Abstract
Hepatocyte growth factor (HGF) has been shown to have anti-fibrotic, pro-angiogenic, and cardioprotective effects; however, it is highly unstable and expensive to manufacture, hindering its clinical translation. Recently, a HGF fragment (HGF-f), an alternative c-MET agonist, was engineered to possess increased stability and recombinant expression yields. In this study, we assessed the potential of HGF-f, delivered in an extracellular matrix (ECM)-derived hydrogel, as a potential treatment for myocardial infarction (MI). HGF-f protected cardiomyocytes from serum-starvation and induced down-regulation of fibrotic markers in whole cardiac cell isolate compared to the untreated control. The ECM hydrogel prolonged release of HGF-f compared to collagen gels, and in vivo delivery of HGF-f from ECM hydrogels mitigated negative remodeling, improved fractional area change (FAC), and increased arteriole density in rat myocardial infarction model. These results indicate that HGF-f may be a viable alternative to using recombinant HGF, and that an ECM hydrogel can be employed to increase growth factor retention and efficacy.
Keywords: myocardial infarction, growth factor, extracellular matrix, decellularization, hydrogel
Introduction
The development of new therapies to treat myocardial infarction (MI) is a pressing clinical need. One of the experimental therapies for MI is cellular cardiomyoplasty, in which cells are transplanted into the infarct or borderzone regions. This approach has met with some success; however, the mechanism of action of these cells is thought to be due in large part to paracrine signaling [1-5]. This has led to work identifying important paracrine factors for treating MI [1-4]. One cytokine that has emerged as a strong candidate is hepatocyte growth factor (HGF), a potent agonist for the tyrosine kinase surface receptor c-MET, with demonstrated anti-apoptotic, pro-angiogenic, anti-fibrotic, and cardioprotective activity in vitro and in vivo [6-8]. HGF has been applied in animal models of heart disease and shown to provide benefit even in ischemic cardiomyopathy following old MI or hereditary cardiomyopathy [9-11].
Widespread translation of recombinant human HGF (rh-HGF) for cardiovascular disease has however been hindered by two factors: ease of manufacturing and sustained delivery of the protein. Mammalian cell culture methods required for rh-HGF are challenging and expensive, and the protein itself is unstable, limiting its clinical development and translation. Protein delivery is also susceptible to rapid diffusion away from the injection site as well as rapid degradation. In this study, we evaluated the therapeutic potential of a stable c-MET agonist, developed through rational and combinatorial protein engineering methods [12], as an alternative to rh-HGF. The dimeric form of this agonist (cd D127N) can be produced at high yield in a yeast expression system [12] and has been shown to have similar potency as rh-HGF in vitro [13].
Immobilization of growth factors in biomaterial scaffolds has been shown to enhance their effect by increasing stability and activity [14]. These systems also mimic the native microenvironment, where heparin-binding growth factors, such as HGF, are retained locally by sulfated sugars in the extracellular matrix (ECM). We previously demonstrated that the sulfated glycosaminoglycan (sGAG) content of an injectable decellularized ECM-derived hydrogel provides a platform for the sequestration and enhanced delivery of basic fibroblast growth factor (bFGF) [15]. In the current study, we tested whether this biomaterial could increase retention and efficacy of the engineered HGF fragment (HGF-f). We first confirmed activity of this HGF-f on cardiac relevant cell types showing activity with rat vascular cells, and increased survival and decreased fibrosis markers in cardiac derived cells. We further demonstrate that the ECM hydrogel increases retention of HGF-f, and showed that a single injection of HGF-f, delivered in the ECM hydrogel, preserved LV geometry, improved fractional area change, and increased vascularization post-MI in a rat occlusion-reperfusion model. These results demonstrate the therapeutic potential of HGF-f and the ECM hydrogel delivery system.
Materials and Methods
All experiments in this study were conducted in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of California, San Diego and the American Association for Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee at UCSD.
HGF-f preparation and characterization
The monomeric form of HGF-f is comprised of the N domain and first kringle domain (NK1) of HGF, and contains seven point mutations that confer increased thermal stability and soluble expression yield [12]. Upon expression in yeast, HGF-f dimers form spontaneously via a disulfide bond formed through a cysteine residue introduced at the N terminus of the NK1 monomer. This c-Met agonist, previously termed cd D127N, is referred to as HGF-f in the current study. Yeast growth and induction media, as well as detailed protein expression and purification methods, were performed as previously described [12]. Briefly, DNA encoding for HGF-f was cloned into the pPIC9K plasmid (Life Technologies, Grand Island, NY) and transformed into P. pastoris strain GS115. Colonies surviving geneticin selection were inoculated and induced with methanol for three days. Yeast cells were pelleted by centrifugation, and the supernatant collected for Ni-NTA affinity chromatography. The elution fractions containing HGF-f were buffer-exchanged into 1×PBS + 500mM NaCl (PBS500) and further purified with size exclusion chromatography using a Superdex 75 10/300 GL (GE Healthcare, Pittsburgh, PA). Protein purity was analyzed using 12% Tris-Glycine SDS-PAGE (Life Technologies, Grand Island, NY). Protein was flash-frozen in 0.1% Tween20 in PBS500 and stored at -80°C. Thawed protein was kept at 4°C and used within three weeks.
In vitro cell experiments
For western blot analysis, primary rat aortic smooth muscle cells (RASMCs) were plated in 6-well plates and grown until 50% confluence. The cells were then starved for 12 hours in basal 4.5 g/L glucose-Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Carlsbad, CA) + 0.1% BSA (Roche, Indianapolis, IN), followed by addition of 1 nM of HGF-f or rh-HGF (R&D Systems, Minneapolis, MN) for ten and thirty minute time points. Cells were then washed with 1× PBS and lysed with 100 μL NP-40 buffer. Proteins from all conditions were quantified using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA) and then diluted to equal concentrations. Equal volumes of each condition were run on an 8% SDS-PAGE gel, followed by Western blot probing for phosphorylated and total downstream targets. After secondary antibody incubation, horseradish peroxidase-catalyzed luminescence was detected with the ChemiDoc XRS System (Bio-Rad, Hercules, CA). Primary antibodies for Western, except for β-tubulin, were all from Cell Signaling (Danvers, MA), β-tubulin antibody was from Covance (Princeton, NJ). pAkt, Akt, and total Met were used at 1:1000 dilution, while pErk and Erk were used at 1:3000 dilution. pMet was used at 1:500 dilution, while β-tubulin was used at 1:5000 dilution. All secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA) and were used at 1:5000 in 4% milk solution. NP-40 lysis buffer was 20 mM Tris pH 8.0, 137 mM NaCl, 10% glycerol, and 1% IGEPAL/NP40.
