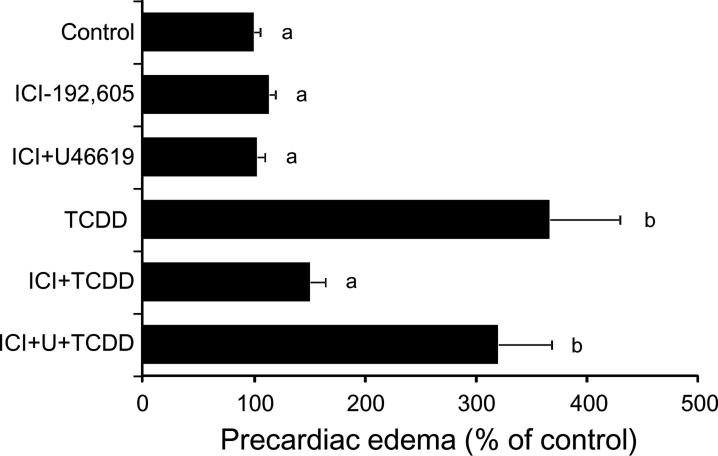
As shown in Fig. 6, a selective TP antagonist, ICI-192,605 (24 μM) inhibited precardiac edema caused by 1 ppb TCDD. Furthermore, the inhibitory effect of ICI-192,605 was almost abolished by a TP agonist, U46619 (30 μM) (Fig. 6). The same concentration of U46619 did not affect basal level of precardiac area or TCDD-induced precardiac edema (Control 100.0±7.8%, U46609 91.8±7.7%: n=24). Neither ICI-192,605 nor ICI-192,605+U46619 affected basal level of precardiac cavity area (Fig. 6).
FIG. 6.
Effects of a thromboxane receptor antagonist and a thromboxane receptor agonist on TCDD-induced precardiac edema. Embryos, treated with a thromboxane receptor (TP) antagonist (24 μM ICI-192,605) and/or a TP agonist (30 μM U46619), were exposed to TCDD (1 ppb) or vehicle alone (Control). Other conditions are the same as given in the FIG. 3 legend. N=22. Values with different letters are significantly different (p < 0.05).
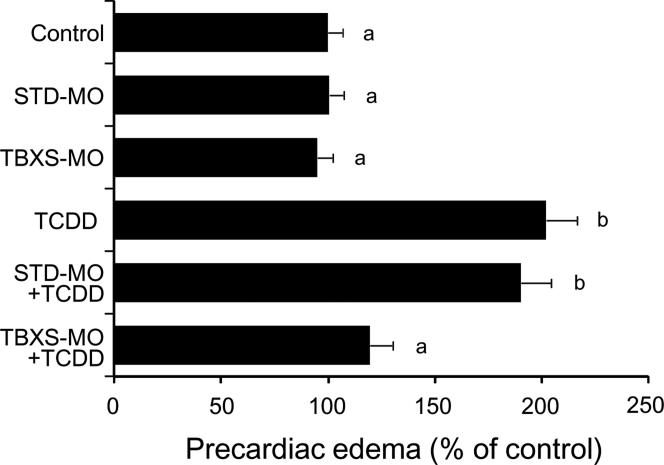
The effect of gene knockdown of thromboxane A synthase 1 (TBXS) on TCDD-induced precardiac edema was evaluated. We intended to block splicing with TBXS-MO, along with standard morphlino (STD-MO) as negative control (Fig. 7). Neither TBXS-MO nor STD-MO showed significant effect on the area of precardiac cavity in unexposed eleutheroembryos at 55 hpf. Injection of TBXS-MO but not STD-MO almost completely blocked precardiac edema caused by 0.5 ppb TCDD in 55 hpf eleutheroembryos (Fig. 7).
FIG. 7.
