Abstract
Objectives
We recently demonstrated a significant correlation between enamel delamination and tooth-level radiation dose in oral cancer patients. Since radiation can induce the synthesis and activation of matrix metalloproteinases, we hypothesized that irradiated teeth may contain active matrix metalloproteinases.
Materials and Methods
Extracted teeth from oral cancer patients treated with radiotherapy and from healthy subjects were compared. Extracted mature third molars from healthy subjects were irradiated in vitro and/or incubated for 0 to 6 months at 37°C. All teeth were then pulverized, extracted, and extracts subjected to proteomic and enzymatic analyses.
Results
Screening of irradiated crown extracts using mass spectrometry identified MMP-20 (enamelysin) which is expressed developmentally in dentin and enamel but believed to be removed prior to tooth eruption. MMP-20 was composed of catalytically active forms at Mr=43, 41, 24 and 22 kDa and was immunolocalized predominantly to the morphological dentin enamel junction. The proportion of different sized MMP-20 forms changed with incubation and irradiation. While the pattern was not altered directly by irradiation of healthy teeth with 70 G, subsequent incubation at 37°C for 3–6 months with or without prior irradiation caused the proportion of Mr=24–22 kDa MMP-20 bands to increase dramatically. Extracts of teeth from oral cancer patients who received >70 Gy radiation also contained relatively more 24 and 22 kDa MMP-20 than those of healthy age-related teeth.
Conclusion
MMP-20 is a radiation-resistant component of mature tooth crowns enriched in the dentin-enamel. We speculate that MMP-20 catalyzed degradation of organic matrix at this site could lead to enamel delamination associated with oral cancer radiotherapy.
Keywords: dentin-enamel junction, matrix metalloproteinase-20, human tooth crown, radiotherapy, oral cancer
INTRODUCTION
Over 600,000 patients worldwide are diagnosed with oral cancer annually(1). Treatment usually involves surgery and radiotherapy with doses of 60–70 Gy(2). After radiotherapy, teeth display increased susceptibility to dental disease with lesions differing in location, appearance, and progression from smooth-surface dental caries(3). The process initiates with an enamel shear fracture(3). We recently demonstrated a direct link between radiation and dentition breakdown and a significant correlation of tooth-level radiation dose with the severity of individual tooth breakdown(4).
The dental-enamel junction is a complex interfacial structure within the human tooth, and despite a lifetime of loading associated with mastication, enamel rarely delaminates from dentin. Although Sognnaes et al. (5) first described an enamel organic matrix layer adjacent to the DEJ in mature molar teeth, this layer remains largely unappreciated (6). Total delamination of the enamel from the dentin is a unique pattern of destruction associated with radiotherapy. While radiation could potentially cause lytic changes in collagen polypeptides, we hypothesize it may stimulate the transcription and activation of MMPs(7) within exposed teeth, which could degrade components of the enamel organic matrix and DEJ, thereby destabilizing the DEJ and leading to enamel delamination.
During tooth development, MMP-20 is secreted by ameloblasts and odontoblasts to facilitate mineralization of enamel and dentin by cleaving extracellular matrix proteins(8–10). However, MMP-20 is believed to be largely proteolytically removed from teeth prior to their maturation(11).
We show here that MMP-20 protein and activity are readily detectable in extracts of impacted mature human third molar crowns from healthy subjects. MMP-20 survives exposure of those teeth to high dose radiation in vitro, although its predominant size shifts to a catalytically active 24 and 22 kDa forms which are similar to that in extracts of crowns from oral cancer patients irradiated in vivo.
MATERIALS AND METHODS
Tooth specimens
Following an IRB approved protocol, pairs of extracted healthy third molars with closed apices and no restorations or caries were collected from the same individual. Extracted teeth were stored for up to 2 weeks in PBS containing 0.002% NaN3 at 4°C prior to being assigned to one of 6 groups. Group 1 (in vitro irradiated) received a total dose of 70 Gy with radiation administered at 2 Gy/day, 5 days a week for 7 weeks to mimic a representative patient radiotherapy schedule. Teeth were stored in PBS containing 0.002% NaN3 during the radiation treatment time with the solution changed every 2 weeks. Following irradiation, teeth were immediately frozen at −80°C. Groups 2 and 3 were irradiated with 70 Gy using the same protocol as Group 1 and then subsequently incubated for either 3 or 6 months at 37°C in PBS containing 0.002% NaN3 (in vitro irradiated and incubated) prior to freezing. Groups 4 and 5 were only incubated at 37°C for either 3 or 6 months prior to freezing. Group 6 was frozen without irradiation or incubation at 37°C. Storage media was refreshed periodically (once a week) during long-term incubations. Each control tooth was matched with a corresponding treated tooth collected from the same patient. There was no significant difference in age among the in vitro irradiated and control teeth with an average age of 20 years. A separate group of extracted teeth from oral cancer patients who received an average total dose of 65 Gy during the course of radiotherapy was also included in the study (in vivo irradiated). The latter group was compared with a group of healthy third molars that were extracted form age-related patients with an age range of 50–78 years old. Following treatment, all teeth were stored at −80°C until processed. Prior to protein extraction, roots and pulp were removed from each tooth, and crowns were then processed.
Protein extraction
Individual crowns were flash-frozen, pulverized, and extracted for 72 h at 4°C with 0.05 M Tris-buffer (pH7.5), containing 4 M guanidine hydrochloride, 0.5 M EDTA, and a mixture of protease and phosphatase inhibitors(12). Samples were centrifuged and supernatants dialyzed against 5% (v/v) acetic acid or 0.05% SDS at 4°C using 6–8 kDa molecular weight cut-off dialysis tubing (Spectrum Laboratories, Rancho Dominguez, CA). Extracts were aliquoted, lyophilized and stored frozen. Protein concentration was determined using the Non-Interfering™ Protein Assay kit (G-Biosciences, Saint Louis, MO).