Neonatal cardiomyocytes were isolated from 1 to 2 day old Sprague-Dawley rats using the Neonatal Cardiomyocyte Isolation System (Worthington Biomedical Corporation, Lakewood, NJ). The cardiomyocytes were cultured in DMEM, 10% fetal bovine serum (Hyclone), and 0.5% Penicillin/Streptomycin (P/S, Invitrogen, Carlsbad, CA) antibiotic. Prior to cell seeding, all tissue culture surfaces were collagen coated with 1 mg/mL collagen diluted in 0.1 M acetic acid. Cells were cultured at 37°C and 5% CO2.
For 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays, the crude cell isolate was pre-plated for 2 hours at 37°C to remove the majority of the cardiac fibroblast population. The suspension was collected and plated at 250,000 cells per well of a collagen coated 48-well plate. Cells were allowed to adhere for 24 hours after which the cells were switched to serum free DMEM and 0.5% P/S for another 24 hours. The experimental groups (untreated or 1 nM HGF-f) were then sterile filtered and added to the wells in serum free media (n = 6/group). The MTT assay was performed after 24 hours in culture and absorbance was analyzed in a SynergyTM 4 Multi-Mode Microplate Reader BioTEK plate-reader at 540 nm.
For gene expression assays, the crude cell isolate (without pre-plating) was plated directly into a collagen coated 24-well plate at 500,000 cells per well (n = 3/group). Cells were cultured the same as previously stated for the MTT assay. RNA was isolated 24 hours after the treatment was added via the RNEasy isolation kit (Qiagen). cDNA was synthesized with via SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). Gene expression was measured with quantitative polymerase chain reaction using SYBR Green PCR Master Mix (Life Technologies). Rat specific primers for MMP-1 (F: 5′-TGGCAACCCATTACCAAGTCA-3′, R: 5′-AGAGCTCCTCTGTTGACCCT-3′), MMP-9 (F: 5′-CGTGGCCTACGTGACCTATGA-3′, R: 5′-TGCACCGCTGAAGCAAAAG-3′), TGFβ-1 (F: 5′-AGGACCTGGGTTGGAAGTGG-3′, R: 5′-AGTTGGCATGGTAGCCCTTG-3′) and GAPDH (F: 5′-ACTTTGGCATCGTGGAAGGG-3′, R: 5′-ACTTGGCAGGTTTCTCCAGG-3′) were designed (Invitrogen, Carlsbad, CA) and used at a 10 μM final concentration in 20 μL reactions. Samples were loaded in the CFX96 PCR Detection System (BioRad, Hercules, CA). Gene expression was normalized to GAPDH.
Extracellular matrix preparation
Porcine pericardial ECM matrix was prepared as previously described [16]. Briefly, porcine pericardium was decellularized with sodium dodecyl sulfate, and then lyophilized and milled into a powder and stored frozen. Prior to use, the milled samples were partially pepsin-digested in 0.1 M HCl and then buffered to pH 7.4. This injectable form of the ECM was then diluted in 1× PBS to a concentration of 6 mg/ml for injection into animals. When relevant, HGF-f was added to the 1× PBS at an appropriate concentration in order to deliver 10 μg of peptide in 75 μL of ECM.
Heparin binding and hydrogel retention
HGF-f was injected onto a heparin affinity column (GE Healthcare Biosciences, Pittsburgh, PA) and analyzed using a Varian Prostar chromatography system, run for 100 minutes at a flow rate of 0.25 mL/min with a gradient of PBS + 2M NaCl. The salt concentration needed to elute the protein was estimated from the time of elution. To evaluate HGF-f retention from biomaterials in vitro, sets (n = 6) of 200 μL ECM (6 mg/mL) or collagen (2.5 mg/mL) gels were formed overnight in microcentrifuge tubes, as previously described [15]. At these concentrations ECM-derived and collagen gels are similar with respect to mechanical properties and pore size. [15] 10 μg of HGF-f was added to both materials before gelation. After gelation, gels were rinsed to remove any unincorporated HGF-f, 200 μL of PBS was added to each sample, and gels were incubated at 37 °C. Supernatants were collected and exchanged every 24 hours over 5 days, at which point bacterial collagenase (Worthington Biomedical Corporation, Lakewood, NJ) at 100 U/mL in a 0.1 M Tris-base, 0.25 M CaCl2 solution, pH 7.4, was added to the gels for 4 hours at 37 °C to degrade the collagen. To release remaining HGF-f, 100 μL of a 1.5 M NaCl solution was added to each tube and incubated for 1 hour. Ni-NTA conjugated to horseradish peroxidase allowed for identification of HGF-f through its C-terminal hexahistidine tag. HGF-f in the rinse was used to determine the amount remaining in the gels, and cumulative release over time was calculated as a percent of the remaining HGF-f in the gels after the rinse step.
Rat occlusion-reperfusion model
Left coronary artery temporary occlusion was performed on female Sprague Dawley rats (225-250 g) under aseptic conditions, as previously described [15, 17]. Briefly, the heart was exposed using a left anterior thoracotomy, and the artery was occluded for 25 minutes using a 6-0 silk suture at 1-2 mm below the left atrial appendage. The chest was closed and the animals were allowed to recover. Buprenorphine was administered subcutaneously at 5 mg/kg to prevent postoperative pain and Lactated Ringer's Solution (3 cc) was given subcutaneously to the animals to prevent dehydration.
