Abstract
Background
In utero undernutrition is associated with obesity and insulin resistance, although its effects on skeletal muscle remain poorly defined. Therefore, in the current study we explored the effects of in utero food restriction on muscle energy metabolism in mice.
Methods
We used an experimental mouse model system of maternal undernutrition during late pregnancy to examine offspring from undernourished dams (U) and control offspring from ad libitum fed dams (C). Weight loss of 10 wk old offspring on a 4 wk 40% calorie restricted diet was also followed. Experimental approaches included bioenergetic analyses in isolated mitochondria, intact (permeabilized) muscle and at the whole body level.
Results
U have increased adiposity and decreased glucose tolerance compared to C. Strikingly, when U are put on a 40% calorie restricted diet they lose half as much weight as calorie restricted controls. Mitochondria from muscle overall from U had decreased coupled (state 3) and uncoupled (state 4) respiration and increased maximal respiration compared to C. Mitochondrial yield was lower in U than C. In permeabilized fiber preparations from mixed fiber type muscle U had decreased mitochondrial content and decreased adenylate free leak respiration, fatty acid oxidative capacity, and state 3 respiratory capacity through complex I. Fiber maximal oxidative phosphorylation capacity did not differ between U and C but was decreased with calorie restriction.
Conclusions
Our results reveal that in utero undernutrition alters metabolic physiology through a profound effect on skeletal muscle energetics and blunts response to a hypocaloric diet in adulthood. We propose that mitochondrial dysfunction links undernutrition in utero with metabolic disease in adulthood.
Keywords: obesity, metabolism, fetal programming, mitochondria
Introduction
Obesity risk in adulthood can be influenced by events during intrauterine life. The developmental programming hypothesis holds that adverse influences during critical periods in development permanently alter tissue structure and function, which in turn increases disease risk. Hales and Barker demonstrated a strong association of low birth weight with cardiovascular disease and type 2 diabetes mellitus (T2DM) risk and hypothesized that this resulted from the offspring developing a thrifty phenotype in utero in anticipation of life with limited food (1, 2). Epidemiological studies in humans and animal models show that during the prenatal period, it is crucial to achieve optimal nutrition as both low and high birth weights are associated with risk of metabolic disease (3). In the current study, we have used a mouse model of low birth weight generated through 50% food restriction of mouse dams during the third week of gestation (4). Initial studies using this mouse model reported that offspring of undernourished pregnancies develop progressive, severe glucose intolerance by 6 months of age, beta cell dysfunction, and increased lipogenic gene expression and adipocyte size (4, 5).
Disordered skeletal muscle metabolism is associated with the adverse metabolic complications of obesity and T2DM, and has not been investigated in this model of obesity. Skeletal muscle in obese individuals exhibits reduced oxidative capacity, increased glycolysis, mitochondrial dysfunction, and a shift in fiber type distribution towards more glycolytic fibers (6–10). Healthy individuals with low birth weight have been shown to have abnormalities in muscle including decreased mass, reduced oxidative capacity, increased glycolytic capacity, and a lower proportion of oxidative type I fibers (11–13). It is well known that muscle is highly adaptable and responds to environmental and physiological challenges by changing its size, composition, and aerobic capacity (14, 15). Therefore, we hypothesized that the increased susceptibility to obesity and glucose intolerance in low birth weight mice is due in part to dysfunctional muscle mitochondrial energetics. This is supported by observations in rats showing that a low protein diet during pregnancy is associated with decreased mitochondrial DNA content in muscle of offspring (16) and that growth restriction by bilateral uterine artery ligation in late gestation causes decreased ADP-stimulated respiration in muscle mitochondria (17). No studies to date have examined muscle mitochondrial energetics in animals having low birth weight as a result of maternal food restriction and none have assessed the response of the adult offspring to a hypocaloric diet. Although links are well established between low birth weight and increased susceptibility to obesity and T2DM, the mechanisms by which maternal food restriction alters the long-term metabolic health of offspring remain to be fully understood.
Materials and Methods
Animals
All experiments were performed according to the principles and guidelines of the Canadian Council of Animal Care and the study was approved by the Animal Care Committee, University of Ottawa. Animals were housed with controlled temperature, humidity, and light-dark cycle (0600h – 1800h). Virgin female ICR mice (Harlan, Indianapolis, IN; age 6–8 wk) were paired with male ICR mice (Harlan; age 6–8 wk). Pregnancies were dated by vaginal plug (day 0.5) and pregnant mice were housed individually with ad libitum access to standard rodent chow (T.2018, Harlan Teklad, Indianapolis, IN, USA). On day 12.5 of pregnancy, dams were randomly assigned to either a control or an undernutrition group. Dams in the undernutrition group were 50% food restricted from days 12.5 to 18.5, based on the food intake of gestational day matched controls. At birth, all mothers were given ad libitum access to chow. On post-natal day 1, litters were equalized to eight, with additional pups being removed randomly from the litter. Pups were weaned at age three wk and mice were housed in groups of 3–4 from age 3 to 10 wk. At age 8 wk, mice were separated into individual cages and at age 10 wk mice were randomly assigned to either a 40% calorie restricted group (D01092702: Research Diets) or an ad libitum control group. The calorie restricted diet was formulated to ensure that the nutrient intake is normal despite decreased energy intake; it is thus used to study effects of calorie restriction rather than food restriction. Therefore, at 14 wk of age 4 groups of mice were studied: in utero undernourished offspring fed ad libitum postnatally (U-L), in utero undernourished offspring that were calorie restricted for 4 wk during adulthood (U-R), control offspring fed ad libitum (C-L), and control offspring that were calorie restricted for 4 wk during adulthood (C-R). Calorie restricted mice were fed daily at 16:00. Female offspring were used. End point determinations were performed with mice from at least four different litters per group, with no more than two mice obtained from the same litter. Mice were fasted overnight prior to euthanizing.
Indirect calorimetry and activity
At ages 10 and 14 wk, randomly selected mice from each group were placed in a 4-chamber open-circuit indirect calorimeter (Columbus Instruments, Columbus, OH) (18). Chambers were configured with dual axis (X, Y) detection of motion using infrared photocells (Columbus Instruments; Columbus, OH) to measure activity. Measurements were conducted over 24h after 2h of acclimatization. The respiratory exchange ratio (RER) was calculated as VCO2/VO2. Activity was calculated as total number of laser beam breaks.