LC-tandem MS
Extracts were electrophoresed on 4–20% gradient gels and then fixed. Each gel lane was cut lengthwise into a series of 1 mm slices and then reduced and alkylated followed by digestion with trypsin. Extracted peptides were analyzed by capillary LC-tandem MS (Thermo Finnigan LTQ) using a 50 μM i.d. × 8 cm long capillary column packed with Phenomenex Jupiter C18 reversed phase matrix resolved with a linear gradient of acetonitrile (13). The instrument was operated in the data-dependent mode in which one mass spectrum and eight collision induced dissociation spectra were acquired per cycle. Data were analyzed using Mascot protein identification software (Matrix Science, Ltd.), with manual inspection of peptide matches for validation.
Western blotting
Extracts were hydrated in SDS sample buffer containing 8 M urea and an excess of DTT(14) and heated at 95°C for 10 min prior to loading on mini-gels (pre-poured long-life Precise 4–20% gradient gels). Electrophoresis was achieved at 50 V and migration of unknowns was calculated relative to that for Coomassie-blue pre-stained globular standard proteins (Bio-Rad, Inc.). Proteins were electrophoretically transferred at 100 V for 2 h to PVDF membranes in chilled 0.01M N-cyclohexyl-3-aminopropanesulfonic acid buffer, pH 11.0 containing 10% methanol. After transfer, membranes were washed 4X in Tris-buffered saline, pH 8.0 containing 0.01% Tween 20 (TBTS) and then blocked for 1 h with mixing in Blotto (5% non-fat dry milk in TBTS). Membranes were mixed overnight with (1/2000) anti-MMP-20 antibody (#M-5934, affinity purified N-terminal IgG, rabbit, Sigma-Aldrich) and then washed 4 times with TBTS. Secondary antibody (1/10,000 horseradish peroxidase conjugated goat anti-rabbit IgG antibody, Bio-Rad) was incubated in the dark with membranes for a minimum of 2 h prior to extensive washing with TBTS and development of the resultant chemiluminescence signal using a SuperSignal West Dura Extended Duration kit (Pierce Chemical Co.). Digital images were captured using a Fuji LAS4000 imager system.
Zymography
Extracts were hydrated without DTT and boiling, and electrophoresed on either 12.5% casein or gelatin gels (Bio-Rad), or a 12% gel containing 0.1 mg/ml of recombinant porcine amelogenin that was kindly provided by Dr. Janet Oldak's lab. After running, gels were developed as previously described(15). Casein and gelatin gels were visualized with 0.2% Coomassie Brilliant Blue R-250 (Sigma-Aldrich); amelogenin containing gels were stained with SYPRO Ruby (Bio-Rad).
Immunoprecipitation
Extracts were rehydrated in PTO buffer and MMP-20 was immunoprecipitated using an antibody against the N-terminus of MMP-20 (Sigma-Aldrich) as described(16). Non-immune rabbit IgG (Sigma-Aldrich) served as a negative control. Immunoprecipitates were pelleted, washed, and eluted with loading buffer containing 10% (v/v) DTT for use in western blotting or with loading buffer without DTT for zymography. A Clean-Blot™ IP Detection Kit (1:200, Thermo Fisher) was used to detect immunoprecipitated bands by western blotting.
Confocal Immunofluorescence Studies
Mesiodistal sections (0.3 – 0.5 mm in thickness) were cut from six healthy third molars obtained from individual patients with an average age of 20 years old. Sections were mounted on #1 chambered borosilicate coverglass slides (Lab-Tek, Rochester, NY) and demineralized in 10% EDTA (pH 7.4), containing 4% formalin or 4% p-formaldehyde and 0.002% sodium azide (Sigma-Aldrich) for 2 weeks.
Sections were then washed with Buffer A and then blocked for 2 h in Buffer B containing avidin D blocking solution (Vector Labs, Burlingame, CA). Sections were then incubated overnight at room temperature with primary antibody (1:200, rabbit poly-clonal anti-MMP20 antibody, Sigma-Aldrich) diluted in Buffer B containing biotin blocking solution (Vector Labs). Control sections were incubated in parallel with purified non-immune serum rabbit IgG (1:200, Sigma-Aldrich) under the same conditions. Sections were then incubated for 1 h with biotin conjugated-protein A (1:10,000, Millipore) in Buffer B, washed and then incubated with streptavidin labeled with AlexaFluor594 (1ug/ml, Jackson Laboratories, West Grove, PA) in Buffer B for 1 h.
Finally, slides were imaged with a confocal laser scanning microscope (CLSM; Leica TCS SP5 II, Wetzlar, Germany) using the resonant scanner at 405 and 594 nm. Image formats were 1024 × 1024 pixels and confocal image z-stacks were analyzed using ImageJ software. The 405 nm channel was subtracted from the 594 nm channel to remove the inherent autofluorescence of the enamel organic layer from the final images.
RESULTS
LC-tandem MS identifies protein fragments derived from MMP-20 in extracts of in vitro irradiated crowns
Crown extracts were subjected to gel electrophoresis and then individual gel slices were trypsinized and analyzed by LC-tandem mass spectrometry in order to identify candidate MMPs for further study. Interestingly, characteristic MMP-20 peptides were identified at 24–22 and 19 kDa (Table 1). Based upon the broad peptide coverage reflected in the 22–24 kDa region, we presume this peak represents several overlapping degraded protein fragments co-migrating in the gel. Since this analysis did not detect other MMPs, we then carried out a more complete analysis of MMP-20 in tooth extracts.