Echocardiography
Transthoracic echocardiography was performed one week post-MI to perform baseline measurements of cardiac function, and again at 4 weeks post-injection (5 weeks post-MI). Animals were anesthetized using 5% isoflurane and then administered at the lowest level of isoflurane necessary to maintain anesthesia during imaging (1-1.5%). Parasternal long axis images were obtained by an experienced sonographer using a GE Vivid i system with a 12 MHz transducer. LV volumes were calculated using a modified Simpson's Rule Method of Discs. Ejection fraction (EF) and fractional area change (FAC) were calculated as the diastolic minus systolic measurements divided by diastolic measurement using LV volumes and areas, respectively. FAC was calculated based on areas measured (end-diastolic and end-systolic) in parasternal long-axis views since the infarcts were largely apical. The ECG was continuously monitored during image acquisition to record heart rate and ensure there were no noticeable arrhythmias. An experienced cardiac sonographer blinded to the treatment groups performed all image acquisition and analysis.
Injection surgery
Animals were randomized one week after MI, and injected with either 75 μl of ECM hydrogel (n = 9), HGF-f in ECM hydrogel (n = 10), HGF-f in saline (n = 10), or saline (n = 12) using a previously described procedure [15, 17, 18]. Briefly, a similar procedure for anesthesia and analgesia as in the MI surgery was performed. A lateral incision was made below the xyphoid process, followed by an incision through the diaphragm. The pericardium was partially removed and the anterolateral portion of the heart was exposed. An intramyocardial injection of material or saline into the infarct area was performed using a 27 G needle. Presence of the injected material was verified by temporary discoloration of the tissue. The chest was then closed and the animal was allowed to recover.
Histology and immunohistochemistry
Animals were euthanized immediately after post-treatment echocardiography (5 weeks post-MI) by an IP injection of Fatal Plus (936 mg/kg). Hearts were excised, arrested in a solution containing 25 mM NaHC03, 2 mM CaCl2, 5 mM Dextrose, 2.7 mM MgS04, 22.8 mM KCl, 121.7 mM NaCl, and 20 mM 2,3 butanedione monoxime, and fresh frozen in Tissue Tek O.C.T. compound. Due to freezing artifacts, which prevented proper analysis, histological and immunological examination was performed on 4 to 8 hearts per group. Cryosections sections were stained with Masson's trichrome to visualize the infarct. To assess fibrosis, five slides evenly distributed throughout the infarct were selected from each heart and scanned at 20× magnification using an Aperio ScanScope CS2 slide scanner (Leica). The ‘Positive Pixel Count V9’ algorithm within ImageScope (Aperio) software was used to detect non-nuclear blue staining. Interstitial fibrosis was measured in non-infarcted septal wall regions. Infarct fibrosis percentage was determined from 20× images within the infarct region.
To assess vascular density, immunohistochemistry was performed on three slides evenly spaced through the infarct region, according to previously described methods [15, 19]. Briefly, cryosections were fixed with acetone, incubated with anti-smooth muscle actin antibody (Dako, Carpinteria, CA; 1:75 dilution) and FITC labeled isolectin (Vector Laboratories, Burlingame, CA; 1:100 dilution), and then stained with Alexa Fluor 568 anti-mouse antibody (Invitrogen, Carlsbad, CA; 1:2000 dilution). Nuclei were visualized with fluorescent Hoescht 33342. Images were taken with a 10× objective. Arterioles with lumen diameters in the range of 10 to 100 μm in the infarct area were quantified as previously described [20]. Capillary density was quantified by comparing the area stained positive for capillaries divided by the total area of the infarct region through a custom macro in ImageJ.
Cross-sectional area of cardiomyocytes was assessed as previously described [21, 22]. Briefly, cryosections were fixed with acetone, incubated with an anti-laminin antibody (Abcam, Cambridge, MA; 1:100 dilution) and stained with AlexaFluor 488 goat anti-rabbit (Invitrogen, Carlsbad, CA; 1:500 dilution). Nuclei were stained with Hoechst 33342. Four images (200×) of the remote myocardium were captured for three different slides of each heart using Axio Vision software. Cross-sectional areas of cardiomyocytes with centered nuclei were outlined and their area was measured using ImageJ. Each group had n>300 cells.
Statistical analysis
Differences between in vitro data were assessed using a student's t-test. Baseline and post-treatment echocardiographic measures were assessed using a two-tailed paired t-test. All other statistical analysis was performed using one-way ANOVA followed by Bonferroni's multiple comparisons test. All measurements were reported mean ± SEM. Significance was accepted at p < 0.05.
Results
In vitro activity of HGF-f
Previous work with the engineered HGF-f protein demonstrated activation of c-MET receptor in canine [12] and human cells [13]; thus, before proceeding with a rodent MI study, we first validated biological efficacy in a relevant cell type. At a concentration of 1 nM, HGF-f stimulated the phosphorylation of c-MET and its downstream targets Akt and Erk in RASMCs similar to rh-HGF (Figure 1). Full length rh-HGF has previously been shown to have cardioprotective effects in vivo post-infarct [7]. To confirm that HGF-f has cardioprotective activity, we tested its ability to increase viability of serum-starved rat neonatal cardiomyocytes. Treating cells with HGF-f resulted in a significant 22% increase in metabolic activity compared to the untreated control (Figure 2A).
Figure 1. Stimulation of c-MET and downstream pathways in RASMCs.