Glucose tolerance testing
Oral glucose tolerance tests were performed on mice fasted for 6h. Glucose (2 mg/g body weight) was given by gavage at time 0. Saphenous vein blood was collected at 0, 15, 30, 60 and 120 min and glucose concentration was determined by a glucometer (Bayer, Canada).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) of 14 wk old mice was performed as in Du et al (19) with modifications detailed below. 4 mice at a time were euthanized with sodium pentobarbital and then imaged. Coronal spin-echo T1-weighted sequences were obtained from head to anus with a Siemens 3-T MRI system using a standard human head coil. Adipose tissue volume was calculated from the spin-echo MR images with volume segmentation for adipose tissue. Axial and coronal images were obtained for each mouse and the average of these images was calculated.
Histology
At 14 wk of age, the soleus and tibialis anterior (TA) muscles were isolated for histology. Sections were stained for myosin heavy chain (MHC) isoforms and cytochrome c oxidase (COX) activity (20, 21). Analyses were performed using ImageJ software (NIH).
Muscle homogenate
Quadriceps muscles were flash frozen in liquid N2. Muscle was homogenized on ice in a lysis buffer (20 mM Tris-HCl, 1% Triton X-100, 50 mM NaCl, 250 mM sucrose, 2% β-mercaptoethanol, 50 mM NaF, 5 mM NaPP, 1mM Na3VO4, and protease inhibitors) and spun at 14 000 g for 20 min at 4°C. Protein content was measured using a bicinchoninic acid assay and samples were stored at −80°C.
Western blotting
Homogenate and isolated mitochondria were subjected to standard Western blotting procedures. For additional information, including antibodies used see Supplemental Information. Band intensity was quantified using Image J (NIH) and normalized to complex IV for mitochondrial samples or α-tubulin for homogenate samples.
Mitochondrial isolation
Mitochondria from pooled forelimb, hindlimb, and pectoral muscles were isolated using the method described by Chappell and Perry (22) with modifications (23). For additional details, see Supplemental Information. Mitochondria were kept on ice and used for bioenergetic determinations within 2h. Protein concentration was measured using the Bradford assay.
Mitochondrial bioenergetics
Isolated mitochondria were studied using the Seahorse XF24 (Seahorse Bioscience Inc., USA) using a method adapted from (24), as described (25). Oxygen consumption rate (OCR) was determined in quintuplicate under state 2 conditions (10 mM pyruvate and 2 mM malate) prior to sequential additions of ADP (0.1 mM), oligomycin (2.5 μg/ml), FCCP (8 μM), and antimycin A (4 μM) to assess state 3, state 4O, maximal, and non-mitochondrial respiration, respectively. OCR was corrected by subtracting non-mitochondrial values (after addition of antimycin A).
High resolution respirometry
In a separate cohort of mice, the white and red gastrocnemius (wGAS and rGAS) were removed and fibers permeabilized with 50 μg/ml saponin. Respiration was determined in duplicate and at 37°C in MIRO5 (0.5 mM EGTA, 3 mM MgCl2.6H2O, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 110 mM D-sucrose, 0.1% BSA, 60 mM lactobionic acid; pH 7.1) using the Oxygraph-2k (Oroboros, Austria). Malate (2 mM) and octanoyl carnitine (200 μM) were added to determine adenylate free leak respiration (LN). ADP + Mg2+ (5 mM) were subsequently added to determine maximal electron flow through electron-transferring flavoprotein (ETF) and fatty acid oxidative capacity (PETF). Submaximal state 3 respiratory capacity through complex I (PCI) was determined following the addition of pyruvate (5 mM) and glutamate (10 mM). Succinate (10 mM) and ADP + Mg2+ (5 mM) were then added to determine maximum oxidative phosphorylation capacity (PCI+CII). Cytochrome c (10 μM) was added to test the integrity of the mitochondrial outer membrane. Only preparations in which addition of cytochrome c caused <10% increase in respiration were included. Antimycin A (2.5 μM) was added to inhibit complex III and terminate respiration to determine nonmitochondrial oxygen consumption. All values were corrected for residual oxygen consumption.
Statistical analyses
All measures were analyzed using GraphPad Prism 5.0 (La Jolla, CA). Data between groups at 10 wk of age were compared using an unpaired Student’s t-test. Body weight over time and glucose tolerance data were analyzed using two-way repeated measures ANOVA with Bonferroni post-hoc tests. All data between groups at 14 wk of age were analyzed using a two-way ANOVA and Bonferroni post-hoc tests. Values are given as mean ± SEM. P<0.05 was considered significant.
Results
Low birth weight offspring have decreased glucose tolerance
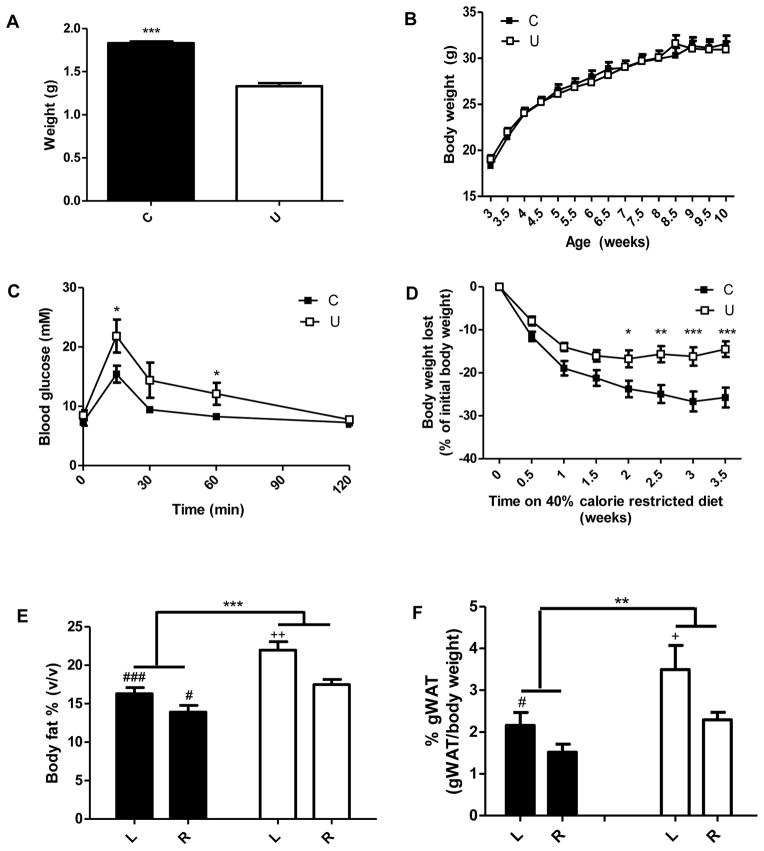
Body weights of offspring from undernourished dams (U) were 27% lower on post-natal day 1 compared to offspring from control dams (C) (Figure 1A). The body weight of U was similar to C by three wk of age and their growth curves and food intake afterwards did not differ significantly (Figure 1B and Figure S1A, respectively). U had decreased glucose tolerance at 10 wk of age (Figure 1C), which became progressively worse by 14 wk (Figure S1B). Tail and femur lengths were not different between U and C, indicating no difference in linear growth. There was also no difference in liver or heart weights (Table S1; 10 wk and Table S2; 14 wk).