Protection from TCDD-induced precardiac edema by knockdown of thromboxane synthase. After injection of either a splicing-target type morpholino antisense oligonucleotide against thromboxane synthase (TBXS-MO) or a negative control morpholino (STD-MO), embryos were exposed to vehicle or 0.5 ppb TCDD. Other conditions are the same as given in the Fig. 3 legend. N=26-36. Values with different letters are significantly different (p < 0.05).
3.5. Effects of c-mpl knockdown on TCDD-induced precardiac edema
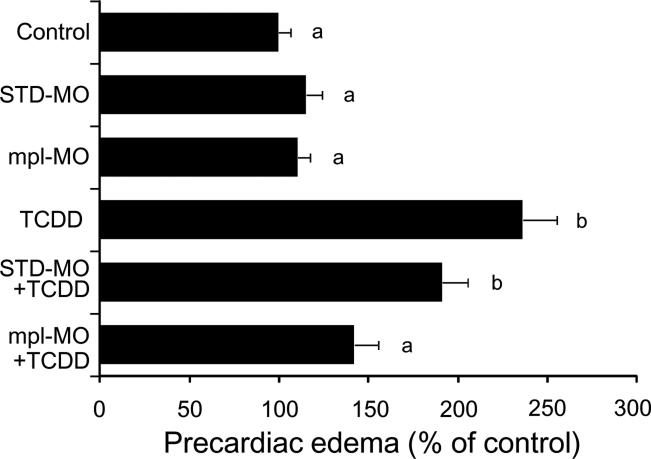
Thrombocytes are nucleated blood cells in fish species and are functionally homologous to mammalian platelets in being the dominant reservoir of thromboxane synthesis (Lloyd-Evans et al., 1994). A receptor for thrombopoietin, c-mpl, is absolutely required for thromboxane maturation in developing zebrafish (Lin et al., 2005). Thus, we addressed the effects of knockdown of c-mpl (mpl-MO) on TCDD-induced precardiac edema. The mpl-MO used in the present study was shown to be effective in reducing the number of thrombocytes in developing zebrafish (Lin et al., 2005). As shown in Fig. 8, mpl-MO markedly inhibited precardiac edema by TCDD without any effect on the control value. STD-MO was without effect on either control or TCDD-induced response.
FIG. 8.
Protection from TCDD-induced precardiac edema by c-mpl knockdown. After injection of either a splicing-target type morpholino antisense oligonucleotide against c-mpl (mpl-MO) or a negative control morpholino (STD-MO), embryos were exposed to vehicle or 0.5 ppb TCDD. Other conditions are the same as given in the Fig. 3 legend. N=35-41. Values with different letters are significantly different (p < 0.05).
3.6. Expression of transcripts of COX2b during zebrafish development and effects of TCDD exposure
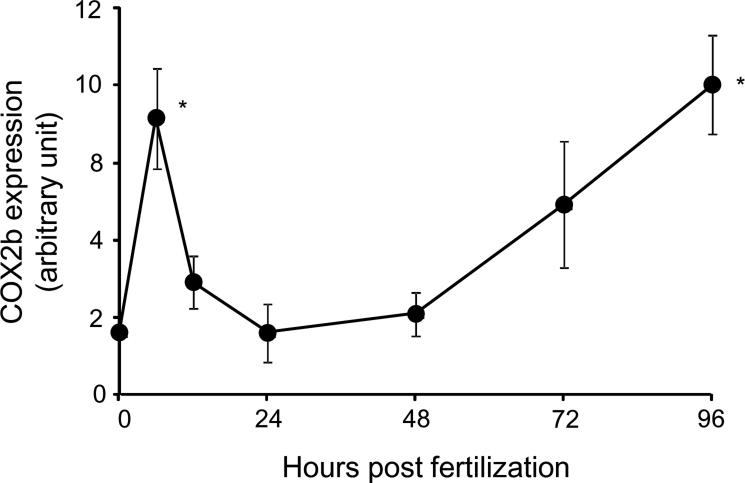
There was limited information on COX2b expression in early developmental stages of zebrafish. As shown in Fig. 9, COX2b transcripts were detected in embryos just after fertilization, suggesting maternal origin. The abundance of transcripts abruptly rose to a peak level at 6 hpf, decreased to an initial level by 24 hpf, and gradually increased again toward 96 hpf. Thus, COX2b transcripts were present in eleutheroembryos during the time at which TCDD caused precardiac edema.
