Abstract
Impaired signaling by granulocyte/macrophage-colony stimulating factor (GM-CSF) drives the pathogenesis of two diseases (autoimmune and hereditary pulmonary alveolar proteinosis (PAP)) representing over ninety percent of patients who develop PAP syndrome but not a broad spectrum of diseases that cause PAP by other mechanisms. We previously exploited the ability of GM-CSF to rapidly increase cell-surface CD11b levels on neutrophils (CD11bSurface) to establish the CD11b stimulation index (CD11b-SI), a test enabling the clinical research diagnosis of impaired GM-CSF signaling based on measuring CD11bSurface by flow cytometry using fresh, heparinized blood. (CD11b-SI is defined as GM-CSF-stimulated- CD11bSurface minus unstimulated CD11bSurface divided by un-stimulated CD11bSurface multiplied 100.) Notwithstanding important and unique diagnostic utility, the test is sensitive to experimental conditions that can affect test performance. The present study was undertaken to optimize and standardize CD11b-SI test for detecting impaired GM-CSF signaling in heparinized human blood specimens from PAP patients. Results demonstrated the test was sensitive to choice of anticoagulant, pretesting incubation on ice, a delay between phlebotomy and test performance of more than one hour, and the concentration GM-CSF used to stimulate blood. The standardized CD11b-SI test reliably distinguished blood specimens from autoimmune PAP patients with impaired GM-CSF signaling from those of health people with normal signaling. Intra-subject differences were smaller than inter-subject differences in repeated measures. Receiver operating characteristic curve analysis identified a CD11b-SI test result of 112 as the optimal cut off threshold for diagnosis of impaired GM-CSF signaling in autoimmune PAP for which the sensitivity and specificity were both 100%. These results support the use of this standardized CD11b-SI for routine clinical identification of impaired GM-CSF signaling in patients with autoimmune PAP. The CD11b-SI may also have utility in clinical trials of novel therapeutic strategies targeting reduction in GM-CSF bioactivity now under evaluation for multiple common autoimmune and inflammatory disorders.
Keywords: Pulmonary alveolar proteinosis, CD11b, Neutrophil, Granulocyte/macrophage-colony stimulating factor, Flow cytometry, Diagnosis
1. Introduction
Granulocyte/macrophage-colony-stimulating factor (GM-CSF) is a cytokine with pleiotropic effects on myeloid cells including stimulation of the survival, proliferation, and differentiation myeloid progenitors, as well as augmentation (“priming”) of host defense functions of mature macrophages and neutrophils (Lieschke and Burgess, 1992; Condliffe et al., 1998). Although normally present at low or undetectable levels in vivo (Uchida et al., 2009), GM-CSF is critical to the normal functioning of blood neutrophils (Uchida et al., 2007) and alveolar macrophages (Trapnell and Whitsett, 2002). In humans, disruption of GM-CSF signaling by high levels of neutralizing GM-CSF autoantibodies is associated with the development of autoimmune pulmonary alveolar proteinosis (autoimmune PAP), a rare lung disease characterized by surfactant accumulation, respiratory failure, and increased infections (Kitamura et al., 1999; Trapnell et al., 2003; Uchida et al., 2004; Uchida et al., 2007). However, GM-CSF autoantibodies are present at low levels in healthy people (Uchida et al., 2009) and levels do not correlate with disease severity in PAP patients (Seymour et al., 2003). This apparent paradox is rectified by the concept of a critical threshold of GM-CSF autoantibodies above which GM-CSF-dependent functions (like surfactant clearance by alveolar macrophages) are reduced to zero regardless of the magnitude of the increase above the critical threshold (Bendtzen et al., 2007). Disruption of GM-CSF receptor function by recessive CSF2RA or CSF2RB mutations causes hereditary PAP, which is histologically indistinguishable from autoimmune PAP (Martinez-Moczygemba et al., 2008; Suzuki et al., 2008; Suzuki et al., 2010). PAP also occurs in a heterogeneous group of diseases either as a consequence of an underlying clinical condition presumably affecting the alveolar macrophage function (secondary PAP) (Ishii et al., 2009) or by mutations in genes involved in surfactant production (e.g., SFTPB, SFTPC, ABCA3, TTF1) (congenital PAP, and PAP associated with interstitial lung disease)(Nogee, 2010; Whitsett et al., 2004). In genetically modified mice, GM-CSF deficiency causes PAP (Dranoff et al., 1994) while GM-CSF overexpression causes a syndrome of macrophage accumulation, tissue damage, and death (Lang et al., 1987). Increased GM-CSF bioactivity has been implicated in the pathogenesis of rheumatoid arthritis, multiple sclerosis, and other inflammatory and autoimmune diseases and evaluation of GM-CSF antagonist therapy is underway in human clinical trials for multiple clinical indications (Hamilton, 2008). These observations indicate that GM-CSF bioactivity is tightly controlled in healthy people, that loss of tight control is involved in the pathogenesis of multiple diseases and suggest the utility of a clinical test to measure impaired GM-CSF signaling in humans.