Figure 1.
In utero undernutrition is associated with low birth weight, decreased glucose tolerance, decreased weight loss in response to calorie restriction and increased adiposity. A) Weight of the offspring at 1 day of age. Offspring from 7 dams per group were weighed. Student’s t-test, *** = p<0.0001, n=50–87. B) Bi-weekly body weight of offspring. Two way-repeated measures ANOVA, not significant, n=34. C) Blood glucose concentrations before and for 2 h after oral glucose administration (2 mg/g body weight). Oral glucose tolerance test was performed at age 10 wk. Two-way repeated measures ANOVA with Bonferroni post-hoc test, * = p<0.05, n=6–8. D) Change in bodyweight on a 40% calorie restricted diet started at 10 wk of age. Values are presented as percent of body weight prior to caloric restriction. Two-way repeated measures ANOVA with Bonferroni post-hoc test, * = p<0.05, ** = p<0.01, *** = p<0.001, n=11. E and F) Increased adiposity in 14 wk old offspring fed ad libitum (L) and after a 4 week 40% calorie restriction (R). Quantification of adipose tissue volume from spin-echo MR images with volume segmentation for total adipose tissue, n=4–5 (E), and amount of gonadal white adipose tissue (gWAT) expressed as a percentage of total body weight (F), n=8. Two-way ANOVA with Bonferroni post-hoc test, ** = p<0.01, *** = p<0.001, # = p<0.05 (C vs. U), ### = p<0.001 (C vs. U), + = p<0.05 (L vs. R), ++ = p<0.01 (L vs. R). Black = C (control offspring), white = U (in utero undernourished offspring). Values are mean ± SEM.
In utero undernutrition is associated with increased adiposity and blunted weight loss in response to calorie restriction
Given our interest in weight loss response to hypocaloric diets (21, 26), we studied whole body and skeletal muscle metabolism in offspring in both ad libitum and calorie restricted states. At 10 wk of age, mice were put on a 40% calorie restriction diet for 4 wk. With calorie restriction, U lost significantly less weight than controls, losing 15% and 26% of initial body weights, respectively (Figure 1D). Absolute weight loss was ~50% less in U than C. Body fat was quantified using MRI (Figure 1E). Body fat percent was greater in ad libitum and calorie restricted U compared to ad libitum and calorie restricted C. Correspondingly, fat free mass was decreased in U compared to C. U had greater gonadal white adipose tissue (gWAT) weights at 10 and 14 wk compared to C (Figure S1C and 1F respectively). Differences in body composition were not due to differences in heart or liver weight as indicated above (Table S2) and there were no noticeable differences in organ size at time of sacrifice or on MRI images.
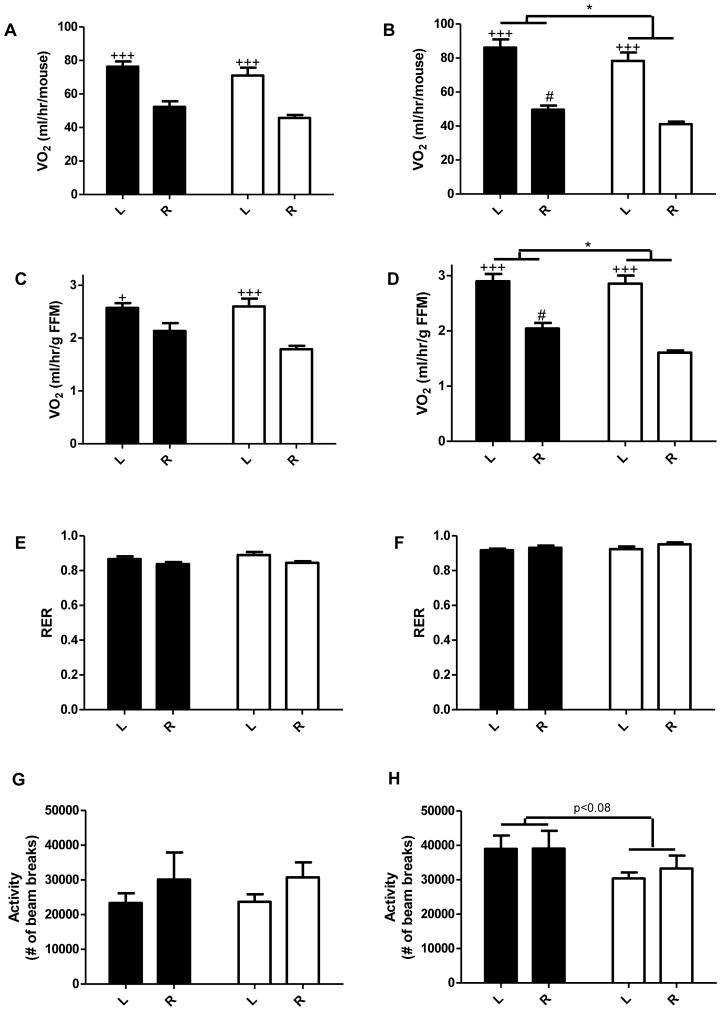
In utero undernutrition decreases metabolic rate in the dark phase
Indirect calorimetry and physical activity monitoring were conducted in the dark phase, when mice are awake and active, and the light phase, when mice typically sleep and are less active. At 10 wk, there was no significant difference in oxygen consumption, RER, or activity (Figure S2). At 14 wk, oxygen consumption per mouse was decreased in U in the dark phase, including a significant decrease in U-R vs. C-R (Figure 2B). Oxygen consumption per mouse was not different in the light phase (Figure 2A). Given the differences in adiposity and that adipose tissue is metabolically much less active than other tissues, oxygen consumption was also normalized to fat free mass (FFM). Results expressed per gram of FFM showed the same significant differences (Figure 2C, 2D). Thus, data suggest that U have a decreased metabolic rate in the dark phase compared to C, particularly when mice are calorie restricted. No differences were observed in RER suggesting that U and C metabolize similar relative amounts of carbohydrates and fats (Figure 2E, 2F). There was a trend for decreased physical activity in U in the dark phase (Figure 2H). Physical activity was not different in the light phase (Figure 2G).
Figure 2.