FIG. 9.
COX2b expression in early life stage of zebrafish. Expression levels of COX2b transcripts in various developmental stages of whole zebrafish eleutheroembryos (from 0 to 96 hpf) were determined by qRT-PCR. N=4 or 5 per group. Thirty to fifty whole embryos or eleutheroembryos were used for each group. Asterisk indicates statistical significance compared to control at each stage (p < 0.05).
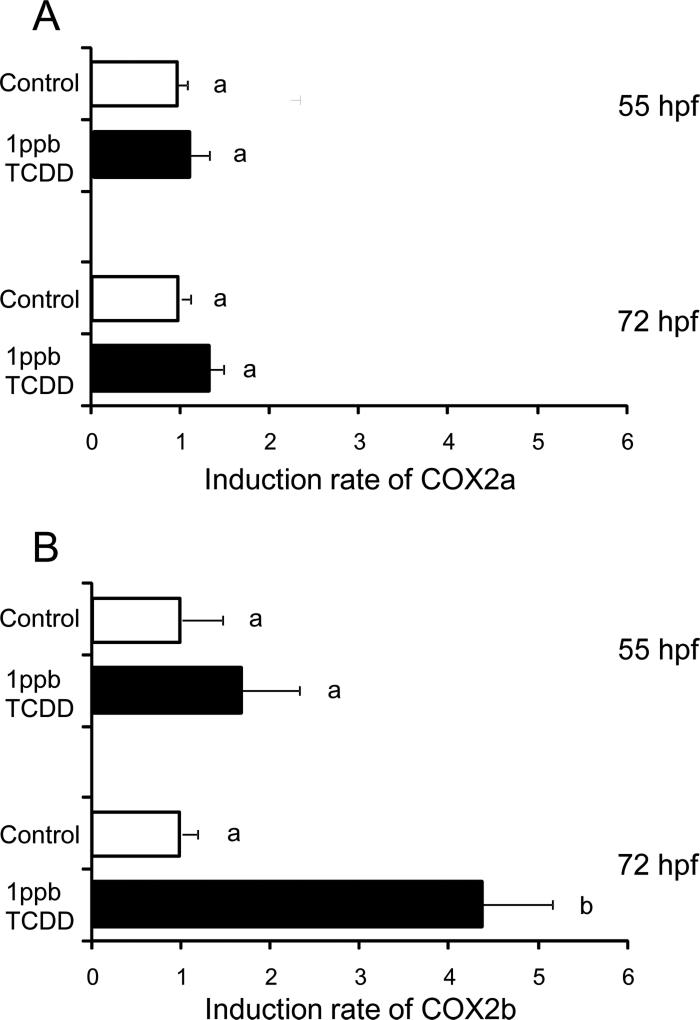
The effect of TCDD on COX2b expression was examined in comparison with COX2a (Fig. 10). TCDD (1 ppb) did not affect COX2a expression levels at 55 hpf or 72 hpf (Fig. 10A). By contrast, COX2b expression was markedly induced by the same concentration of TCDD at 72 hpf, although the effect was not significant for 55 hpf eleutheroembryos (Fig. 10B).
FIG. 10.
Induction of COX2b but not COX2a by TCDD in whole zebrafish eleutheroembryos. Expression levels of COX2a (A) and COX2b (B) transcripts in whole zebrafish eleutheroembryos at 55 hpf and 72 hpf were determined by qRT-PCR. Embryos were exposed to 1 ppb TCDD (solid black bars) or 0.1% DMSO (solvent control, open bars) from 24 hpf until the determination. Results are expressed as mean ± SEM N= 5 per group. Thirty eleutheroembryos were used for each group. Values with different letters are significantly different (p < 0.05).