CD11b is an cell-adhesion molecule normally present in resting neutrophils within pre-formed granules that is translocated to the cell surface upon neutrophil activation (Graves et al., 1992) including after exposure to an increased concentration of GM-CSF (Condliffe et al., 1998). Previously, we exploited this endogenous priming mechanism in developing the CD11b stimulation index (CD11b-SI), a test to measure impaired GM-CSF signaling in human blood specimens (Uchida et al., 2007). In this assay, fresh, heparinized whole blood is incubated with and without GM-CSF and the mean fluorescence of CD11b on CD16Hi leukocytes is measured by flow cytometry to determine the level of cell-surface CD11b on neutrophils (CD11bSurface). The GM-CSF stimulated increase in CD11bSurface (i.e., the stimulation index or SI), is large and readily detected in blood specimens from healthy individuals and zero or severely reduced in patients with autoimmune PAP or hereditary PAP (Uchida et al., 2007; Suzuki et al., 2008; Uchida et al., 2009). In the present study, we evaluated and optimized the experimental conditions of the CD11b-SI assay and then validated the test using clinical specimens from patients previously diagnosed with autoimmune PAP and from healthy people.
2. Methods
2.1. Participants
The institutional review board of the Cincinnati Children's Hospital Medical Center (CCHMC) and the University of Tokyo Graduate School of Medicine approved the study. All participants or their legal guardians gave written informed consent, and minors gave assent. Participates included 10 individuals referred for evaluation or treatment of autoimmune PAP diagnosed based on clinical and radiographic findings; an open lung biopsy, transbronchial lung biopsy, or cytologic analysis of bronchoalveolar lavage cells and fluid; and a positive GMAb test performed as described (Uchida et al., 2014). We also studied 34 healthy control subjects who were nonsmokers with no history of major illness and symptom-free at the time of enrollment in the study. Data for some individuals were previously reported (Uchida et al., 2007; Uchida et al., 2009, Han et al., 2009).
2.2. Reagents
Primary antibodies included FITC- or PE-conjugated anti-human CD11b, PE-conjugated anti-human CD16 (Miltenyi Biotec K.K., Tokyo, Japan); FITC-rat IgG (isotype control) (abcam, Cambridge, UK); anti-human CD11b monoclonal (BioLegend, San Diego, CA); anti-human phospho-STAT5 (Millipore, Billerica, MA). Secondary detection antibody (anti-rat IgG-HRP(HAF005)) was from R&D systems (Minneapolis, MN) and anti-mouse IgG-HRP was from GE Healthcare (Little Challfont Buckinghamshire, UK). HRP-conjugated anti-actin antibody was from Santa Cruz Biotechnology (Dallas, TX). E. coli-derived recombinant human GM-CSF was from ATGen (Seongnam, South Korea). Recombinant cytokines, and other proteins included G-CSF, C5a, Interleukin (IL)-6, IL-8, IL-10, Interferon (IFN)-β, IFN-γ (all from Wako, Osaka, Japan).
2.3. Flow Cytometry
Evaluation of neutrophils by flow cytometry was performed as previously described (Uchida et al., 2007). Briefly, 200 μL of whole blood in a 1.5 ml polypropylene tube was incubated (37°C, 30 minutes) with recombinant human GM-CSF (10 ng/mL) or other cytokines / chemokines, placed on ice immediately. An aliquot (50 μL) of each was mixed with 50 μL of ice-cold PBS containing FITC-conjugated anti-human CD11b antibody (4 μL, undiluted) and PE-conjugated anti-human CD16 antibody (4 μL, undiluted) in a polystyrene round-bottom tube (BD Bioscience, San Jose, CA) and incubated (4°C, 30 min, in darkness). After adding 2 mL of FACS lysing solution (BD Biosciences, San Jose, CA), incubation (room temp, 10 min), tubes were centrifuged (300 x g, room temp, 5 min). The cell pellets were re-suspended in 200μL of PBS and stored in 4 °C in the dark until evaluation by flow cytometry. The amount of cell-surface CD11b on neutrophils ([CD11bSurface]) was measured by flow cytometry as mean fluorescent intensity of FITC on CD16Hi cells defined by gating. The CD11b stimulation index (CD11b-SI) was calculated using the following equation
where [CD11bSurface]+GM-CSF represents [CD11bSurface] after incubation with GM-CSF and [CD11bSurface]No GM-CSF represents [CD11bSurface] after incubation without GM-CSF. In some experiments, cytoplasmic CD11b was stained with PE-CD11b (4 μl, undiluted) using the Intracellular Cytokine Staining Kit (eBioscience, San Diego, CA) as per manufacturer's instructions.