In utero undernutrition decreases metabolic rate and activity in the dark phase. Data for 14 wk old mice fed ad libitum (L) and after a 4 wk 40% calorie restriction (R). Data were collected for 24h and averaged over each of the light (Left; A, C, E, G) and dark phases (Right; B, D, F, H) of the day (lights on 6:00–18:00). A–D) Whole body oxygen consumption by indirect calorimetry expressed per mouse (A, B) and per gram of fat free mass (FFM) (C, D). E F) Respiratory exchange ratio (RER), calculated as VCO2/VO2. G, H) Activity expressed as the sum of beam breaks in the x and y direction. Values are mean ± SEM, n=8. Two-way ANOVA with Bonferroni post-hoc test,* = p<0.05, # = p<0.05 (C vs. U), + = p<0.05 (L vs. R)+++ = p<0.001 (L vs. R). Black = C (control offspring), white = U (in utero undernourished offspring).
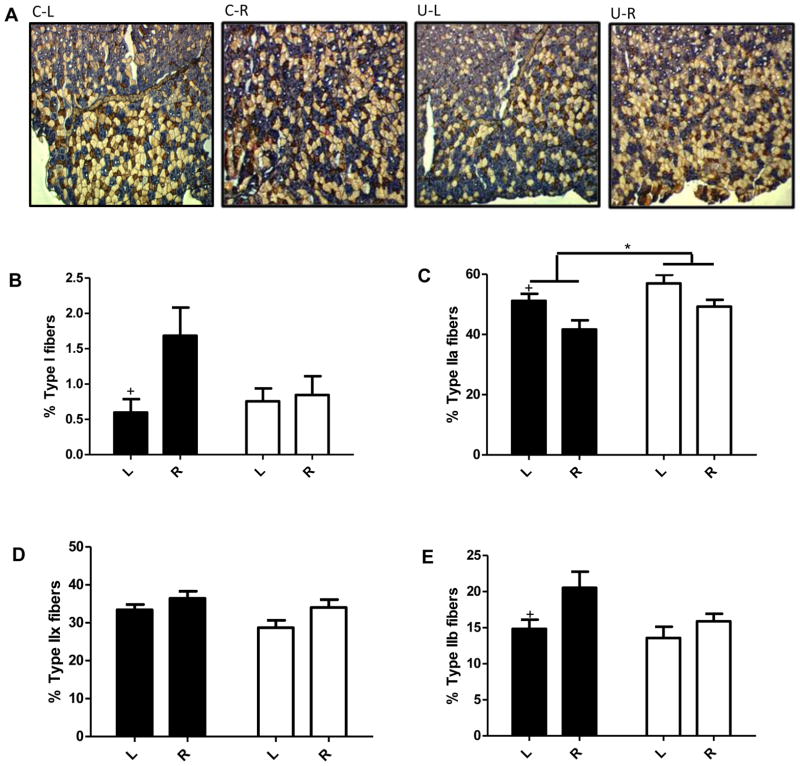
Blunted or absent response to calorie restriction in muscle fiber types and gene transcriptome in U compared to C offspring
The soleus and TA muscles were selected for histological analyses as they differ greatly in fiber type composition. Fiber type proportions were classified based on MHC isoforms. In order of most oxidative to least oxidative (i.e., most glycolytic), mouse muscle contains type I, IIa, IIx, and IIb fibers (27). The soleus is highly oxidative, comprising almost exclusively type I and type IIa fibers. In contrast, the TA is more glycolytic containing a mix of fiber types. There were no differences in fiber type in soleus between U-L and C-L or between U-R and C-R (Figure S4A–4C). Interestingly, in the more glycolytic TA, U and C responded differently to caloric restriction (Figure 3A–3E). In U, calorie restriction did not alter fiber type proportions. In contrast, calorie restriction in C resulted in increased types I and IIb fibers and decreased type IIa fibers. Overall, there was a small but significant increase in the proportion of type IIa fibers in U (5.8% increase U-L vs C-L, 7.6% increase U-R vs C-R). Given these effects, microarray analyses were performed to measure the content of gene transcripts in the TA. Despite robust effects of calorie restriction on the expression of genes involved in skeletal muscle development and oxidative phosphorylation in control mice, gene expression was not drastically different between U and C, especially under conditions of CR. (Figure S3 A, B). We also compared genes that were differentially expressed with calorie restriction in the two groups. Overall, results show that with calorie restriction far fewer genes are differentially expressed in U (U-R vs U-L; 1010) compared C (C-R vs C-L; 2267) (Figure S3C).
Figure 3.
Changes in fiber type in response to calorie restriction are eliminated with in utero undernutrition. Data for 14 wk old mice fed ad libitum (L) and after a 4 wk 40% calorie restriction (R). Fiber type proportions in the tibialis anterior. Muscle sections were stained based on myosin heavy chain expression for type I fibers (red; B), type IIa fibers (blue; C) and type IIb fibers (brown; D). Unstained fibers were counted as type IIx fibers (E). Proportions were calculated as the percent of the total number of fibers with an average of 988±38 fibers counted per image. Representative images are shown in A. Values are mean ± SEM, n=8. Two-way ANOVA with Bonferroni post-hoc test, * = p<0.05, + = p<0.05 (L vs. R). Black = C (control offspring), white = U (in utero undernourished offspring).
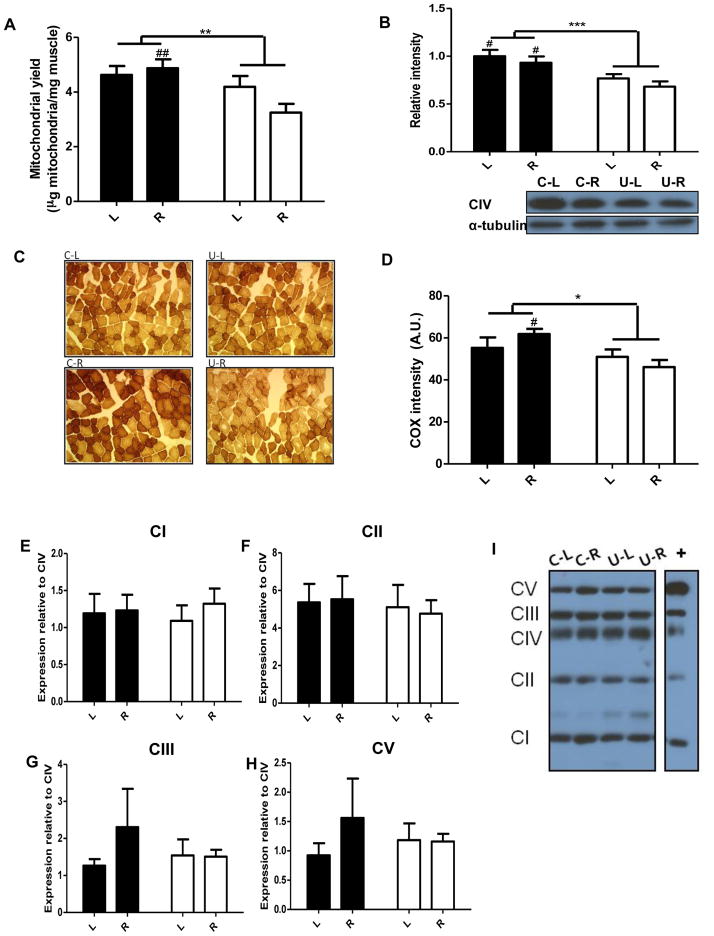
In utero undernutrition results in reduced skeletal muscle mitochondrial content
As mitochondria play a key role in energy metabolism, the above-described differences in weight loss and metabolism could be due in part to differences in mitochondrial content. Crude mitochondrial yield, calculated as total mitochondrial protein from pooled skeletal muscle per unit wet weight, was decreased in U compared to C (Figure 4A). With calorie restriction, mitochondrial content was decreased in U-R compared to C-R. Given the approximate nature of this assessment, we also measured complex IV (cytochrome c oxidase) protein, in quadriceps muscle. Levels were decreased in U compared to C (Figure 4B). To further assess mitochondrial content, sections of soleus and TA were stained for COX activity. In soleus, COX activity did not differ between U and C (Figure S4D–4E). In TA, COX activity was decreased in U compared to C (Figure 4C, 4D). Altogether findings indicate that mitochondrial content is decreased, as indicated by mitochondrial yield of pooled skeletal muscle; however, COX analysis of specific muscles indicates that mitochondrial content is decreased in mixed and low oxidative muscles but not in the highly oxidative soleus muscle.