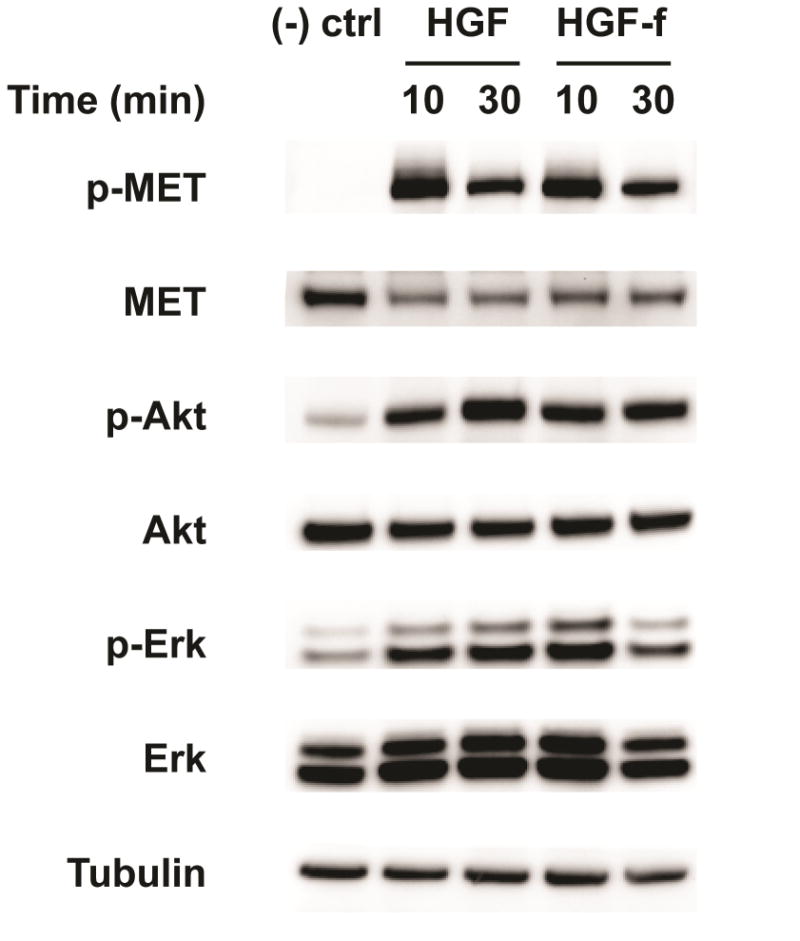
HGF-f or rh-HGF was added to RASMC cultures at a concentration of 1 nM for the indicated timepoints. Phosphorylated (p) and total amounts of c-MET, Akt, and Erk are shown, as analyzed by Western blotting of cell lysates, and demonstrate that HGF-f is capable of activating c-MET and downstream intracellular signaling pathways similar to rh-HGF. Tubulin was used as a control for total cellular protein. (-) ctrl= untreated cells.
Figure 2. In vitro effect of HGF-f on cardiac derived cells.
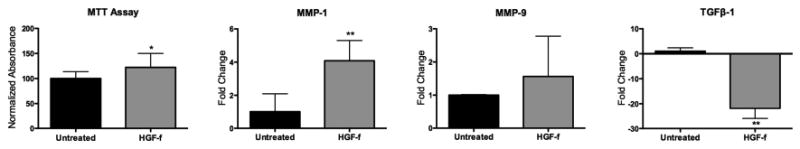
(A) HGF-f increased metabolic activity of serum starved cardiomyocytes by 22% as measured by changes in absorbance. B-D, qRT-PCR gene expression changes in cardiac cell isolate. (B) MMP-1 mRNA expression significantly increased with addition of HGF-f compared to the untreated control. (C) MMP-9 expression levels trended higher with addition of HGF-f though were not significant over the untreated control. D) TGFβ-1 is dwnregulated when HGF-f is added to the culture compared to the untreated group. *p<0.05; **p<0.01.
HGF has been shown to affect the expression of a number of matrix remodeling proteins that mediate fibrosis [23-25]. Thus, we investigated the effects of HGF-f on relevant downstream gene expression levels in a crude neonatal cardiomyocyte isolate, including cardiac fibroblasts, which are involved in matrix remodeling. Matrix metalloproteinase-1 (MMP-1) was significantly upregulated following treatment with HGF-f; however, there was no significant increase in MMP-9 mRNA levels. Interestingly, expression of TGFβ-1, which has been shown to be correlated with fibrosis, was significantly downregulated in the HGF-f group compared to the untreated group (Figure 2B-D).
HGF-f heparin binding and retention in an ECM-derived hydrogel
HGF-f contains the major heparin binding sites identified in HGF and should bind strongly to heparin. A heparin affinity column was used to confirm that heparin binding could occur, and thus be exploited in our ECM hydrogel delivery system. The chromatogram in Figure 3A shows strong binding of HGF-f to heparin, requiring more than a 1 M concentration of NaCl in PBS to elute the protein. In comparison, non-heparin binding proteins elute immediately from the column upon injection (not shown).
Figure 3. HGF-f binds to heparin and ECM hydrogels.
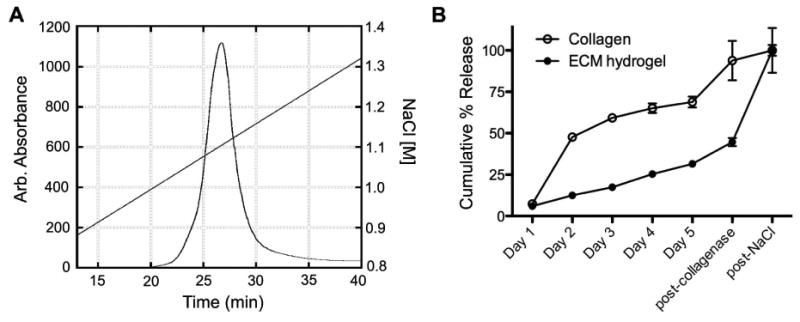
(A) HGF-f was loaded onto a heparin affinity column and eluted using a NaCl gradient. The chromatogram corresponds to protein elution from the column, as measured by absorption at 280 nm. HGF-f elutes as a single peak in phosphate buffered saline with an additional 1.1 M NaCl, indicating strong binding to heparin. (B) Relatively rapid diffusion of HGF-f is observed from collagen hydrogels (open circles), while slow release is seen from ECM hydrogels (solid circles). Nearly complete release of HGF-f from collagen hydrogels is seen post-collagenase treatment, while treatment with 1.5 M NaCl solution is needed to fully dissociate HGF-f from the ECM hydrogels. Data is mean ± SEM.
We next examined the ability of the ECM-derived hydrogel to bind and retain HGF-f. In collagen gels, which do not contain any sGAGs for growth factor binding, 50% of HGF-f was released within the first day and continued to diffuse from the gels over days 2-5, followed by nearly complete release after collagenase treatment (Figure 3B). No additional HGF-f release was seen after addition of a high salt solution (NaCl). In contrast, slow sustained release of HGF-f was observed from the ECM hydrogels over 5 days, followed by a small efflux after collagenase treatment. The majority of HGF-f was then released from ECM hydrogels following treatment with NaCl, which disrupts electrostatic interactions (Figure 3B).