Table 1.
Identification of 23 kDa and 19 kDa MMP-20 fragments in gel bands by LC-MS after polyacrylamide gel electrophoresis of in vitro irradiated tooth crown extracts which were incubated for 3 months at 37°C.
| MMP-20 peptide position | Tryptic peptide sequence identified | 24-22 kDa bands | 19 kDa band |
|---|---|---|---|
| 110–116 | LFPGEPK | X | |
| 129–140 | YTPSMSSVEVDK | X | |
| 141–159 | AVEMALQAWSSAVPLSFVR | X | |
| 187–210 | GTLAHAFAPGEGLGGDTHFDNAEK | X | |
| 252–260 | NPYGFHLPK | X | |
| 265–273 | GIQALYGPR | X | |
| 357–363 | GTAYFFK | X | X |
| 372–380 | GFQMQGPPR | X | X |
| 381–389 | TIYDFGFPR | X | X |
| 390–401 | HVQQIDAAVYLR | X | X |
| 464–476 | YDTEKEDVVSVVK | X | X |
Western blotting confirms presence of MMP-20
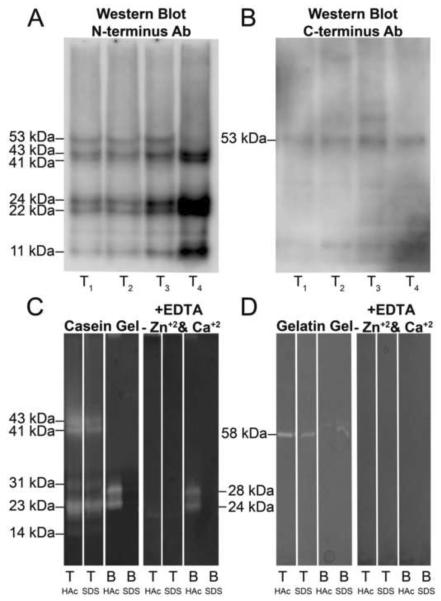
To confirm the presence of MMP-20 in the crown extracts, four individual control crown extracts were subjected to Western blot analysis for the N-terminus and the C-terminus of MMP-20. Immunoreactive bands were detected at approximately 53, 43, 41, 24, 22 and 11 kDa with an antibody against the N-terminal domain of MMP-20 (Fig. 1A). In contrast, only the 53 kDa band was detected with a C-terminal domain specific antibody (Fig. 1B). The 53 kDa band is similar in size to the pro-form of human MMP-20 and represented approximately 18% of the average density of all the bands. A 43 and 41 kDa doublet corresponded to the expected sizes for active MMP-20 and represented about 24% of the total band density. Immunoreactive bands at 24, 22 and 11 kDa represent fragments of MMP-20 presumed to be missing the C-terminal domain. Average total relative densities for these bands were 27%, 20%, and 11%, respectively.
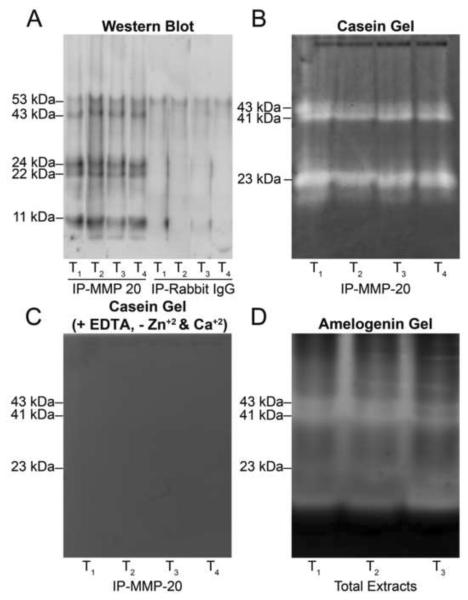
Figure 1. A and B. Western blot for MMP-20 in extracts from crowns of four individual mature human teeth.
Four tooth crowns were individually extracted and processed, and are referred to as T1–T4. A, Six bands were detected at 53, 43, 41, 24, 22 and 11 kDa with an antibody against the N-terminal domain of MMP-20. These bands corresponded in size to the pro-form (53 kDa), activated doublet forms (43 and 41 kDa) and fragments (24, 22 and 11 kDa) of MMP-20. B, Only the pro-form (53 kDa) of MMP-20 was detected by an antibody against the C-terminal domain of MMP-20. Molecular weight estimates were made reference to globular standards run on the same gel. C and D. Casein gel zymography shows extracts of human dental crowns, but not of bone, are enriched in MMP-20 bands. C, left panel, Casein gel zymography demonstrates MMP-20 like bands are present in extracts of human tooth crowns but not from rat bone. KEY: T, crown extracts exchanged into and lyophilized from 5% HAc or 0.05% SDS, respectively; B, rat calvarial bone extracts exchanged into and lyophilized from 5% HAc or 0.05% SDS, respectively. C, Right panel, Negative control for casein zymography (with EDTA and without zinc and calcium supplementation). Please refer to KEY above. D, Left panel, Gelatin gel zymography reveals a single 58 kDa band in extracts of human tooth crowns. Please refer to KEY above. D, Right panel, Negative control for gelatin zymography (with EDTA and without zinc and calcium supplementation). Please refer to KEY above.