Figure 4.
The effect of in utero undernutrition on skeletal muscle mitochondrial content from 14 wk old mice fed ad libitum (L) and after a 4 wk 40% calorie restriction (R). A) Crude mitochondrial yield of pooled skeletal muscle, n=6. B) Protein expression of mitochondrial complex IV (CIV) in quadriceps muscle homogenate, representative Western blot on bottom and quantification above, n=8. C,D) Tibialis anterior muscle sections were stained for cytochrome c oxidase (COX) activity, representative images (C) and quantification (D). Fiber intensity was calculated for an average of 213 ± 14 fibers per mouse, n=5. E–I) Protein expression of mitochondrial complexes I, II, III, and V relative to complex IV in isolated mitochondria, representative Western blot (I) and quantification (E–H), n=6. Values are mean ± SEM. Two-way ANOVA with Bonferroni post-hoc test, *=p<0.05, ** = p<0.01, *** = p<0.001, # = p<0.05 (C vs. U), ## = p<0.01 (C vs. U). Black = C (control offspring), white = U (in utero undernourished offspring).
In utero undernutrition alters mitochondrial bioenergetics in skeletal muscle
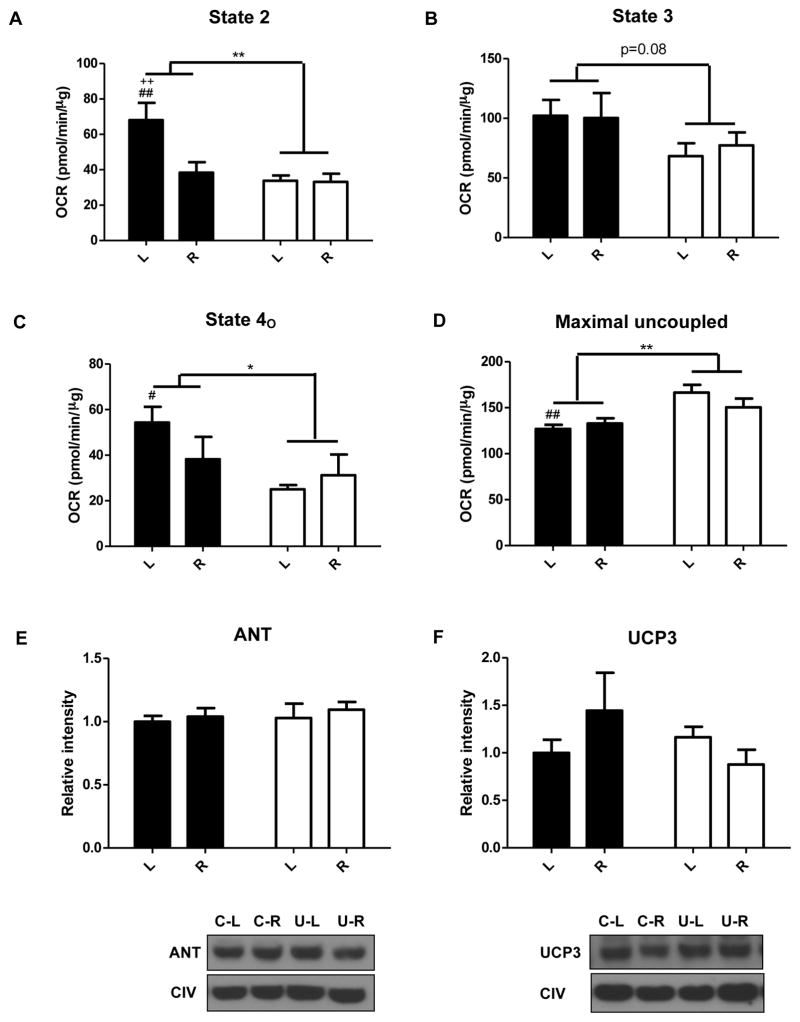
Bioenergetic determinations were conducted on mitochondria from pooled forelimb, hindlimb, and pectoral muscles. State 2 (Figure 5A) and state 4O (Figure 5C) respiration rates were decreased in U with a trend for a decrease in state 3 respiration (Figure 5B). Intriguingly, FCCP-induced maximal respiration was higher in U vs C (Figure 5D). More specifically, in mitochondria from the ad libitum fed U mice, state 4 and state 4O respiration were decreased while maximal respiration was increased compared to C (U-L vs. C-L). Interestingly, in the calorie restricted state there were no differences in mitochondrial oxygen consumption (U-R vs. C-R). We then assessed the relative proportion of the mitochondrial protein complexes in mitochondria. The relative levels of complexes I, II, III, and V compared to complex IV did not differ between the groups (Figure 4E–4I). Since state 4O respiration was decreased in U, we also assessed protein levels of adenine nucleotide translocase (ANT) and uncoupling protein 3 (UCP3), but found no differences (Figure 5E and 5F, respectively).
Figure 5.