2.4. Western blotting
Heparinized whole blood was incubated with or without GM-CSF (10ng/mL, 37°C, 15min) and red blood cells were lysed with lysing buffer (BD Pharmlyse™, BD Biosciences, San Jose, CA). After washing with ice-cold PBS, cells were extracted with protein extraction buffer (M-PER® #78501) containing protease inhibitor cocktail (0.5% v/v), phosphatase inhibitor cocktail (1% v/v) (all from Thermo Scientific, Rockford, IL), and EDTA (5 mM). Protein extracts were suspended in sample loading buffer, layered onto AnykD gradient gels (Bio-rad, Hercules, CA), separated by gel electrophoresis (100 V, 150 minutes) and transferred (Transblot™, Bio-rad, Hercules, CA) onto PVDF membranes (Immobilon, Merck Millipore, Billerica, MA), per manufacturer's instructions. Membranes were incubated with either murine anti-CD11b monoclonal antibody (10 μl from the vial diluted in 500 μl of immunoblotting buffer (Uchida 2009) (BioLegend, San Diego, CA) or murine anti-phospho- STAT5 antibody (5 μl from the vial diluted in 1000 μl of immunoblotting buffer (Millipore, Billerica, MA) and detection was done using ECL plus™ (GE healthcare, Little Chalfont, UK). Band intensity of CD11b was quantified by Image Quant TL Analysis Toolbox Ver 7.0 (GE Healthcare, Pittsburgh, PA).
2.5. Statistical Analysis
Numerical data were evaluated for a normal distribution using the Kolmogorov-Smirnov test and for equal variance using the Levene median test; parametric data are presented as mean (±SD) and nonparametric data are presented as median and interquartile range (IQR). Statistical comparisons of parametric data were made with Student's t-test for two-group comparisons and with one-way analysis of variance with post hoc analysis according to the Holm-Sidak method for multiple-group comparisons or Dunnett's method for multiple comparisons versus control. Nonparametric data were compared with the use of the Mann-Whitney rank-sum test. Intra- individual percent variation was calculated as the average, for all measured values from each person, of the value minus the mean of all values for an individual divided by the mean and multiplied by 100. Inter-individual percent variation was calculated as the average, for all individuals, of the final value for each individual minus the mean for all individuals divided by the mean for all individuals and multiplied by 100. Receiver operating characteristic (ROC) curve and all other statistical analyses were done using SigmaPlot software, version 12 (Systat Software, SanJose, CA). P values less than 0.05 were considered to indicate statistical significance and are denoted by asterisks (P<0.05 - *; P<0.01 - **; P<0.001 - ***). All experiments were repeated at least twice with similar results.
3. Results
3.1. Optimization of CD11b-SI components and procedure
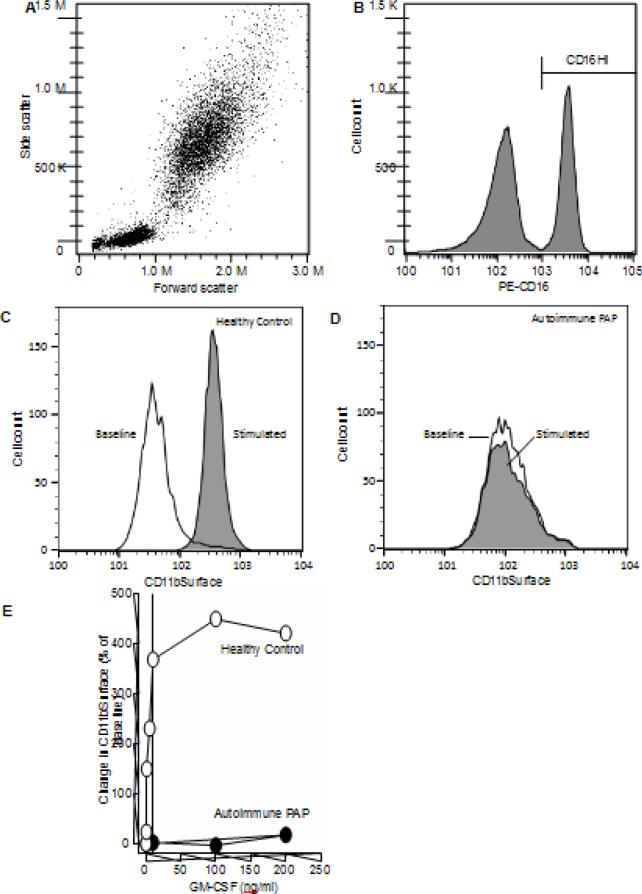
Neutrophils in fresh heparinized whole bloodidentified by CD16Hi immunostaining (Figure 1A-B) had detectable levels of cell-surface CD11b (CD11bSurface) at baseline (i.e., without stimulation) in both healthy individuals (1C) and autoimmune PAP patients (1D). GM-CSF stimulated a significant increase in CD11bSurface in blood from healthy individuals (Figure C) but not in blood from autoimmune PAP patients (Figure D). The change from baseline CD1bSurface increased with GM-CSF concentration and reached saturation at ~10 ng/mL in healthy people and was severely blunted with little increase in patients with autoimmune PAP (Figure 1E). To exploit this GM-CSF-stimulated change in CD11bSurface as a measure of impaired GM-CSF signaling in clinical samples, we first optimized experimental conditions to establish a standardized test to measure impaired GM-CSF signaling in whole blood.