Gel zymography reveals MMP-20 bands are catalytically active
Prominent doublet bands at 43 and 41 kDa and one centered at 23 kDa were observed along with two weaker bands at 31 and 14 kDa [lane 1, Fig. 1C (left panel)]. Consistent with an MMP assignment for these bands, no activity was observed in the presence of EDTA and in the absence of exogenous zinc and calcium ions [lane 1, Fig. 1C (right panel)]. The three major bands observed at 43, 41 and 23 kDa in casein zymography are those predicted for active forms of MMP-20, whereas two weak bands at 31 and 14 kDa do not correspond to known fragments of MMP-20. When extracts were analyzed by gelatin zymography, only one band was visible at 58 kDa [lanes 1 and 2, Fig. 1D (left panel)].
Comparison of crown extracts with those for rat calvarial bone showed that the latter did not contain analogous MMP-20 bands [compare lane 1 with lanes 3 and 4, Fig. 1C (left panel)]. Furthermore, caseinolytic bands at 24 and 28 kDa detected in bone extracts were not zinc and calcium dependent metalloproteinases [B lanes, Fig. 1C], while we presume doublet gelatinolytic bands at 60 and 62 kDa are MMP-2 [B lanes, Fig. 1D (left panel)]. Finally, since more caseinolytic and gelatinolytic activity was recovered when crown and bone extracts were exchanged into and lyophilized from 5% acetic acid rather than from 0.05% SDS [compare lanes 1 and 2, Fig. 1C (left panel)], all subsequent extractions utilized this methodological step.
MMP-20 survives high dose irradiation of teeth in vitro and in vivo
To determine whether MMP-20 is altered by high dose in vitro irradiation of teeth, we carried out two studies.
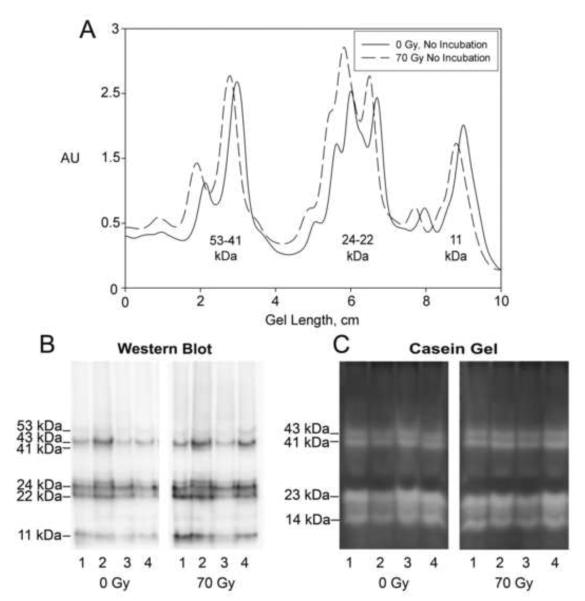
First, mature third molars were irradiated in vitro with 70 Gy over a course of 7 weeks. These teeth, along with their non-irradiated matched-pair controls, were then immediately frozen, pulverized, extracted, and subjected to comparative western blotting and casein gel zymography (Fig. 2). Western blotting of these extracts showed that the relative amount and size of recovered MMP-20 bands were not significantly changed (Figs. 2A and B). Likewise, relative distribution of MMP-20 caseinolytic activity was not altered by radiation exposure (Fig. 2C). Thus high dose irradiation does not have an immediate short-term effect on the relative protein structure or catalytic activity of MMP-20 present within teeth.
Figure 2. Comparison of extracts from non-irradiated control teeth with those from in vitro irradiated teeth demonstrates that the protein composition of MMP-20 is unchanged and that MMP-20 retains its activity.
A, Representative densitometric scans for MMP-20 bands after western blotting demonstrate no significant change in the banding pattern for MMP-20 in in vitro irradiated teeth as compared to their paired controls. B, Western blot for MMP-20 in extracts from control and in vitro irradiated teeth. C, Casein gel zymography on a series of extracts shows MMP-20 activity profile in the in vitro irradiated teeth is similar to that in the matched pair controls. KEY: Numbering of gel lanes refers to corresponding, individual tooth extracts.
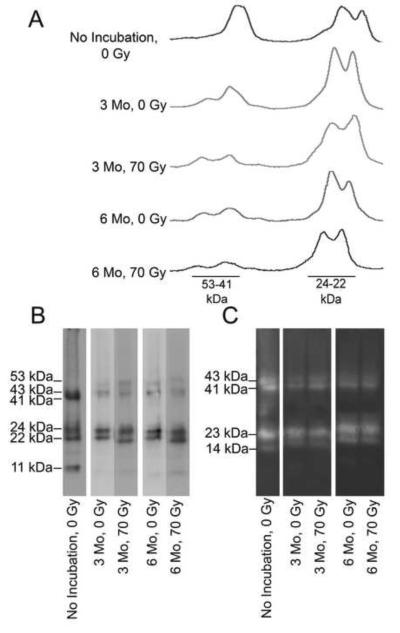
To study the combined effect of radiation and incubation on MMP-20, groups of in vitro irradiated teeth were also subsequently incubated at 37°C for either 3 or 6 months and then compared to paired non-irradiated control teeth incubated at 37°C for either 3 or 6 months. Western blotting showed that the 23 kDa MMP-20 band was the most prominent immunoreactive band after radiation and/or 37°C incubation (Figs. 3A and B). When similarly loaded gel lanes were compared by densitometry after western blotting, the region near 23 kDa was relatively unchanged while the 41/43 kDa doublet decreased (Fig. 3A, B, and C). Visual inspection of zymographic images revealed a similar assessment, e.g., the amount of active 23 kDa MMP-20 fragment was relatively unchanged by long term incubation at 37°C alone or when combined with high dose irradiation (Fig. 3C). In contrast, lower amounts of active 41/43 kDa MMP-20 were observed after treatment.