In utero undernutrition alters mitochondrial bioenergetics in skeletal muscle from 14 wk old mice fed ad libitum (L) and after a 4 wk 40% calorie restriction (R). Data are shown for State 2 (A), State 3 (B), State 4O (C), and Maximal respiration (D). OCR = Oxygen consumption rate. E,F) Protein expression of ANT (E) and UCP3 (F) in isolated mitochondria, representative Western blots (top) and quantification (bottom). Complex IV was used as a loading control. Values are mean ± SEM, n=6. Two-way ANOVA with Bonferroni post-hoc test,* = p<0.05, ** = p<0.01, # = p<0.05 (C vs. U), ## = p<0.01 (C vs. U), ++ = p<0.01 (L vs. R). Black = C (control offspring), white = U (in utero undernourished offspring).
In utero undernutrition alters energetics in permeabilized fibers from white gastrocnemius but not red gastrocnemius
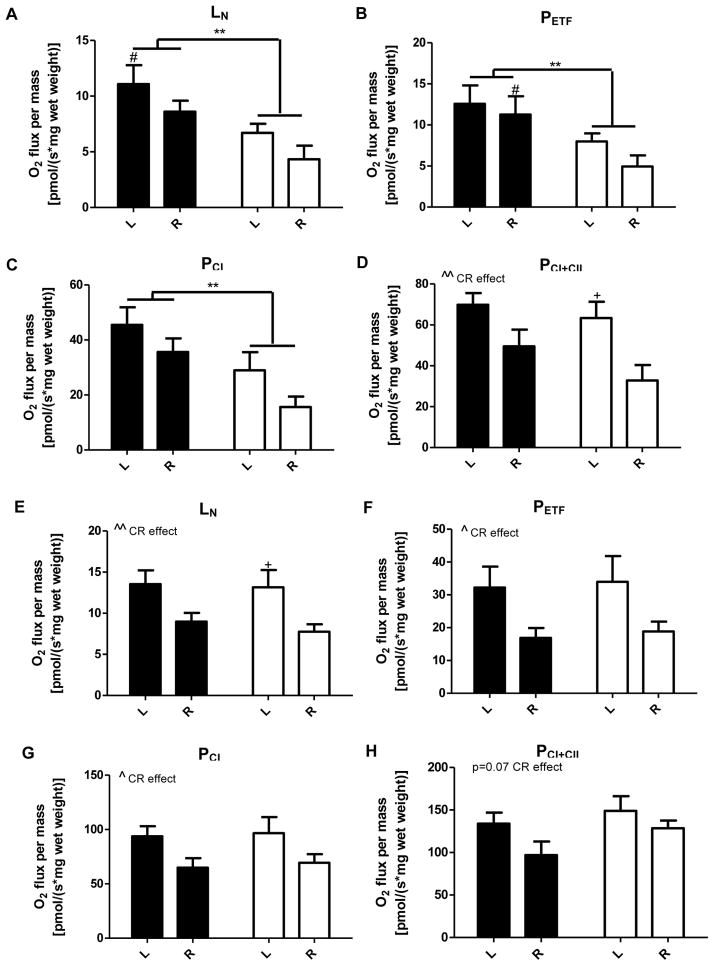
To further assess energetic differences in muscle, we performed high resolution respirometry on permeabilized muscle fibers of the more glycolytic wGAS and the more oxidative rGAS. wGAS fibers from U had decreased adenylate free leak respiration (Figure 6A), fatty acid oxidative capacity (Figure 6B), and state 3 respiratory capacity through complex I (Figure 6C). Maximal oxidative phosphorylation capacity did not differ between U and C but was significantly decreased with calorie restriction (Figure 6D). In contrast, respiration in fibers from rGAS was not altered in U (Figure 6E–6H). However, calorie restriction decreased adenylate free leak respiration (Figure 6E), fatty acid oxidative capacity (Figure 6F), and state 3 respiratory capacity through complex I (Figure 6G) with a trend for a decrease in maximal oxidative phosphorylation capacity (p=0.07; Figure 6H). Similar differences between U and C were also measured in wGAS and rGAS at 10 wk of age (Figure S5).
Figure 6.
In utero undernutrition alters energetics in permeabilized fibers from white gastrocnemius (wGAS) but not red gastrocnemius (rGAS). O2 flux in permeabilized fibers from wGAS (A–D) and rGAS (E–H) from 14 wk old mice fed ad libitum (L) and after a 4 wk 40% calorie restriction (R). Data are shown for adenylate free leak respiration (LN; A, E), maximal electron flow through electron-transferring flavoprotein and fatty acid oxidative capacity (PETF; B, F), submaximal state 3 respiratory capacity through complex I (PCI; C, G), and maximum oxidative phosphorylation capacity (PCI+CII; D, H). Values are mean ± SEM, n=6–8, *=p<0.05, **=p<0.01, #=p<0.05 (C vs. U), +=p<0.05 (L vs. R) ), ^=p<0.05 (calorie restriction (CR) effect), ^^p<0.01 (CR effect). Black = C (control offspring), white = U (in utero undernourished offspring).
Discussion
Although there is an increasing appreciation of the role of in utero nutrition in modifying the susceptibility for metabolic disease, its effects on skeletal muscle have remained largely unexplored. In utero undernutrition has been linked to obesity and insulin resistance in animal models and is associated with metabolic disease in humans (3). In the current study, we used a mouse model of maternal undernutrition to show that low birth weight is associated with impaired skeletal muscle bioenergetics. Most notable is our demonstration of a negative impact of maternal undernutrition on skeletal muscle mitochondrial energetics and the capacity for weight loss. Specifically, maternal undernutrition altered muscle physiology (e.g., fiber content) and down-regulated mitochondrial content and energetics, in two experimental systems (i.e., isolated mitochondria and permeabilized muscle fibers). This occurred in predominantly mixed muscles (quadriceps, TA, wGAS), but not in highly oxidative muscles (soleus and rGAS).
The developmental programming hypothesis proposes that food restriction in utero causes adaptations to maintain energy homeostasis that favour survival in an environment with limited nutrient supply, resulting in a thrifty phenotype. However, with post natal nutrient excess, these adaptations made in utero become detrimental, increasing susceptibility to obesity and glucose intolerance (1). Here we extend this hypothesis and demonstrate that low birth weight offspring when calorie restricted in adulthood, are resistant to weight loss due apparently to the thrifty metabolic mechanisms programmed in utero.