4. Discussion
The present results identify a previously unrecognized condition caused by TCDD, termed precardiac edema, and show the usefulness of precardiac edema for investigating the mechanisms of TCDD toxicity. Compared to conventional pericardial edema (Teraoka et al., 2003b), precardiac edema caused by TCDD showed a much higher rate of change and superior detectability, which enabled us to address the effects of treatment with other chemicals and gene knockdown at lower concentrations of TCDD and at an earlier stage of embryonic development, 2 dpf (Fig. 2). As toxicants can cause secondary toxic effects, it is important to identify whenever possible the primary effects involved and the earliest developmental stage when they appear. For example, ascorbic acid inhibited TCDD-induced precardiac edema at 55 hpf in the present study (Table 1), while we were not able to obtain significant effects of antioxidants on pericardial edema in TCDD-exposed 72 hpf eleutheroembryos. As prostaglandin pathways play important roles in heart development that occurs by 3 dpf (Scherz et al., 2008), measurement of precardiac edema in earlier stages was required to address the involvement of COX2 in TCDD-induced edema in this study. Together with the effects of AHR2-KD and ARNT1-KD on TCDD-induced edema, resemblance of the TCDD concentration-response relationship for precardiac edema at 2 dpf in the current study to that for pericardial edema at 3 dpf reported previously (Teraoka et al., 2003b) suggests shared mechanisms of these endpoints of cardiovascular toxicity in developing zebrafish.
Present study suggests the involvement of COX2 in TCDD-induced precardiac edema. TCDD-induced precardiac edema was prevented by NS398, a selective COX2 inhibitor (Fig. 3). The same responses were also obtained for conventional pericardial edema by TCDD in some cases. However, blockage of TCDD-induced pericardial edema was not consistently observed; the results varied among batches of embryos that were collected from different females, perhaps due to obligatory role of COX2 in heart valve formation by 3 dpf (Scherz et al., 2008). In the present study, we showed that knocking down COX2b, but not COX2a, prevented zebrafish eleutheroembryos from developing TCDD-induced precardiac edema (Fig. 4), suggesting an important role specifically of the COX2b subtype. COX2b transcripts are expressed during the early stage of development when TCDD-induced precardiac edema was first detected (Fig. 9). We also found COX2b induction by TCDD in 72 hpf eleutheroembryos, but not COX2a (Fig. 10). There also was a trend of COX2b induction at 55 hpf, although this was not statistically significant. Local induction of COX2b at 55 hpf in circulation system could be obscured by fluctuating basal expression in other tissues. Tissue localization of COX2b, as well as COX2a, should be examined in future studies, A single study reported that COX2a was detected within sprouting gill arteries and vasculature of the intestine of 4 dpf eleutheroembryos (Grosser et al., 2002). Recently, it was reported that PCB126 induced both COX2a and COX2b in 4 dpf eleutheroembryos (Jönsson et al., 2012), which is reminiscent of the results of Dong et al. (2010), who reported COX2 induction by TCDD and the correlation between COX2 induction and pericardial edema in developing medaka. The present results provide important evidence for the involvement of COX2b in precardiac edema formation around heart by TCDD.
Real-time qPCR with whole eleutheroembryos showed that COX2b expression was detected in eggs just after fertilization and reached a temporal peak at 6 hpf when vasculature is not yet present (Fig. 9), suggesting an unidentified role in gastrulation, not associated with survival. We previously found a similar peak in COX1 expression at 12 hpf (Teraoka et al., 2009). The role of COX2b as well as COX2a in hematopoietic differentiation has been suggested in developing zebrafish (Yeh et al., 2009). However, hematopoietic differentiation in zebrafish embryos occurs from 22 to 40 hpf.