Figure 3. A comparison of the effect of time, temperature and radiation shows that the Mr=22-24 kDa MMP-20 doublet fragments are most the stable.
A, Representative densitometric scans for MMP-20 bands after western blotting show that the Mr=22-24 kDa MMP-20 fragments are the most stable to radiation damage and subsequent incubation at 37°C. Please refer to the Key below. B, Representative western blot for MMP-20 shows that the Mr=22-24 kDa MMP-20 fragments are the most stable to radiation damage and subsequent incubation at 37°C. crown extracts of teeth that have been treated differently in regards to the time of incubation at 37°C vs. storage at −80°C, and radiation. Please refer to the Key below. C, Casein gel zymographic analysis demonstrates that Mr=22-24 kDa MMP-20 fragments are the most stable to radiation damage and subsequent 37°C incubation. KEY: No incubation: tooth was frozen immediately at − 80°C; 3 Mo: tooth was incubated at 37°C for 3 months. 6 Mo: tooth was incubated at 37°C for 6 months. 0 Gy: tooth was not irradiated; 70 Gy: tooth received a total radiation dose of 70 Gy.
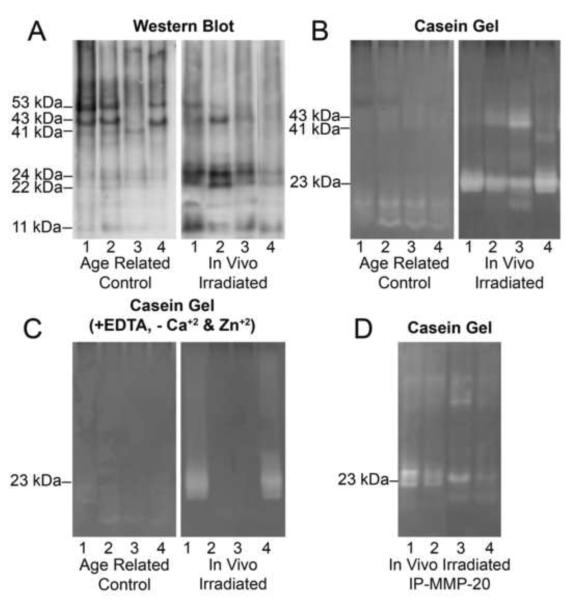
In the second study, we compared extracts from teeth of oral cancer patients that were calculated to have received an average dose of 65 Gy of irradiation (in vivo irradiated teeth) with extracts from age appropriate controls. Patient ages were 41–53 years at the time of irradiation and the time between radiotherapy and extraction ranged from 4 to 11 years. Control subject ages ranged from 50–78 years. In contrast to controls which were enriched in the 53/43 kDa forms of MMP-20 (Fig. 4A), in vivo irradiated crowns are enriched in the ~23 kDa MMP-20 form. When these extracts were assayed for caseinolytic activity with gel zymography, most of the activity in the in vivo irradiated extracts was also localized at 23 kDa (Fig. 4B). Interestingly, when the in vivo irradiated extracts were assayed with EDTA and without exogenous zinc and calcium, a broad unidentified 23 kDa band was observed in half of the extracts (Fig. 4C) which resembled a similar sized unidentified protease in bone extracts (Fig. 1C). Subsequent immunoprecipitation studies showed that the 23 kDa fragment was the most prominent MMP-20 detected, although overall recovery from one out of four teeth was lower (Fig. 4D).
Figure 4. Comparison of extracts from in vivo irradiated teeth with those from age related control teeth demonstrates Mr=22-24 kDa MMP-20 forms are enriched in the in vivo irradiated teeth.
A, Western blot for MMP-20 in extracts from in vivo irradiated teeth shows that MMP-20 is mainly present as Mr=22-24 kDa doublet bands as compared to controls where it is mainly present as Mr= 53 and 43 kDa forms. B, Casein zymography on a series of extracts shows minimal activity in control teeth as compared to in vivo irradiated teeth where activity was observed as a broad Mr= 23kDa band. C, Caseinolytic activity was observed in some of the in vivo irradiated tooth extracts in the presence of EDTA and the absence of zinc and calcium supplementation. D, Extracts from in vivo irradiated teeth were immunoprecipitated with anti-MMP-20 antibodies and then subjected to Casein gel zymography. KEY: Lane numbers refer to extracts of corresponding individual irradiated teeth.
Immunoprecipitation supports identity of MMP-20 bands from casein zymography
Crown extracts were immunoprecipitated using an antibody against the N-terminal domain of MMP-20 and then immunoblotted. Five bands were detected at 53, 42, 22, 19 and 11 kDa, whereas no bands were detected in controls with non-immune IgG (Fig. 5A).
Figure 5. Results of immunoprecipitation with anti-N-terminal MMP-20 antibody.
Extracts T1–T4 were each derived from four individual crowns. A, MMP-20 immunoprecipitated from extracts of four individual teeth. Five bands at 53, 42, 24, 22 and 11 kDa were detected in immunoprecipitates. No bands (except for minor reactivity at 53 kDa) were observed in the negative controls using non-immune rabbit IgG. B, Casein zymography on immunoprecipitates identified an active doublet at 43 and 41 kDa and a doublet band at 24 and 22 kDa. C, No caseinolytic activity was observed in the presence of EDTA and the absence of zinc and calcium supplementation. D, Amelogenin zymography of immunoprecipitates identified an active doublet at 43 and 41 kDa and a band at 23 kDa.