Cardiac function in rat MI model
After the baseline and screening echocardiograms, 41 animals underwent injections of saline (n = 12), ECM hydrogel (n = 9), HGF-f in ECM hydrogel (n = 10), or HGF-f in saline (n = 10). One animal in the HGF-f in saline group died due to complications closing the diaphragm after the injection surgery. Four animals from the saline group, one animal from the ECM hydrogel group, four animals from the HGF-f in ECM group, and two animals from the HGF-f in saline group were additionally excluded due to lack of infarction upon histological analysis, for final animal numbers of 6-8 per group. A single dose of 10 μg of HGF-f was chosen for this initial study based on our previous work with this model and other studies using HGF in small animal models [26]. Treatment one week post-MI allows for the initial inflammation to subside, avoids the window in which an intramyocardial injection is risky in patients, and mimics an appropriate timeline in humans.
No significant differences in echocardiographic measurements were observed between groups at pre-injection baseline (1 week post-MI). Given the variability with the infarct model, paired t-tests were used to analyze the echocardiographic data so that each animal served as its own internal control. By 4 weeks post-injection, saline-injected control animals deteriorated significantly with respect to LV diastolic (LVAd) and systolic (LVAs) areas and end-diastolic (EDV) and end-systolic (ESV) volumes (Table 1), indicative of negative LV remodeling. No other group changed significantly with respect to LV geometry measurements, suggesting all treatment groups were capable of preventing negative LV remodeling. There was a significant increase (p <0.05) in the fractional area change (FAC), a measure of cardiac function, in the HGF-f in ECM hydrogel group over the course of the study (Table 1). In contrast, other treatment groups showed no significant increases in this measurement of cardiac function (Table 1). Delivery of HGF-f in the ECM hydrogel resulted in a trend towards an increased EF, although these results did not achieve statistical significance (Table 1). A graphical display of changes in FAC and EF is shown in Figure 4.
Table 1. Echocardiographic data.
| Saline | ECM hydrogel | HGF-f in ECM hydrogel | HGF-f in saline | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Pre-injection | 4 weeks post-injection | Pre-injection | 4 weeks post-injection | Pre-injection | 4 weeks post-injection | Pre-injection | 4 weeks post-injection | |
| FAC, % | 41.3 ± 3.1 | 39.7 ± 3.2 | 41.0 ± 2.3 | 42.3 ± 1.2 | 38.7 ± 1.8 | 43.3 ± 2.2* | 35.4 ± 3.8 | 35.6 ± 4.1 |
| LVAd, mm2 | 65.1 ± 2.3 | 73.0 ± 3.4* | 67.4 ± 1.6 | 72.5 ± 2.7 | 63.1 ± 4.0 | 74.2 ± 1.8 | 71.2 ± 4.1 | 74.2 ± 4.8 |
| LVAs, mm2 | 38.2 ± 2.3 | 43.9 ± 2.9* | 39.8 ± 1.8 | 41.7 ± 1.4 | 38.5 ± 2.4 | 42.2 ± 2.4 | 46.5 ± 5.3 | 48.5 ± 6.1 |
| EF, % | 58.9 ± 2.6 | 55.9 ± 3.7 | 61.7 ± 2.4 | 59.6 ± 2.1 | 59.0 ± 2.5 | 63.2 ± 2.5 | 53.1 ± 5.6 | 51.5 ± 5.4 |
| EDV, mm3 | 268 ± 19 | 337 ± 30* | 307 ± 15 | 336 ± 17 | 325 ± 41 | 358 ± 20 | 327 ± 30 | 355 ± 44 |
| ESV, mm3 | 112 ± 13 | 146 ± 19* | 119 ± 11 | 132 ± 6 | 118 ± 14 | 137 ± 15 | 165 ± 33 | 181 ± 43 |
Data were compared with a paired t-test so that each animal served as its own internal control.
Significance indicated by asterisks on the post-injection values represent a statistically significant change (p < 0.05) between pre-injection baseline (1 week post-MI) and 4 weeks post-injection (5 weeks post-MI). Data is mean ± SEM.
Figure 4. Effect on cardiac function.

Absolute change in FAC (A) and EF (B) is displayed. Data is mean ± SEM.
Increased neovascularization, decreased interstitial fibrosis, and decreased cardiomyocyte diameter
An increase in neovasculature was observed in the HGF-f in ECM hydrogel group. Capillary density trended higher (Figure 5A), while arteriole density was significantly increased (p < 0.0001) within the infarct region with HGF-f treatment in the ECM hydrogel compared to HGF-f delivered in saline, the ECM hydrogel alone, or saline alone (Figure 5B-F). There was a slight, though not significant, increase in arteriole density after injection of HGF-f alone compared to the ECM hydrogel and saline controls. We also observed a trend toward reduction of interstitial fibrosis in treated hearts compared to control animals, although this was not significant. The percent interstitial fibrosis in the saline control was significant greater than healthy animals (p < 0.05), while there was no significant difference between healthy animals and all three treated groups (Figure 6A-C). Infarct fibrosis ranged from 66 to 78% in all groups and was not significantly different between groups. As another indicator of negative LV remodeling, we also assessed the average cross-sectional area of cardiomyocytes in the remote myocardium. All three treated groups had significantly smaller cardiomyocytes compared to the saline control; however, only the ECM hydrogel alone and HGF-f delivered in the ECM hydrogel were not statistically different than healthy hearts (Figure 6D,E).
Figure 5. Vessel density.
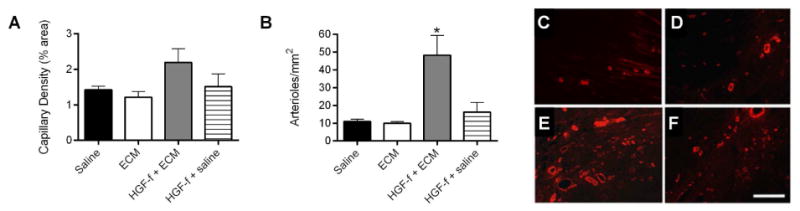
Quantification of neovasculature in the infarct region indicates a trend towards an increase in capillary density (A) and a significant increase in arteriole density (B) with HGF-f delivered from ECM hydrogels (*p < 0.05). Data is mean ± SEM. Administration of HGF-f alone had a small effect in arteriole formation, though it was not significantly greater than the ECM hydrogel alone or the saline control. Representative images of vascularized areas of infarcts from animals injected with saline (C), ECM (D), HGF-f in ECM (E), and HGF-f in saline (F) illustrate the differences in arteriole density, here stained for α-smooth muscle actin (red). Scale bar is 200μm.