A TP selective antagonist, ICI-192,605, was very effective in inhibiting TCDD-induced precardiac edema and this inhibitory effect was blocked by U46619, a TP selective agonist (Fig. 6). As the complete mRNA sequence of TP in zebrafish is not available at present, we focused on thromboxane synthesis enzyme, TBXS. TCDD-induced precardiac edema was significantly protected by TBXS-MO (Fig. 7), which was also effective in preventing mesencephalic circulation failure by TCDD (Teraoka et al., 2009). These results suggest involvement of the thromboxane pathway in TCDD-induced precardiac edema, as well as in mesencephalic circulation failure in developing zebrafish. Thrombocytes are the nucleated equivalent of platelets in fish and other non-mammalian vertebrates and play important roles in synthesis, storage and release of thromboxane and in blood coagulation (Lloyd-Evans et al., 1994). Thrombopoietin binding to c-mpl, the sole known receptor for thrombopoietin, plays an important role in thrombocyte production by increasing progenitor maturation along the thrombocyte lineage (Lin et al., 2005). The present results show that knocking down c-mpl inhibited TCDD-induced precardiac edema formation (Fig. 8). This observation supports the idea that thromboxane release by TCDD may have led to edema in developing zebrafish.
We previously reported that TCDD-induced mesencephalic circulation failure was blocked by knocking down COX2a expression in 50 hpf eleutheroembryos (Teraoka et al., 2009). Knockdown of COX2b did not affect TCDD-induced circulation failure in the present study (Fig. 5). Conversely, our current results showed the involvement of COX2b, but not COX2a, in the TCDD-induced precardiac edema response in 55 hpf eleutheroembryos (Fig. 4). This could be a tissue specific difference (heart/adjoining vessels versus mesencephalic vein), or perhaps a stage specific one (55 hpf versus 50 hpf).
In mammals, augmentation of vascular permeability by prostaglandins has been mainly explained by their vasomotor activity. Thromboxane is a potent vasoconstrictor serving as an inhibitor for edema caused by inflammatory substances (Halushka et al., 1989). However, zebrafish larvae do not have functional vascular smooth muscle until they are juvenile fish about one month of age (Georgijevic et al., 2007; Santoro et al., 2009). This suggests that zebrafish larvae respond to thromboxane through another mechanism. Previously, we reported that TCDD raised vascular permeability to albumin in dorsal midbrain, which was blocked by AHR2 knockdown and antioxidant treatment in 50 hpf zebrafish eleutheroembryos (Dong et al., 2004). It was reported that thromboxane A2 (TBXA2) increases endothelial permeability by promoting NF-kB mediated production of IL-8 in human microvascular endothelial cells (Kim et al., 2010). Early stages of the angiogenic process are often accompanied by enhanced vascular permeability. We found that exposure of zebrafish embryos to TCDD caused morphological changes in brain blood vessels including neovascularization (Teraoka et al., 2010). Otherwise, low concentrations of TP agonists induce the angiogenesis in vitro, while high doses of TP agonists produce a biphasic response (Nie et al., 2000; Benndorf et al., 2008). There also is evidence suggesting that TBXA2 has opposite effects on angiogenesis depending upon the thromboxane receptor isoforms, a and b, in human endothelial cells (Ashton and Ware, 2004; Michel et al., 2006). At present, properties of zebrafish TP receptor are unknown. Further studies will be needed to examine the mode of the involvement of thromboxane in TCDD-induced edema formation.
Unlike effects on mesencephalic circulation, a TP agonist, U46619, did not mimic TCDD in causing precardiac edema in the present study. This implies the involvement of other factors in evoking edema. Oxidative stress caused by TCDD is one candidate, as antioxidant inhibited precardiac edema caused by TCDD, as well as mesencephalic circulation failure (Dong et al., 2004). Dioxins increase mitochondrial respiration-dependent reactive oxygen production through AHR (Senft et al., 2002). Alternatively, reactive oxygen species could be generated from uncoupling of CYP1A by dioxins and PCBs (Bondy and Naderi, 1994; Schlezinger et al., 2006). At present, an adequate method to generate oxidative stress in specific regions of zebrafish larvae has not been established. Thus, whether oxidative stress plays a pivotal role in causing TCDD-induced precardiac edema awaits the analysis of target tissue for oxidative stress.