Fig. 1.
Evaluation of CD11bSurface on the neutrophil in the whole blood: an assay to measure the GM-CSF-stimulated increase in cell-surface CD11b on neutrophils in whole blood. A. Representative leukocyte cytogram. Whole blood was processed and evaluated by flow cytometry as described in the Methods. B. Identification of neutrophils by gating on CD16. Immunostained leukocytes were gated for phycoerythrin (PE)-fluorescence to identify neutrophils as a distinct CD16Hi population. C-D. Quantification of neutrophil cell-surface CD11b (CD11bSurface) for a healthy control (C) and a patient with autoimmune PAP (D). Representative histogram of the fluorescence intensity in neutrophils from healthy control. open area – no GM-CSF stimulation; filled area – after GM-CSF stimulation. E. Percent change in CD11bSurface for healthy individual (HC) and patient with autoimmune pulmonary alveolar proteinosis (PAP).
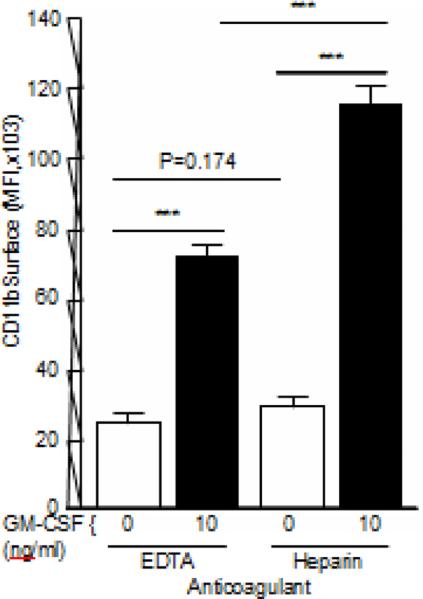
The effect of anticoagulant on CD11bSurface was evaluated in fresh whole blood collected into either EDTA- or heparin-containing phlebotomy specimen tubes. Baseline, un-stimulated CD11bSurface was not different among blood specimens collected using EDTA or heparin (P=0.174). In contrast, the GM-CSF-stimulated increase in CD11bSurface was smaller when EDTA was used for phlebotomy compared to samples collected in heparin (Figure 2).
Fig. 2.
Effect of anticoagulant on baseline and GM-CSF-stimulated CD11bSurface. Fresh blood was collected from healthy people into ethylenediaminetetraacetic acid (EDTA) – or sodium heparin-containing phlebotomy tubes and the mean fluorescence intensity (MFI) of CD11b on CD16Hi-gated neutrophils (CD11bSurface) was measured after stimulation with 10 ng/ml GM-CSF using the CD11b-SI test as described in the Methods. Bars represent the mean ± SD for n = 3 per condition. Comparisons were made by ANOVA with Dunnett's test; *** = P<0.001.