Figure 6. Interstitial fibrosis and cardiomyocyte cross-sectional area.
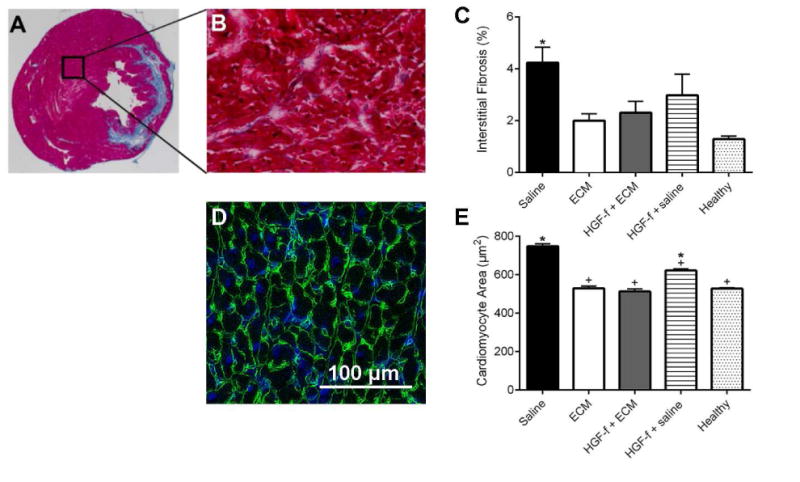
A representative Masson's trichrome stained section is shown in (A) with a higher magnification view of the remote myocardium shown in (B), illustrating interstitial fibrosis. (C) Quantification of the percent collagen content per image area in the remote myocardium (septal wall). The saline control was significantly greater than healthy animals (*p<0.05); no significant differences were observed between all three treated groups and the healthy animals. (D) Representative image of remote myocardium stained with an anti-laminin antibody, showing cardiomyocyte cross-sectional area. (E) Quantification of cardiomyocyte cross-sectional area (n = >300 cells per group; *p<0.0001 compared to healthy; +p<0.0001 compared to saline). All of the treated groups significantly reduced cardiomyocyte size compared to saline; however, cardiomyocytes in the HGF-f in saline group were statistically larger than healthy hearts. Data is mean ± SEM.
Discussion
The mechanism of action of cell therapy for treating MI and heart failure is now largely acknowledged to occur via paracrine signaling rather than transplanted cells incorporating and contributing to cardiac muscle regeneration [27]. Thus, in an effort to simplify the therapeutic approach, individual paracrine factors responsible for the positive outcomes are starting to be identified and evaluated. HGF is one such factor; initial success with this growth factor in increasing angiogenesis and reducing fibrosis in the liver [28, 29] led to its use in ischemic diseases such as peripheral artery disease and myocardial infarction [6-9, 26, 30, 31]. Though pre-clinical studies have been successful, and some work with HGF has moved into the clinic, rh-HGF has remained expensive and challenging to manufacture and is highly unstable [32]. Thus, HGF mimetics that are more stable and easier to produce present tangible opportunities to harness the therapeutic potential of HGF for clinical translation and commercialization. With this work, we demonstrate the in vitro and in vivo potency of a new engineered HGF fragment for the treatment of MI, specifically evaluating how delivery in an ECM hydrogel post-MI affects global cardiac function, vascularization of the infarct region, and myocardial fibrosis.
Combinatorial and rational protein engineering methods were previously used to develop a stable c-MET receptor agonist that emulates the function of full-length HGF and can be produced in high yields [12, 13]. We confirmed that this engineered HGF fragment, HGF-f, retained the ability to bind heparin using affinity chromatography. Accordingly, HGF-f exhibited slow release from ECM hydrogels, which contain sGAGs for increased retention and activity of heparin binding growth factors [15]. In addition, HGF-f was shown to induce receptor activation and biochemical signaling pathways in RASMCs, as evaluated by phosphorylation of c-MET and the downstream targets Akt, and Erk. These results were not unexpected, as the dimeric nature of the engineered HGF fragment likely mediates c-MET receptor dimerization and activation of cell signaling pathways, which ultimately lead to cell proliferation and migration [33].
HGF has previously been shown to prevent cell death and promote angiogenesis in the kidney, lung and heart [34-37], as well as enhance repair in the heart post-MI [7]. Thus, we sought to determine whether HGF-f could confer similar benefits as HGF in ischemic myocardium. The effect of HGF-f on cardiac cells was first evaluated in vitro via investigating the survival of serum-starved neonatal cardiomyocytes. A statistically significant increase in the metabolic activity of serum-starved cardiomyocytes was observed when cells were treated with HGF-f, indicating that the engineered protein promotes cardiomyocyte survival. We also evaluated the potential effect of HGF-f on fibrosis in vitro, by analyzing its effects on a crude cell isolate mixture of cardiomyocytes and cardiac fibroblasts, the cells responsible for much of the ECM composition in the heart. MMP-1 mRNA was significantly upregulated with addition of HGF-f compared to the untreated control; MMP-9 transcription was also upregulated, though the difference was not significant. Both MMP-1 and MMP-9 have been shown to be important in reducing fibrosis in multiple tissues [38-40]. The addition of HGF-f was also shown to dramatically downregulate TGFβ-1 expression. It is well documented that TGFβ-1 expression leads to an increase in fibrosis in over 15 tissues including the heart [41-45], and therefore these results suggest that HGF-f can act to reduce fibrosis.
Similar to previous work with bFGF [15], we show that an ECM derived hydrogel can sequester and prolong delivery of HGF-f, presumably through heparin-binding sites. In vivo results support our original hypothesis that delivery of HGF-f from an ECM hydrogel would be beneficial post-MI by preserving or improving cardiac function, increasing vascularization, and reducing fibrosis. The LV of saline-injected animals dilated as typical of post-MI negative LV remodeling. The expansion in LV areas and volumes was not seen in any of the treated groups, suggesting negative remodeling was inhibited. However, only delivery of HGF-f from the ECM hydrogels was capable of significantly improving cardiac function, as shown by an improvement in FAC. The results of this study support the potential of HGF-f, when delivered in an ECM hydrogel, for treating MI. The limitations of this study include a relatively small sample size and the use of echocardiographic imaging rather than MRI, limiting resolution of differences in functional data. In addition, alternative doses and injection time were not evaluated. These parameters will be important to optimize to fully evaluate the maximum efficacy and translational potential of HGF-f.