When immunoprecipitates were further characterized by casein zymography, only doublet bands at 43 and 41 kDa, and a band at 23 kDa, were detected (Fig. 5B). Comparison of these findings with those from Figure 1C revealed that immunoprecipitation had removed weak bands at 31 and 14 kDa while retaining bands at 43/41 and 23 kDa. While we also expected to see caseinolytic activity at 53 kDa, the size of pro-MMP-20, we could not identify any published report which showed active 53 kDa MMP-20 in enamel extracts from mature teeth. We therefore conclude that the 53 kDa MMP-20 band detected in Western blotting may be catalytically inactive. Consistent with their MMP character, the catalytic activity of 43/41 and 23 kDa bands was dependent on added zinc and calcium ions (Fig. 5C). Analyses with immunoprecipitates indicate that extracts of mature human crowns are enriched in three catalytically active fragments of MMP-20.
Extracts were also tested for their capacity to cleave amelogenin, a physiological substrate of MMP-20. As shown in Figure 5D, recombinant amelogenin was cleaved by MMP-20, yielding three bands at 43, 41 and 23 kDa which were identical in size to the MMP-20 bands detected by casein zymography (Fig. 5B).
MMP-20 is present within the enamel matrix, the dentin-enamel junction, and dentin tubules
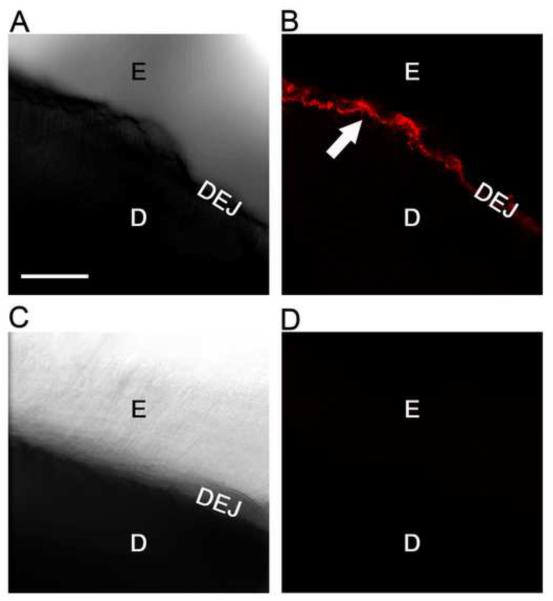
In view of our focus on enamel, we carried out confocal microscopic immunofluorescent labeling of MMP-20 in cropped crown sections in which all but several millimeters of dentin was removed with a motorized burr. Average age of the subjects from whom the teeth were obtained was 20 years old. Immunofluorescently labeled MMP-20 protein was localized strongly to the dentin-enamel junction as evidenced by a close correspondence of these images with those of the optically visible DEJ in bright field (see arrow, Fig. 6 A and B). The apparent width of the DEJ varies slightly in these images due to the tangential nature of the cutting plane across the section. Weaker immunostaining for MMP-20 was also detectable in dentin tubules adjoining the DEJ and in the adjacent enamel organic matrix layer (Fig. 6 A and B). In contrast, controls using non-immune IgG were devoid of fluorescent staining.
Figure 6. Confocal microscopy reveals MMP-20 is enriched in the dentin enamel junction within mature human teeth.
A, Ten micron thick stacked bright field image (10X) of a demineralized cropped crown section immunostained for MMP-20, showing enamel (E), dentin (D) and dentin enamel junction (DEJ). B, Fluorescent field image (10X) of same section shown in A showing that MMP-20 (pseudo colored in red) is strongly immunostained at the DEJ (white arrow). Weaker reactivity is evident in the adjoining enamel matrix and adjacent dentin tubules. C, Ten micron thick stacked bright field negative control image (10X) of a demineralized crown section immunostained similarly but with purified non-immune rabbit IgG. D, Fluorescent field image (10X) of the crown section shown in C showing no staining. Scale bar=100 microns (all images same magnification).
DISCUSSION
The data presented here support the following statements. Based on mass spectrometry, immunoblotting, and gel zymography, MMP-20 represents the most prominent matrix metalloproteinase in extracts of in vitro irradiated and incubated mature tooth crowns, as well as paired control crowns. Second, MMP-20 in mature human crowns is comprised of three catalytically active forms at Mr=43, 41, and 23 kDa, although a presumed precursor at 53 kDa and a smaller 11 kDa form were also detected immunologically. Third, the proportion of different sized MMP-20 forms in tooth extracts is dependent upon patient history and tooth treatment. Specifically, the pattern of MMP-20 bands was not directly changed by exposure of control teeth to 70 Gy. However, for teeth subjected to radiation and incubation at 37°C for 3 and 6 months, the proportion of the 23 kDa MMP-20 form increased dramatically. Interestingly, extracts of teeth from oral cancer patients (ages 41–53) who received >60 Gy radiation and were removed some years later, also largely contained 23 kDa MMP-20 form as compared to their age-related controls (ages 50–78) which mainly contained the higher molecular weight forms of MMP-20 (i.e., 43–41 kDa and 53 kDa). Fourth, within enamel, MMP-20 protein was localized to the dentin enamel junction, to the enamel matrix, and to adjacent dentin tubules. Our in vitro results indicate that the Mr=23 kDa fragment of MMP-20 is a radiation-resistant component of mature tooth crowns; similar analyses of teeth irradiated in vivo also identify the 23 kDa fragment as the predominant MMP-20 species. Due to its broad substrate specificity, neutral pH range, and morphological enrichment at the DEJ, we hypothesize that 23kDa MMP-20 could proteolytically degrade the protein components of the enamel-dentin interface following irradiation, which may contribute to pathologic enamel delamination observed after oral radiotherapy.