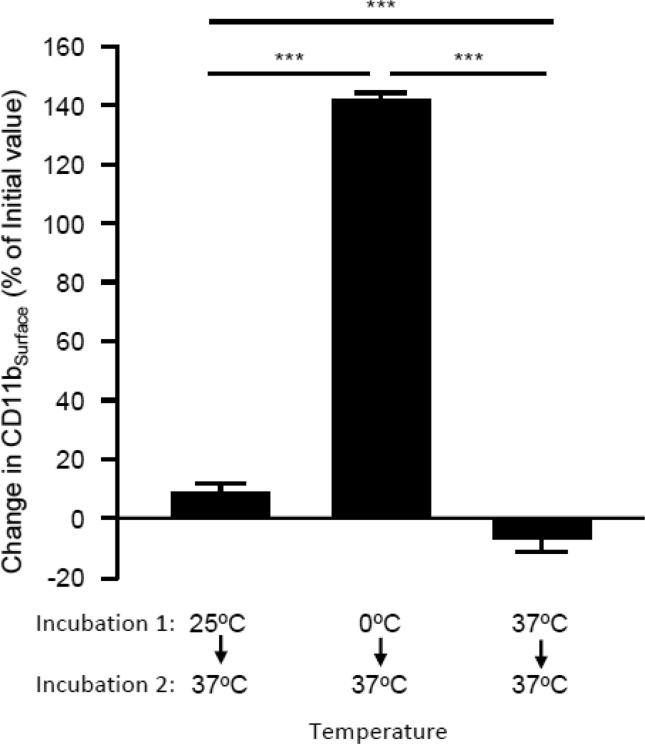
Blood is frequently collected at room temperature and maintained / evaluated at room temperature, on ice, or at 37 °C, depending on assay requirements. Therefore, we evaluated the effect of temperature on baseline CD11bSurface using fresh whole blood collected at room temperature (~25°C), and then maintained briefly at different combinations of these three temperatures. When blood was incubated at 25 °C and then at 37 °C (30 min each) or maintained at 37 °C for 60 min, baseline CD11bSurface was unaffected (Figure 3). However, when blood was incubated at 0°C and then at 37 °C (30 min each), CD11bSurface increased by 140% (Figure 3). Consequently, subsequent studies were conducted with blood maintained at room temperature before evaluation.
Fig 3.
Effect of change in the temperature at which blood was maintained before testing on the CD11bSurface. Fresh, heparinized whole blood was collected from healthy people by phlebotomy and immediately incubated at 0°C, 25°C, or 37°C for 30 min (incubation period 1) followed immediately by incubation at 37°C for 30 min (incubation period 2) and then CD11bSurface was measured as described in the Methods. For each sample, the CD11bSurface at the end of incubation period 2 was subtracted from the CD11bSurface at the end of incubation period 1, divided by the CD11bSurface at the end of incubation period 1 and multiplied by 100 percent. Positive values indicate an increase in CD11bSurface after incubation period 2 compared to after incubation period 1. Bars represent the mean ± SD for n = 3 per condition. Comparisons were made by ANOVA with Dunnett's test; *** = P<0.001.
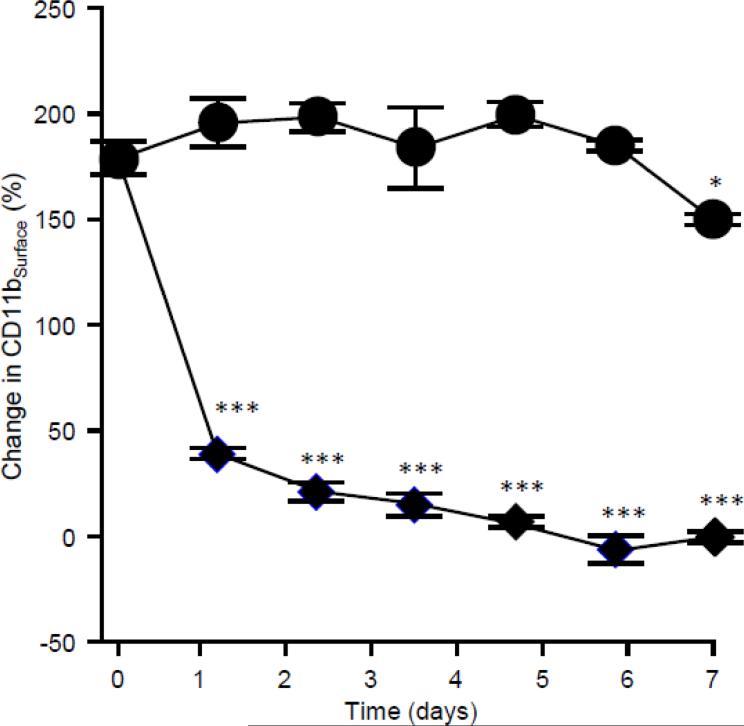
Next we evaluated potential effects of time before blood specimen evaluation on baseline CD11bSurface. The ability of neutrophils to exhibit an increase in CD11bSurface after incubation of heparinized whole blood with 10 ng/mL GM-CSF compared to baseline CD11bSurface (henceforth, change in CD11bSurface (%)) was robust immediately after phlebotomy but markedly diminished after 1 day and declined further thereafter (Figure 4). In contrast, when blood was stimulated and then stained and fixed immediately after phlebotomy, flow cytometric analysis could be performed for up to seven days with little to no effect on the change in CD11bSurface (Figure 4).