One effect of HGF-mediated c-MET activation is increased angiogenesis and reduced fibrosis. In fact, when endogenous HGF is blocked in small animal occlusion-reperfusion studies, infarct size and mortality rates increase significantly [46]. Here, our data supports the conclusion that HGF-f acts similarly to HGF in that arteriole density in the infarct region of hearts that received an injection of HGF-f in the ECM hydrogel was significantly increased over controls. There was a slight, but non-significant increase with the delivery of HGF-f alone. There was also an apparent, but not significant, decrease in interstitial fibrosis in all treated animals. Though not specifically investigated in this study, differences in fibrosis in the remote myocardium may have resulted due to a reduction in wall stress due to changes in the remodeling process in treated hearts. A reduction in negative LV remodeling is further indicated by the observed decrease in cardiomyocyte cross-sectional area.
The data throughout our study show increased biological effects when HGF-f is delivered in the ECM hydrogel. These findings are consistent with our previous work with the ECM hydrogel and bFGF [15], and may be due to the prolonged presence or enhanced stability of sequestered HGF-f in the matrix material. The ECM hydrogel may also provide a platform to present HGF-f to resident cells in a manner similar to how endogenous HGF is sequestered in the native cardiac ECM through sGAGs. Other injectable biomaterials that complex HGF via affinity interactions with sulfated groups have shown a similar enhancement of function over delivery of the growth factor alone, both in hindlimb ischemia [26] and MI models [30]. The presentation of a supportive extracellular environment may also be important for cell infiltration post-MI; it has been shown that cardiac progenitor cells are present in higher numbers in failing human hearts, but the harsh environment prevents their positive contribution to repair [47]. Utilizing a biomaterials scaffold derived from ECM components may provide a new template for constructive remodeling, thereby facilitating cellular infiltration such as neovascularization. In addition, ECM derived hydrogels are amenable to catheter based transendocardial injections [17, 48], and therefore, this potential therapy could be applied using a minimally invasive approach.
Conclusions
This work establishes proof-of-concept for using a novel engineered HGF fragment as an alternative to full-length rh-HGF for treating MI. Promising results of in vitro and small animal studies indicate that delivery of HGF-f in an ECM hydrogel may provide benefit post-MI by increasing neovascularization and inhibiting negative LV remodeling. Prolonged delivery and/or presentation from the ECM hydrogel was required, as injection of HGF-f alone did not significantly improve cardiac function post-MI, or enhance neovascularization. With this work, HGF-f is established as a promising candidate for future investigation, and presents tractable opportunities as an alternative to HGF for treating cardiovascular disease.
Acknowledgments
This research was supported in part by National Institutes of Health (NIH) through R01HL113468, R01CA151706, and 5UL1TR000100.
Footnotes
Author conflict of interest: Dr. DeMaria is on the scientific advisory board of Ventrix, Inc. Dr. Christman is co-founder, board member, and holds equity interest in Ventrix, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beohar N, Rapp J, Pandya S, Losordo DW. Rebuilding the damaged heart: the potential of cytokines and growth factors in the treatment of ischemic heart disease. J Am Coll Cardiol. 2010;56:1287–1297. doi: 10.1016/j.jacc.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burchfield JS, Dimmeier S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair. 2008;1:4. doi: 10.1186/1755-1536-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liehn EA, Postea O, Curaj A, Marx N. Repair after myocardial infarction, between fantasy and reality: the role of chemokines. J Am Coll Cardiol. 2011;58:2357–2362. doi: 10.1016/j.jacc.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Sakai K, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26(Suppl 1):188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 7.Ueda H, Nakamura T, Matsumoto K, Sawa Y, Matsuda H. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc Res. 2001;51:41–50. doi: 10.1016/s0008-6363(01)00272-3. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T, Mizuno S, Matsumoto K, Sawa Y, Matsuda H. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. J Clin Invest. 2000;106:1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Matsumoto K, Mizuno S, Sawa Y, Matsuda H. Hepatocyte growth factor prevents tissue fibrosis, remodeling, and dysfunction in cardiomyopathic hamster hearts. Am J Physiol Heart Circ Physiol. 2005;288:H2131–2139. doi: 10.1152/ajpheart.01239.2003. [DOI] [PubMed] [Google Scholar]
- 10.Taniyama Y, Morishita R, Aoki M, Hiraoka K, Yamasaki K, Hashiya N, et al. Angiogenesis and antifibrotic action by hepatocyte growth factor in cardiomyopathy. Hypertension. 2002;40:47–53. doi: 10.1161/01.hyp.0000020755.56955.bf. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Takemura G, Kosai K, Yuge K, Nagano S, Esaki M, et al. Postinfarction treatment with an adenoviral vector expressing hepatocyte growth factor relieves chronic left ventricular remodeling and dysfunction in mice. Circulation. 2003;107:2499–2506. doi: 10.1161/01.CIR.0000065579.19126.B8. [DOI] [PubMed] [Google Scholar]