A key finding was that in response to a 4 wk 40% calorie restriction, U lost 50% less weight than C. This is particularly interesting given documented weight loss variation in diet-adherent obese women in a clinical weight loss program (21, 26, 28). The latter studies showed that obese diet resistant subjects (i.e., in the lowest quintile for weight loss) had decreased ‘energy-wasting’ mitochondrial proton leak respiration, decreased UCP3 mRNA, down-regulation of genes involved in oxidative phosphorylation and glucose and fatty acid metabolism, and decreased oxidative muscle fibers in muscle compared to diet sensitive subjects (21, 26). Consistent with this, pathway enrichment analysis of blood gene expression profiles showed down-regulation of the oxidative phosphorylation pathway in the diet resistant subjects prior to the hypocaloric diet program (28). While many studies have examined variation in weight gain, few studies have focused on variation in weight loss. Therefore, the latter studies show that mitochondrial energetics are more efficient and fiber composition less ‘oxidative’ in muscle of diet resistant women compared to diet-sensitive women. In the current study we document diet resistance in a murine epigenetic model and findings are consistent with the possibility that environmental factors and epigenetic mechanisms may contribute at least in part to weight loss resistance in humans.
Skeletal muscle accounts for ~20% of resting metabolic rate in humans and its mass and oxidative capacity are positively related to resting energy expenditure (29, 30). Given that muscle is an important determinant of whole body metabolism and insulin sensitivity, reductions in muscle mass are important. We found that offspring of undernourished dams (U) have reduced lean body mass and increased fat mass, as calculated from MRI. This alteration in body composition is a common phenotype associated with low birth weight in humans and animal models (4, 31–34). This reduction in muscle mass may disrupt systemic metabolism and contribute to the decreased glucose tolerance and adult disease risk in this model. Metabolic rate was decreased in U in the dark phase after calorie restriction, as compared to C. This could explain, in part, why U mice lose less weight under this condition. There was a trend towards decreased physical activity in U in the dark phase,; however, physical activity was not significantly different after calorie restriction. These findings suggest that with caloric restriction, U have an increased ability to conserve energy.
We also examined mitochondrial content in skeletal muscle. Overall, mitochondrial content is decreased in U as assessed by mitochondrial yield from pooled skeletal muscle. Moreover mitochondrial content is decreased in mixed fiber muscles as assessed by COX activity staining in TA and by COX protein levels in quadriceps but remains unchanged in soleus as assessed by COX activity staining. Mitochondrial energetics were then assessed in mitochondria and permeabilized muscle fibers. In utero undernutrition altered mitochondrial energetics with mitochondria from U having decreased state 2, state 3 (trend), and state 4O respiration rates but an increased maximal respiration rate. These differences were not due to decreased expression of UCP3 or ANT or to a decrease in the relative proportion of the mitochondrial complexes but may be a result of their increased activity. Respiration in fibers from wGAS extended these results in showed that U have decreased adenylate free (i.e., proton leak) respiration, fatty acid oxidative capacity, and complex I driven state 3 respiration. Maximal oxidative phosphorylation however was not different in permeabilized fibers. Thus U have decreased muscle mitochondrial content while isolated mitochondria have increased maximal respiratory capacity. Although skeletal muscle from U has a high capacity for oxygen consumption, under resting conditions energy expenditure is decreased. Therefore, in utero undernutrition results in not only a decrease in mitochondrial content but also decreased respiration in muscle fibers and impaired mitochondrial respiration. Taken together, findings explain, at least in part, the dramatically blunted weight loss with calorie restriction in U. Given the central role that muscle plays in whole body metabolism, we suggest that muscle mitochondrial dysfunction caused by in utero undernutrition contributes to obesity, weight loss resistance and glucose intolerance in adulthood. The impact of in utero undernutrition on other highly metabolic tissues, such as liver, remains to be examined.
Interestingly, in utero undernutrition did not affect respiration in fibers from rGAS, although calorie restriction had a more profound effect in rGAS compared to wGAS. Thus alterations in metabolism that occur with in utero undernutrition are partly dependent on muscle type. In utero undernutrition also increased the proportion of type IIa fibers in the TA and eliminated the change in fiber type proportions induced by calorie restriction in this muscle. There was no effect of in utero undernutrition on fiber type proportions in soleus. Similarly, COX activity was decreased in the TA of U, but not in the soleus. The increase in type IIa fibers in this mouse model is similar to the fiber type shift towards more glycolytic fibers and a lower oxidative capacity observed in muscle from obese individuals with T2DM (6, 9, 10). Moreover weight loss resistant obese patients have a greater proportion of glycolytic fibers than weight loss sensitive patients (21). Taken together, results indicate that in utero undernutrition causes metabolic dysfunction in more glycolytic muscle, such as wGAS and TA, with minimal effects in oxidative muscle, such as rGAS and soleus. Gene expression analysis of the TA showed that despite robust effects of calorie restriction on expression of genes involved in muscle development and OXPHOS in control mice, expression was not drastically different between U and C, especially under conditions of CR (Figure S3). Overall, it is apparent that calorie restriction has less of an effect on the transcriptome in U than C muscle. This is intriguing in light of the decreased weight loss and lack of change in fiber type induced by calorie restriction in U compared to C.
It has been shown in the same model of in utero undernutrition that the prevention of rapid catch-up growth in early life prevents the development of obesity and glucose intolerance in adulthood (35). In a study of low birth weight rats that were undernourished throughout gestation, exercise in adulthood was shown to eliminate prenatally induced obesity (36). These findings combined with our results suggest that, in low birth weight offspring, calorie restriction in early life is more effective than in adulthood to oppose obesity. Moreover, a physical activity intervention in adulthood may be more effective than calorie restriction to reduce obesity associated with low birth weight. This finding could be particularly important when designing weight loss programs for obese diet-resistant adults. Further studies are needed.
In summary, our results show that in utero undernutrition impacts muscle energetics by altering mitochondrial content and oxidative functions. This is supported by several observations. First, adult mice from undernourished pregnancies have increased adiposity and when challenged with calorie restriction, have a dramatically decreased weight loss. Secondly, muscle mitochondrial content is decreased in mixed fiber muscles with in utero undernutrition. Thirdly, in utero undernutrition resulted in decreased respiration in isolated mitochondria and permeabilized fibers. Taken together, results suggest that the decreased mitochondrial content and activity in mixed fiber muscles contribute to increased adiposity and impaired glucose tolerance and, when faced with dietary energy deficit there is an apparent heightened metabolic efficiency. Overall, findings suggest that low birthweight offspring may have developed a protective mechanism in utero for species survival in times when energy supply is restricted. As low birth weight is a significant risk factor for obesity and T2DM, understanding how it alters skeletal muscle is important for both prevention and therapy.
Supplementary Material
Acknowledgments
We would like to thank Jian Xuan for technical assistance with animal work, Linda Jui for preparation and staining of histological slides, and Gabrielle Côté for assistance with counting muscle fibers. This research was supported through grants from Canadian Institutes of Health Research (MOP57810, MEH; MOP258677, AB), National Institutes of Health (P20MD000175, DK088319; SG) and American Heart Association (AHA10SDG4230068; SG).