Fig. 4.
Effect of time between GM-CSF stimulation/fixation and evaluation by flow cytometry on the change in CD11bSurface. Fresh, heparinized blood was collected from healthy people, incubated with or without GM-CSF within 1 hour of phlebotomy, staining with antibodies to CD11bSurface, and fixed (closed symbols) or stored at room temperature for various times (open symbols) before stimulation, immunostaining and analysis by flow cytometry as described in the Methods. Symbols represent the mean ± SD for n = 3 per point. Comparisons to the corresponding value on day zero were made by ANOVA with Dunnett's test; * = P<0.05, *** = P<0.001.
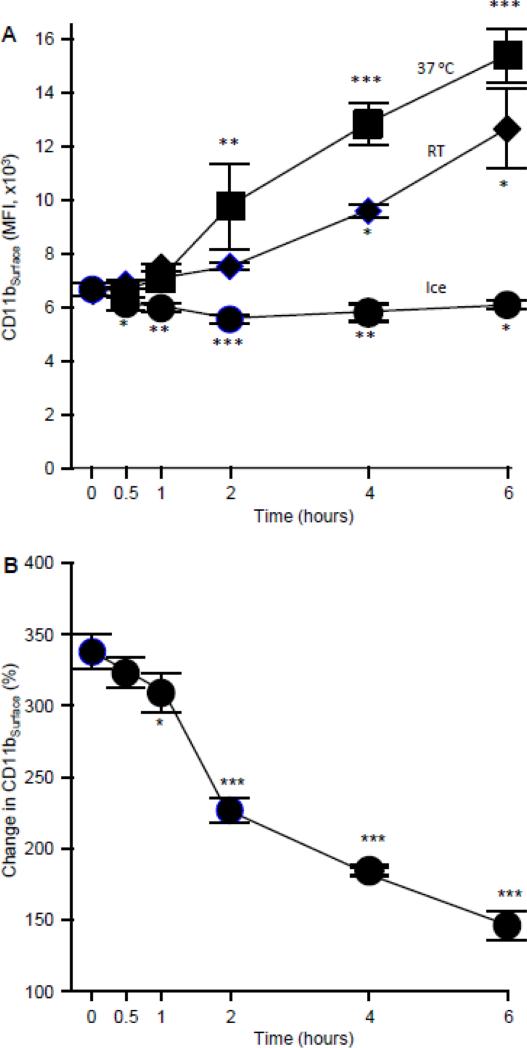
To further explore the effects of time and temperature on the change in CD11bSurface, we evaluated the effects of a short-term delay between phlebotomy and testing for freshly isolated, heparinized whole blood stored at various temperatures. CD11bSurface remained constant for up to 6 hours when blood was kept on ice but increased progressively when blood was kept at room temperature (and even faster when blood was kept at 37 °C) during this time period (Figure 5A). In parallel, the change in CD11bSurface declined over the time period for blood maintained at 25 °C (Figure 5B).
Fig. 5.
Effects of temperature of blood after phlebotomy and delay in analysis on change in CD11bSurface. A. Effect of temperature on CD11bSurface expression levels. Fresh, heparinized blood was collected and maintained at the indicated temperatures and then CD11b expression levels were evaluated as described in the Methods. B. Effect of a short delay prior to analysis on the change in CD11bSurface stimulated with 10ng/mL of GM-CSF. Fresh, heparinized blood was kept at 25°C for various times before evaluation by the change in CD11bSurface as described in the Methods. Symbols represent the mean ± SD for n = 3 per point. Comparisons to the corresponding value at zero hours were made by ANOVA with Dunnett's test; * = P<0.05, ** = P<0.01, *** = P<0.001.