- 12.Jones DS, 2nd, Tsai PC, Cochran JR. Engineering hepatocyte growth factor fragments with high stability and activity as Met receptor agonists and antagonists. Proc Natl Acad Sci USA. 2011;108:13035–13040. doi: 10.1073/pnas.1102561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu CJ, Jones DS, Tsai PC, Venkataramana A, Cochran JR. An engineered dimeric fragment of hepatocyte growth factor is a potent c-MET agonist. FEBS Lett. 2014 doi: 10.1016/j.febslet.2014.11.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 15.Seif-Naraghi SB, Horn D, Schup-Magoffin PJ, Christman KL. Injectable extracellular matrix derived hydrogel provides a platform for enhanced retention and delivery of a heparin-binding growth factor. Acta Biomater. 2012;8:3695–3703. doi: 10.1016/j.actbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng Part A. 2010;16:2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. Journal of the American College of Cardiology. 2012;59:751–763. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–5416. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 21.Chen JF, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2008;105:2111–2116. doi: 10.1073/pnas.0710228105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Song Y, Zhang Y, Xiao H, Sun Q, Hou N, et al. Cardiomyocyte overexpression of miR-27b induces cardiac hypertrophy and dysfunction in mice. Cell research. 2012;22:516–527. doi: 10.1038/cr.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 24.Haruyama T, Ajioka I, Akaike T, Watanabe Y. Regulation and significance of hepatocyte-derived matrix metalloproteinases in liver remodeling. Biochem Biophys Res Commun. 2000;272:681–686. doi: 10.1006/bbrc.2000.2837. [DOI] [PubMed] [Google Scholar]
- 25.Esposito C, Parrilla B, De Mauri A, Cornacchia F, Fasoli G, Foschi A, et al. Hepatocyte growth factor (HGF) modulates matrix turnover in human glomeruli. Kidney Int. 2005;67:2143–2150. doi: 10.1111/j.1523-1755.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 26.Ruvinov E, Leor J, Cohen S. The effects of controlled HGF delivery from an affinity-binding alginate biomaterial on angiogenesis and blood perfusion in a hindlimb ischemia model. Biomaterials. 2010;31:4573–4582. doi: 10.1016/j.biomaterials.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 27.Cheng AS, Yau TM. Paracrine effects of cell transplantation: strategies to augment the efficacy of cell therapies. Semin Thorac Cardiovasc Surg. 2008;20:94–101. doi: 10.1053/j.semtcvs.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Michalopoulos GK. Liver regeneration: molecular mechanisms of growth control. Faseb J. 1990;4:176–187. [PubMed] [Google Scholar]
- 29.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 30.Ruvinov E, Leor J, Cohen S. The promotion of myocardial repair by the sequential delivery of IGF-1 and HGF from an injectable alginate biomaterial in a model of acute myocardial infarction. Biomaterials. 2011;32:565–578. doi: 10.1016/j.biomaterials.2010.08.097. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Yang R, Li W, Ogasawara AK, Schwall R, Eberhard DA, et al. Early treatment with hepatocyte growth factor improves cardiac function in experimental heart failure induced by myocardial infarction. J Pharmacol Exp Ther. 2003;304:654–660. doi: 10.1124/jpet.102.041772. [DOI] [PubMed] [Google Scholar]
- 32.Ross J, Gherardi E, Mallorqui-Fernandez N, Bocci M, Sobkowicz A, Rees M, et al. Protein engineered variants of hepatocyte growth factor/scatter factor promote proliferation of primary human hepatocytes and in rodent liver. Gastroenterology. 2012;142:897–906. doi: 10.1053/j.gastro.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Chirgadze DY, Hepple JP, Zhou H, Byrd RA, Blundell TL, Gherardi E. Crystal structure of the NK1 fragment of HGF/SF suggests a novel mode for growth factor dimerization and receptor binding. Nat Struct Biol. 1999;6:72–79. doi: 10.1038/4947. [DOI] [PubMed] [Google Scholar]
- 34.Michieli P, Cavassa S, Basilico C, De Luca A, Mazzone M, Asti C, et al. An HGF-MSP chimera disassociates the trophic properties of scatter factors from their pro-invasive activity. Nat Biotechnol. 2002;20:488–495. doi: 10.1038/nbt0502-488. [DOI] [PubMed] [Google Scholar]
- 35.Ono M, Sawa Y, Matsumoto K, Nakamura T, Kaneda Y, Matsuda H. In vivo gene transfection with hepatocyte growth factor via the pulmonary artery induces angiogenesis in the rat lung. Circulation. 2002;106:I264–269. [PubMed] [Google Scholar]
- 36.Stewart N, Chade AR. Renoprotective effects of hepatocyte growth factor in the stenotic kidney. Am J Physiol Renal Physiol. 2013;304:F625–633. doi: 10.1152/ajprenal.00504.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang ZJ, Chen B, Sheng Z, Zhang DG, Jia EZ, Wang W, et al. Improvement of heart function in postinfarct heart failure swine models after hepatocyte growth factor gene transfer: comparison of low-, medium- and high-dose groups. Mol Biol Rep. 2010;37:2075–2081. doi: 10.1007/s11033-009-9665-5. [DOI] [PubMed] [Google Scholar]
- 38.Messerli FH. TIMPs, MMPs and cardiovascular disease. Eur Heart J. 2004;25:1475–1476. doi: 10.1016/j.ehj.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Rajendran R, Rajeesh MP, Shaikh S, Shanthi, Pillai MR. Expression of matrix metalloproteinases (MMP-1, MMP-2 and MMP-9) and their inhibitors (TIMP-1 and TIMP-2) in oral submucous fibrosis. Indian J Dent Res. 2006;17:161–166. doi: 10.4103/0970-9290.29870. [DOI] [PubMed] [Google Scholar]
- 40.Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S88–91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li RK, Li G, Mickle DA, Weisel RD, Merante F, Luss H, et al. Overexpression of transforming growth factor-betal and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–881. doi: 10.1161/01.cir.96.3.874. [DOI] [PubMed] [Google Scholar]
- 42.Tomita H, Egashira K, Ohara Y, Takemoto M, Koyanagi M, Katoh M, et al. Early induction of transforming growth factor-beta via angiotensin II type 1 receptors contributes to cardiac fibrosis induced by long-term blockade of nitric oxide synthesis in rats. Hypertension. 1998;32:273–279. doi: 10.1161/01.hyp.32.2.273. [DOI] [PubMed] [Google Scholar]
- 43.Branton MH, Kopp JB. TGF-beta and fibrosis. Microbes Infect. 1999;1:1349–1365. doi: 10.1016/s1286-4579(99)00250-6. [DOI] [PubMed] [Google Scholar]
- 44.Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:2347–2357. doi: 10.1016/s0022-2828(95)91983-x. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi N, Calderone A, Izzo NJ, Jr, Maki TM, Marsh JD, Colucci WS. Hypertrophic stimuli induce transforming growth factor-beta 1 expression in rat ventricular myocytes. J Clin Invest. 1994;94:1470–1476. doi: 10.1172/JCI117485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin H, Wyss JM, Yang R, Schwall R. The therapeutic potential of hepatocyte growth factor for myocardial infarction and heart failure. Curr Pharm Des. 2004;10:2525–2533. doi: 10.2174/1381612043383863. [DOI] [PubMed] [Google Scholar]
- 47.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, et al. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science Translational Medicine. 2013;5:173ra125. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]