In an effort to identify the mechanism responsible for enamel delamination associated with high dose radiotherapy for oral cancer, we have focused on MMPs because radiation has been shown to cause their activation and induce their expression(7) and because of their capacity to degrade components of extracellular basement membrane structures like the DEJ. Initial mass spectrometric screening of irradiated tooth extracts only identified MMP-20. Subsequent immunoprecipitation studies and gel zymography confirmed this assignment and identified three catalytically active forms (43, 41, and 23 kDa) and two inactive forms (53 and 11 kDa). In addition to cleaving amelogenin here, MMP-20 is known to display a broad substrate specificity for basement membrane components fibronectin, collagen type IV, laminin, and tenascin-C(17). Catalytically active MMP-2 and MMP-9 have also been reported to be enriched in dentin(18), although gelatin zymographic analyses here only detected low levels. We speculate that the lower concentrations of MMP-2 and MMP-9 than MMP-20 detected in crown extracts may reflect real quantitative differences in mature teeth or may be due to the latter's inherent greater stability.
MMP-20 expression was shown previously to be restricted developmentally to ameloblasts and odontoblasts in teeth as well as to odontogenic tumor cells(19, 20). Secreted along with enamel matrix proteins during amelogenesis, MMP-20 functions to degrade amelogenin, enamelin, and ameloblastin(8, 21, 22) thus facilitating enamel mineralization(8). MMP-20 also facilitates dentin mineralization in a similar fashion(9, 10). Despite report by Sulkala et al. (23) showing it was present in mature dentin and odontoblasts, MMP-20 is generally believed to be largely degraded and removed from developing teeth before they are fully mineralized(11, 24).
Our results suggest that in vitro proteolytic processing of MMP-20 is different than that in vivo. For example, extracts of young teeth (ages 18–22) yield similar MMP-20 compositions containing 53, 43, 41, 21, and 11 kDa bands. Exposure to high dose radiation alone did not alter this pattern, however, subsequent in vitro incubation for 3 and 6 months at 37°C alone or after high dose radiation shifted toward profiles enriched in 23 kDa MMP-20. In contrast to older control teeth (ages 50–78) and young control teeth (ages 18–22), extracts of teeth from irradiated oral cancer patients (ages 41–53) are enriched predominantly in 23 kDa MMP-20. These findings suggest that MMP-20 in teeth is not affected directly by high dose radiation exposure but rather that prior irradiation favors processing in vivo to yield 23 kDa MMP-20. Based on its broad catalytic specificity, its activity displayed in tooth extracts, and its localization to the dentin enamel junction in mature teeth, we suggest that 23 kDa MMP-20 mediated degradation could play a causative role in enamel delamination associated with oral cancer radiotherapy.
ACKNOWLEDGEMENTS
Supported by NIDCR RO1 grant DE021462. This work is free of any conflicts of interest. We acknowledge use of the confocal microscope at the University Missouri, Kansas City School of Dentistry Confocal Microscopy Core. This facility is supported by the UMKC Office of Research Services, UMKC Center of Excellence in Dental and Musculoskeletal Tissues, and NIH grant S10RR027668”.
ABBREVIATIONS
- Gy
Gray
- MMP-20
matrix metalloproteinase 20 or enamelysin
- MMP
matrix metalloproteinase
- SDS
sodium dodecyl sulfate
- HAc
acetic acid
- DEJ
dentin enamel junction
- LC
liquid chromatographic
- MS
mass spectrometry
- PBS
phosphate buffered saline
- BSA
bovine serum albumin
- PTO
phosphate-Tween-20 ovalbumin buffer
- EDTA
ethylene diaminetetraacetic acid
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
- Buffer A, PBS (pH 7.4)
containing 0.05% Tween 20 and 0.1% BSA
- Buffer B, PBS (pH 7.4)
containing 0.05% Tween 20 and 1% BSA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Overman VP. The Oral Cancer Foundation. International Journal of Dental Hygiene. 2013;7(3):229–30. doi: 10.1111/j.1601-5037.2009.00408.x. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE, Stern D. Oral and Maxillofacial Pathology: A Rationale for Diagnosis and Treatment. Quintessence Co, Inc; Chicago: 2002. pp. 380–81. [Google Scholar]
- 3.Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 4.Walker MP, Wichman B, Cheng AL, Coster J, Williams KB. Impact of Radiotherapy Dose on Dentition Breakdown in Head and Neck Cancer Patients. Pract Radiat Oncol. 2011;1(3):142–48. doi: 10.1016/j.prro.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sognnaes RF. The Organic Elements of the Enamel: II. the Organic Framework of the Internal Part of the Enamel. with special Regard to the Organic Basis for the So-Called Tufts and Schreger's Bands. J Dent Res. 1949;28:549–57. doi: 10.1177/00220345490280060401. [DOI] [PubMed] [Google Scholar]