We establish a new indicator of pericardial edema in developing zebrafish (precardiac edema).
Property of precardiac edema by TCDD is similar to that for conventional pericardial edema.
COX2b (but not COX2a)-thromboxane pathway is involved in precardiac edema by TCDD.
Acknowledgements
Grants-in-Aid for Scientific Research (MEXT/JSPS KAKENHI 21310044 (B) and 24580462 (C)), Grant-in-Aid for MEXT-Supported Program for Strategic Research Foundation at Private Universities, 2008-2012 (Rakuno Gakuen University), Japan (H.T.). Superfund Research Program grant P42ES007381 (AK and JJS). A.K. is supported by a Postdoctoral Fellowship for Research Abroad (no. 820). The sponsors had no involvement in performing or in the decision to publish this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have nothing to disclose.
References
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Ashton AW, Ware JA. Thromboxane A2 receptor signaling inhibits vascular endothelial growth factor-induced endothelial cell differentiation and migration. Circ. Res. 2004;95:372–379. doi: 10.1161/01.RES.0000138300.41642.15. [DOI] [PubMed] [Google Scholar]
- Benndorf RA, Schwedhelm E, Gnann A, Taheri R, Kom G, Didie M, Steenpass A, Ergun S, Boger RH. Isoprostanes inhibit vascular endothelial growth factor-induced endothelial cell migration, tube formation, and cardiac vessel sprouting in vitro, as well as angiogenesis in vivo via activation of the thromboxane A2 receptor: a potential link between oxidative stress and impaired angiogenesis. Circ. Res. 2008;103:1037–1046. doi: 10.1161/CIRCRESAHA.108.184036. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Naderi S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem. Pharmacol. 1994;48:155–159. doi: 10.1016/0006-2952(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): the embryonic vasculature is a physiological target for TCDD-induced DNA damage and apoptotic cell death in Medaka (Orizias latipes). Toxicol. Appl. Pharmacol. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- Cantrell SM, Joy-Schlezinger J, Stegeman JJ, Tillitt DE, Hannink M. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced apoptotic cell death in the embryonic vasculature with embryotoxicity. Toxicol. Appl. Pharmacol. 1998;148:24–34. doi: 10.1006/taap.1997.8309. [DOI] [PubMed] [Google Scholar]
- Dong W, Matsumura F, Kullman SW. TCDD induced pericardial edema and relative COX-2 expression in medaka (Oryzias Latipes) embryos. Toxicol. Sci. 2010;118:213–223. doi: 10.1093/toxsci/kfq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicol. Sci. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Tsujimoto Y, Stegeman JJ, Hiraga T. Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2004;77:109–116. doi: 10.1093/toxsci/kfh023. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Georgijevic S, Subramanian Y, Rollins EL, Starovic-Subota O, Tang AC, Childs SJ. Spatiotemporal Expression of Smooth Muscle Markers in Developing Zebrafish Gut. Dev. Dyn. 2007;236:1623–1632. doi: 10.1002/dvdy.21165. [DOI] [PubMed] [Google Scholar]
- Goldstone HM, Stegeman JJ. Molecular mechanisms of 2,3,7,8-tetrachlorodibenzo-p-dioxin cardiovascular embryotoxicity. Drug Metab. Rev. 2006;38:261–289. doi: 10.1080/03602530600570099. [DOI] [PubMed] [Google Scholar]
- Goodale BC, La Du JK, Bisson WH, Janszen DB, Waters KM, Tanguay RL. AHR2 mutant reveals functional diversity of aryl hydrocarbon receptors in zebrafish. PLoS One. 2012;7:e29346. doi: 10.1371/journal.pone.0029346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser T, Yusuff S, Cheskis E, Pack MA, FitzGerald GA. Developmental expression of functional cyclooxygenases in zebrafish. Proc. Natl. Acad. Sci. USA. 2002;99:8418–8423. doi: 10.1073/pnas.112217799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney PD, Smolowitz RM, Peterson RE, Stegeman JJ. Correlation of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in vascular endothelium with toxicity in early life stages of lake trout. Toxicol. Appl. Pharmacol. 1997;143:256–273. doi: 10.1006/taap.1996.8051. [DOI] [PubMed] [Google Scholar]