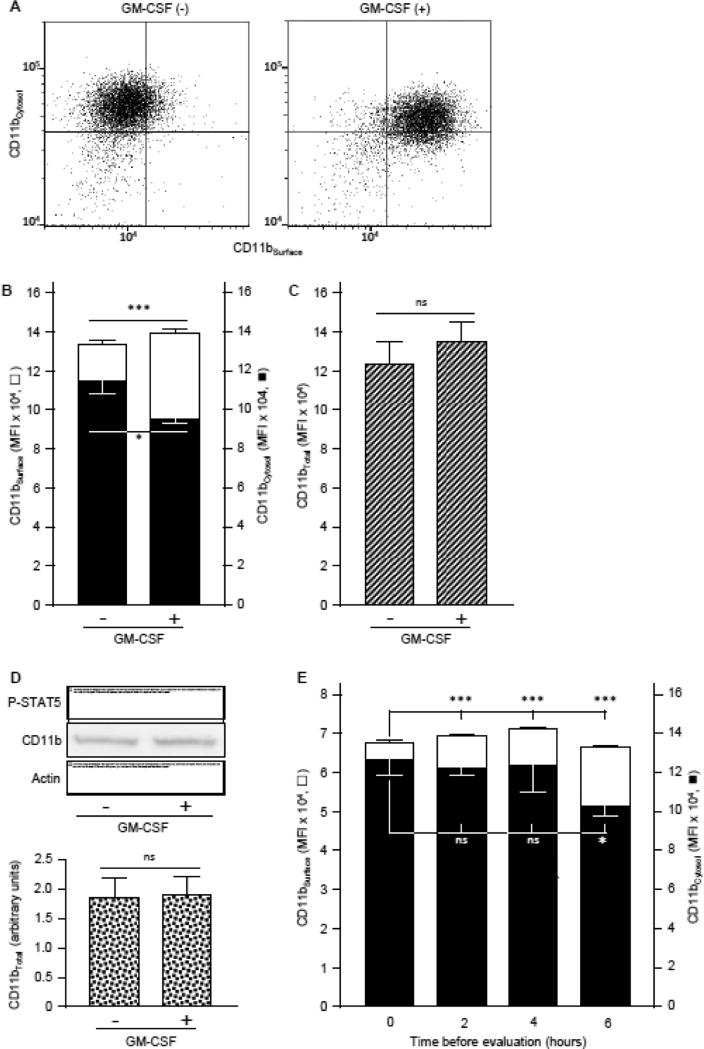
To better understand the mechanism of the GM-CSF-stimulated increase in CD11bSurface, the spatial distribution of CD11b within neutrophils (i.e., in the cytoplasm or on the cell-surface) was evaluated before and after GM-CSF stimulation using several methods. Simultaneous detection of CD11bSurface and cytoplasmic CD11b (CD11bCytosol) in cells double-immunostained with FITC-CD11b or PE-CD11b, respectively (Figure 6A-B), revealed that CD11bSurface increased by 150 % with GM-CSF stimulation (Figure 6C). In contrast, CD11bCytosol decreased by ~11% and CD11bTotal was not different (Figure 6C). CD11bTotal was also unchanged by GM-CSF-stimulation when both CD11bSurface and CD11bCytosol were measured together using the same detection antibody/chromophore (FITC-anti-human CD11b) (Figure 6D) or when measured by western blotting (Figure 6D). Finally, CD11bSurface gradually increased while CD11bCytosol gradually decreased in fresh whole blood maintained at 25 °C for 6 hours (Figure 6E). Together, these results indicate that GM-CSF stimulates pre-formed CD11b located within the neutrophil to translocate to the cell surface after stimulation.
Fig. 6.
GM-CSF-stimulated translocation of CD11b to the neutrophil cell-surface. A,B. Fresh, heparinized blood from healthy people was evaluated for the level of CD11b except that cells were first immunostained with FITC-anti-human CD11b to measure CD11bSurface (open bars), washed, permeabilized and stained with PE-anti-human CD11b to measure cytosolic CD11bCytosol (filled bars) as described in the Methods. A. Representative cytograms of neutrophils stained to show CD11bSurface (horizontal axis) and CD11bCytosol (vertical axis) with or without GM-CSF stimulation (indicated). B. Bars represent the mean ± SD for n = 3 per condition. C. Both CD11bSurface and CD11bCytosol were evaluated with methods described above (legends to Panel A,B) with FITC-anti-human CD11b for both to measure total neutrophil CD11b levels. Bars represent the mean ± SD for n = 3 per condition. D. Quantification of total CD11b by western blotting. Proteins of white blood cell in the whole blood incubated with or without GM-CSF (10 ng/mL) was evaluated by gel electrophoresis and western blotting to quantify CD11b, phosphorylated-STAT5 (as a GM-CSF stimulation control), or actin (as a loading control), and CD11b band intensity quantified by densitometry as described in the Methods. Bars represent mean ± SD for n = 4 determinations per condition. E. Cytosolic CD11b translocation to the neutrophil cell surface with time. Fresh, heparinized blood from healthy people was incubated at room temperature for various times before differential immunostaining to quantify CD11bSurface (open bars) and CD11bCytosol (filled bars) as described above (legend to Panel A). Bars represent the mean ± SD of n = 3 per condition. Comparisons were made by Student's t test (A-C) or ANOVA (D); * = P<0.05, *** = P<0.001.