Scholarships were also awarded from Natural Sciences and Engineering Research Council of Canada (Canada Graduate Scholarship -Doctoral, BB; -Master’s, AC).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Contributions of authors: Conceived and/or designed the work (BB, MEH); performed experiments (BB, MD, GNK, AC, KR); analyzed or interpreting results (BB, SG, MD, AC, AB, KR, ECT, MEP, MEH); wrote the manuscript (BB, MEH). All authors revised the manuscript and approved the final version.
Supplementary information: available at the International Journal of Obesity’s website.
References
- 1.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jimenez-Chillaron JC, Hernandez-Valencia M, Reamer C, Fisher S, Joszi A, Hirshman M, et al. Beta-cell secretory dysfunction in the pathogenesis of low birth weight-associated diabetes: a murine model. Diabetes. 2005;54:702–711. doi: 10.2337/diabetes.54.3.702. [DOI] [PubMed] [Google Scholar]
- 5.Isganaitis E, Jimenez-Chillaron J, Woo M, Chow A, DeCoste J, Vokes M, et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes. 2009;58:1192–1200. doi: 10.2337/db08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 7.Handschin C. Regulation of skeletal muscle cell plasticity by the peroxisome proliferator-activated receptor gamma coactivator 1alpha. J Recept Signal Transduct Res. 2010;30:376–384. doi: 10.3109/10799891003641074. [DOI] [PubMed] [Google Scholar]
- 8.Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, et al. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J Physiol. 2010;588:3567–3592. doi: 10.1113/jphysiol.2010.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- 10.Oberbach A, Bossenz Y, Lehmann S, Niebauer J, Adams V, Paschke R, et al. Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care. 2006;29:895–900. doi: 10.2337/diacare.29.04.06.dc05-1854. [DOI] [PubMed] [Google Scholar]
- 11.Jensen CB, Martin-Gronert MS, Storgaard H, Madsbad S, Vaag A, Ozanne SE. Altered PI3-kinase/Akt signalling in skeletal muscle of young men with low birth weight. PLoS One. 2008;3:e3738. doi: 10.1371/journal.pone.0003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jensen CB, Storgaard H, Madsbad S, Richter EA, Vaag AA. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J Clin Endocrinol Metab. 2007;92:1530–1534. doi: 10.1210/jc.2006-2360. [DOI] [PubMed] [Google Scholar]
- 13.Kensara OA, Wootton SA, Phillips DI, Patel M, Jackson AA, Elia M. Fetal programming of body composition: relation between birth weight and body composition measured with dual-energy X-ray absorptiometry and anthropometric methods in older Englishmen. Am J Clin Nutr. 2005;82:980–987. doi: 10.1093/ajcn/82.5.980. [DOI] [PubMed] [Google Scholar]
- 14.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17:2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 15.Fluck M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159–216. doi: 10.1007/s10254-002-0004-7. [DOI] [PubMed] [Google Scholar]
- 16.Park HK, Jin CJ, Cho YM, Park DJ, Shin CS, Park KS, et al. Changes of mitochondrial DNA content in the male offspring of protein-malnourished rats. Ann N Y Acad Sci. 2004;1011:205–216. doi: 10.1007/978-3-662-41088-2_21. [DOI] [PubMed] [Google Scholar]
- 17.Selak MA, Storey BT, Peterside I, Simmons RA. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am J Physiol Endocrinol Metab. 2003;285:E130–137. doi: 10.1152/ajpendo.00322.2002. [DOI] [PubMed] [Google Scholar]
- 18.Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, et al. SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du H, Dardzinski BJ, O’Brien KJ, Donnelly LF. MRI of fat distribution in a mouse model of lysosomal acid lipase deficiency. AJR Am J Roentgenol. 2005;184:658–662. doi: 10.2214/ajr.184.2.01840658. [DOI] [PubMed] [Google Scholar]
- 20.Estey C, Seifert EL, Aguer C, Moffat C, Harper ME. Calorie restriction in mice overexpressing UCP3: evidence that prior mitochondrial uncoupling alters response. Exp Gerontol. 2012;47:361–371. doi: 10.1016/j.exger.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerrits MF, Ghosh S, Kavaslar N, Hill B, Tour A, Seifert EL, et al. Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J Lipid Res. 2010;51:2394–2404. doi: 10.1194/jlr.P005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chappell JB, Perry SV. Biochemical and osmotic properties of skeletal muscle mitochondria. Nature. 1954;173:1094–1095. doi: 10.1038/1731094a0. [DOI] [PubMed] [Google Scholar]
- 23.Seifert EL, Estey C, Xuan JY, Harper ME. Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem. 2010;285:5748–5758. doi: 10.1074/jbc.M109.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, et al. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mailloux RJ, Xuan JY, Beauchamp B, Jui L, Lou M, Harper ME. Glutaredoxin-2 is required to control proton leak through uncoupling protein-3. J Biol Chem. 2013;288:8365–8379. doi: 10.1074/jbc.M112.442905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harper ME, Dent R, Monemdjou S, Bezaire V, Van Wyck L, Wells G, et al. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes. 2002;51:2459–2466. doi: 10.2337/diabetes.51.8.2459. [DOI] [PubMed] [Google Scholar]
- 27.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Ghosh S, Dent R, Harper ME, Stuart J, McPherson R. Blood gene expression reveal pathway differences between diet-sensitive and resistant obese subjects prior to caloric restriction. Obesity (Silver Spring) 2011;19:457–463. doi: 10.1038/oby.2010.209. [DOI] [PubMed] [Google Scholar]
- 29.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 30.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hediger ML, Overpeck MD, Kuczmarski RJ, McGlynn A, Maurer KR, Davis WW. Muscularity and fatness of infants and young children born small- or large-for-gestational-age. Pediatrics. 1998;102:E60. doi: 10.1542/peds.102.5.e60. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen EL, Malis C, Jensen CB, Jensen JE, Storgaard H, Poulsen P, et al. Altered fat tissue distribution in young adult men who had low birth weight. Diabetes Care. 2005;28:151–153. doi: 10.2337/diacare.28.1.151. [DOI] [PubMed] [Google Scholar]
- 33.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–434. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 34.Woo M, Isganaitis E, Cerletti M, Fitzpatrick C, Wagers AJ, Jimenez-Chillaron J, et al. Early life nutrition modulates muscle stem cell number: implications for muscle mass and repair. Stem Cells Dev. 20:1763–1769. doi: 10.1089/scd.2010.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, et al. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia. 2006;49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 36.Miles JL, Huber K, Thompson NM, Davison M, Breier BH. Moderate daily exercise activates metabolic flexibility to prevent prenatally induced obesity. Endocrinology. 2009;150:179–186. doi: 10.1210/en.2008-1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.