- Halushka PV, Mais DE, Mayeux PR, Morinelli TA. Thromboxane, prostaglandin and leukotriene receptors. Annu. Rev. Pharmacol. Toxicol. 1989;29:213–239. doi: 10.1146/annurev.pa.29.040189.001241. [DOI] [PubMed] [Google Scholar]
- Henry TR, Spitsbergen JM, Hornung MW, Abnet CC, Peterson RE. Early life stage toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish (Danio rerio). Toxicol. Appl. Pharmacol. 1997;142:56–68. doi: 10.1006/taap.1996.8024. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hofsteen P, Plavicki J, Johnson SD, Peterson RE, Heideman W. Sox9b is required for epicardium formation and plays a role in TCDD-induced heart malformation in zebrafish. Mol. Pharmacol. 2013;84:353–360. doi: 10.1124/mol.113.086413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa TO, Griffin KJ, Banerjee U, Herschman HR. The zebrafish genome contains two inducible, functional cyclooxygenase-2 genes. Biochem. Biophys. Res. Commun. 2007;352:181–187. doi: 10.1016/j.bbrc.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson ME, Kubota A, Timme-Laragy AR, Woodin B, Stegeman JJ. Ahr2-dependence of PCB126 effects on the swim bladder in relation to expression of CYP1 and cox-2 genes in developing zebrafish. Toxicol. Appl. Pharmacol. 2012;265:166–174. doi: 10.1016/j.taap.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Hahn ME. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem. J. 2005;392:153–161. doi: 10.1042/BJ20050713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Bae SK, Park HJ, Kim MK, Kim K, Park SY, Jang HO, Yun I, Kim YJ, Yoo MA, Bae MK. Thromboxane A2 increases endothelial permeability through upregulation of interleukin-8. Biochem. Biophys. Res. Commun. 2010;397:413–419. doi: 10.1016/j.bbrc.2010.05.106. [DOI] [PubMed] [Google Scholar]
- Kubota A, Stegeman JJ, Woodin BR, Iwanaga T, Harano R, Peterson RE, Hiraga T, Teraoka H. Role of zebrafish cytochrome P450 CYP1C genes in the reduced mesencephalic vein blood flow caused by activation of AHR2. Toxicol. Appl. Pharmacol. 2011;253:244–252. doi: 10.1016/j.taap.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HF, Traver D, Zhu H, Dooley K, Paw BH, Zon LI, Handin RI. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106:3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans P, Barrow SE, Hill DJ, Bowden LA, Rainger GE, Knight J, Rowley AF. Eicosanoid generation and effects on the aggregation of thrombocytes from the rainbow trout, Oncorhynchus mykiss. Biochim. Biophys. Acta. 1994;1215:291–299. doi: 10.1016/0005-2760(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Michel F, Silvestre JS, Waeckel L, Corda S, Verbeuren T, Vilaine JP, Clergue M, Duriez M, Levy BI. Thromboxane A2/prostaglandin H2 receptor activation mediates angiotensin II-induced postischemic neovascularization. Arterioscler. Thromb. Vasc. Biol. 2006;26:488–493. doi: 10.1161/01.ATV.0000201969.93348.74. [DOI] [PubMed] [Google Scholar]
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M, Fujii-Kuriyama Y. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells. 1997;2:645–654. doi: 10.1046/j.1365-2443.1997.1490345.x. [DOI] [PubMed] [Google Scholar]