- 6.Dusevich V, Xu C, Wang Y, Walker MP, Gorski JP. Identification of a protein-containing enamel matrix layer which bridges with the dentine-enamel junction of adult human teeth. Arch Oral Biol. 2012;57(12):1585–94. doi: 10.1016/j.archoralbio.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strup-Perrot C, Vozenin-Brotons MC, Vandamme M, Benderitter M, Mathe D. Expression and activation of MMP -2, -3, -9, -14 are induced in rat colon after abdominal X-irradiation. Scand J Gastroenterol. 2006;41(1):60–70. doi: 10.1080/00365520510023963. [DOI] [PubMed] [Google Scholar]
- 8.Uskokovic V, Khan F, Liu H, Witkowska HE, Zhu L, Li W, et al. Hydrolysis of amelogenin by matrix metalloprotease-20 accelerates mineralization in vitro. Arch Oral Biol. 2011;56(12):1548–59. doi: 10.1016/j.archoralbio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beniash E, Skobe Z, Bartlett JD. Formation of the dentino-enamel interface in enamelysin (MMP-20)-deficient mouse incisors. Eur J Oral Sci. 2006;114(Suppl 1):24–9. doi: 10.1111/j.1600-0722.2006.00293.x. discussion 39–41, 379. [DOI] [PubMed] [Google Scholar]
- 10.Fanchon S, Bourd K, Septier D, Everts V, Beertsen W, Menashi S, et al. Involvement of matrix metalloproteinases in the onset of dentin mineralization. Eur J Oral Sci. 2004;112(2):171–6. doi: 10.1111/j.1600-0722.2004.00120.x. [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Hu JC, Smith CE, Bartlett JD, Simmer JP. Kallikrein-related peptidase 4, matrix metalloproteinase 20, and the maturation of murine and porcine enamel. Eur J Oral Sci. 2011;119(Suppl 1):217–25. doi: 10.1111/j.1600-0722.2011.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorski JP, Kremer EA, Chen Y, Ryan S, Fullenkamp C, Delviscio J, et al. Bone acidic glycoprotein-75 self-associates to form macromolecular complexes in vitro and in vivo with the potential to sequester phosphate ions. J Cell Biochem. 1997;64(4):547–64. [PubMed] [Google Scholar]
- 13.Keightley JA, Shang L, Kinter M. Proteomic analysis of oxidative stress-resistant cells: a specific role for aldose reductase overexpression in cytoprotection. Mol Cell Proteomics. 2004;3(2):167–75. doi: 10.1074/mcp.M300119-MCP200. [DOI] [PubMed] [Google Scholar]
- 14.Gorski JP, Kremer EA, Chen Y. Bone acidic glycoprotein-75 self-associates to form large macromolecular complexes. Connect Tissue Res. 1996;35(1–4):137–43. doi: 10.3109/03008209609029184. [DOI] [PubMed] [Google Scholar]
- 15.Kremer EA, Chen Y, Suzuki K, Nagase H, Gorski JP. Hydroxyapatite induces autolytic degradation and inactivation of matrix metalloproteinase-1 and -3. J Bone Miner Res. 1998;13(12):1890–902. doi: 10.1359/jbmr.1998.13.12.1890. [DOI] [PubMed] [Google Scholar]
- 16.Gorski JP, Griffin D, Dudley G, Stanford C, Thomas R, Huang C, et al. Bone acidic glycoprotein-75 is a major synthetic product of osteoblastic cells and localized as 75- and/or 50-kDa forms in mineralized phases of bone and growth plate and in serum. J Biol Chem. 1990;265(25):14956–63. [PubMed] [Google Scholar]
- 17.Vaananen A, Srinivas R, Parikka M, Palosaari H, Bartlett JD, Iwata K, et al. Expression and regulation of MMP-20 in human tongue carcinoma cells. J Dent Res. 2001;80(10):1884–9. doi: 10.1177/00220345010800100501. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86(5):436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 19.Takata T, Zhao M, Uchida T, Wang T, Aoki T, Bartlett JD, et al. Immunohistochemical detection and distribution of enamelysin (MMP-20) in human odontogenic tumors. J Dent Res. 2000;79(8):1608–13. doi: 10.1177/00220345000790081401. [DOI] [PubMed] [Google Scholar]
- 20.Turk BE, Lee DH, Yamakoshi Y, Klingenhoff A, Reichenberger E, Wright JT, et al. MMP-20 is predominately a tooth-specific enzyme with a deep catalytic pocket that hydrolyzes type V collagen. Biochemistry. 2006;45(12):3863–74. doi: 10.1021/bi052252o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamakoshi Y, Hu JC, Fukae M, Yamakoshi F, Simmer JP. How do enamelysin and kallikrein 4 process the 32-kDa enamelin? Eur J Oral Sci. 2006;114(Suppl 1):45–51. doi: 10.1111/j.1600-0722.2006.00281.x. discussion 93–5, 379–80. [DOI] [PubMed] [Google Scholar]
- 22.Iwata T, Yamakoshi Y, Hu JC, Ishikawa I, Bartlett JD, Krebsbach PH, et al. Processing of ameloblastin by MMP-20. J Dent Res. 2007;86(2):153–7. doi: 10.1177/154405910708600209. [DOI] [PubMed] [Google Scholar]
- 23.Sulkala M, Larmas M, Sorsa T, Salo T, Tjaderhane L. The localization of matrix metalloproteinase-20 (MMP-20, enamelysin) in mature human teeth. J Dent Res. 2002;81(9):603–7. doi: 10.1177/154405910208100905. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett JD, Simmer JP. Proteinases in developing dental enamel. Crit Rev Oral Biol Med. 1999;10(4):425–41. doi: 10.1177/10454411990100040101. [DOI] [PubMed] [Google Scholar]