- Nie D, Lamberti M, Zacharek M, Li L, Szekeres K, Tang K, Chen Y, Honn KV. Thromboxane A2 regulation of endothelial cell migration, angiogenesis, and tumor metastasis. Biochem. Biophys. Res. Commun. 2000;267:245–251. doi: 10.1006/bbrc.1999.1840. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald HM, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit. Rev. Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Heideman W, Peterson RE. ARNT2 is not required for TCDD developmental toxicity in zebrafish. Toxicol. Sci. 2004;82:250–258. doi: 10.1093/toxsci/kfh235. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: TCDD developmental toxicity in zebrafish requires ARNT1. Mol. Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Plavicki J, Hofsteen P, Peterson RE, Heideman W. Dioxin inhibits zebrafish epicardium and proepicardium development. Toxicol. Sci. 2013;131:558–567. doi: 10.1093/toxsci/kfs301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Pesce G, Stainier DY. Characterization of vascular mural cells during zebrafish development. Mech. Dev. 2009;126:638–649. doi: 10.1016/j.mod.2009.06.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development. 2008;135:1179–1187. doi: 10.1242/dev.010694. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Struntz WD, Goldstone JV, Stegeman JJ. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat. Toxicol. 2006;77:422–432. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Senft AP, Dalton TP, Nebert DW, Genter MB, Puga A, Hutchinson RJ, Kerzee JK, Uno S, Shertzer HG. Mitochondrial reactive oxygen production is dependent on the aromatic hydrocarbon receptor. Free Radic. Biol. Med. 2002;33:1268–1278. doi: 10.1016/s0891-5849(02)01014-6. [DOI] [PubMed] [Google Scholar]
- Stegeman JJ, Goldstone JV, Hahn M. Perspectives on zebrafish as a model in environmental toxicology. Zebrafish. 2010;29:367–439. [Google Scholar]
- Teraoka H, Dong W, Hiraga T. Zebrafish as a novel experimental model for developmental toxicology. Congenit. Anom. 2003a;43:123–132. doi: 10.1111/j.1741-4520.2003.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Teraoka T, Dong W, Ogawa S, Tsukiyama S, Okuhara Y, Niiyama M, Ueno N, Peterson RE, Hiraga T. 2,3,7,8-Tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: Altered regional blood flow and impaired lower jaw development. Toxicol. Sci. 2002;65:192–199. doi: 10.1093/toxsci/65.2.192. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Dong W, Tsujimoto Y, Iwasa H, Endoh D, Ueno N, Stegeman JJ, Peterson RE, Hiraga T. Induction of cytochrome P450 1A is required for circulation failure and edema by 2,3,7,8-tetrachlorodibenzo-p-dioxin in zebrafish. Biochem. Biophys. Res. Commun. 2003b;304:223–228. doi: 10.1016/s0006-291x(03)00576-x. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of the cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol. Appl. Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Ogawa A, Kubota A, Stegeman JJ, Peterson RE, Hiraga T. Malformation of certain brain blood vessels caused by TCDD activation of Ahr2/Arnt1 signaling in developing zebrafish. Aquat. Toxicol. 2010;99:241–247. doi: 10.1016/j.aquatox.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1alpha. Mol. Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- Walker MK, Peterson RE. Aquatic toxicity of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. Plenum Press; New York: 1994. pp. 347–387. [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR.: 1995. [Google Scholar]
- Whitlock JP., Jr. Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Willett KL, Wassenberg D, Lienesch L, Reichert W, Di Giulio RT. In vivo and in vitro inhibition of CYP1A-dependent activity in Fundulus heteroclitus by the polynuclear aromatic hydrocarbon fluoranthene. Toxicol. Appl. Pharmacol. 2001;177:264–271. doi: 10.1006/taap.2001.9296. [DOI] [PubMed] [Google Scholar]
- Yeh JR, Munson KM, Elagib KE, Goldfarb AN, Sweetser DA, Peterson RT. Discovering chemical modifiers of oncogene-regulated hematopoietic differentiation. Nat. Chem. Biol. 2009;5:236–243. doi: 10.1038/nchembio.147. [DOI] [PMC free article] [PubMed] [Google Scholar]