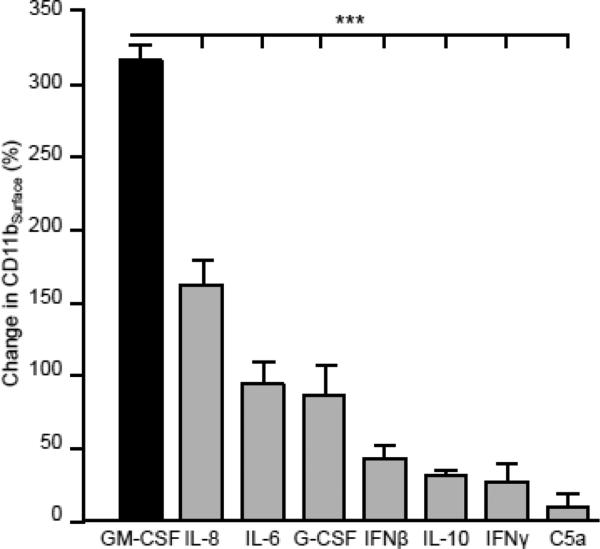
The specificity of the change in CD11bSurface was evaluated by comparing the change in CD11bSurface stimulated by GM-CSF to that caused by a wide range of pro- and anti-inflammatory mediators. Compared to baseline, GM-CSF stimulated a greater increase in CD11bSurface than did IL-8, IL-6, G-CSF, interferon IFN-β, IL-10, IFN-γ, and complement fragment 5a (Figure 7).
Fig. 7.
Capacity of GM-CSF and other neutrophil activators to increase CD11bSurface. Fresh, heparinized blood from healthy people was incubated without or with GM-CSF or other cytokines, chemokines, and inflammatory molecules including interleukin 8 (IL-8), IL-6, granulocyte-colony stimulating factor (GCSF), interferon β (IFNβ), IL-10, IFNγ, and complement fragment 5a (C5a), 10ng/mL each as indicated, and the percent increase in stimulated over baseline CD11bSurface as described in the Methods. Comparisons were made by ANOVA using the Holm-Sidak method for multiple comparisons; *** = P< 0.001.
Taken together, these results indicate that GM-CSF specifically stimulates neutrophils causing a robust and rapid translocation of pre-formed, intracytoplasmic CD11b to the cell surface.
3.2. CD11b-SI Test performance
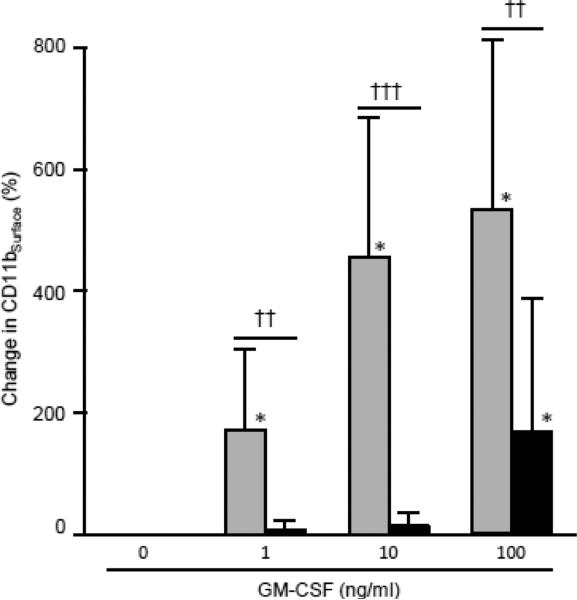
Next, we evaluated the ability of the standardized test to identify impaired GM-CSF signaling in whole blood in patients with autoimmune PAP in whom GM-CSF signaling is known to be impaired by high concentrations of neutralizing GM-CSF autoantibodies and in healthy people in whom GM-CSF signaling in blood leukocytes is present and readily detectable (Uchida et al., 2009). The baseline level of CD11bSurface in autoimmune PAP patients was higher than in healthy individuals (101.4 ± 63.2, 170.2 ± 75.4, n= 25, 10, respectively, p=0.010; not shown). At the standardized concentration for testing (10 ng/mL), GM-CSF stimulated a mean increase from baseline in CD11bSurface of ~450% in 22 healthy people but no increase in 5 patients with autoimmune PAP (Figure 8). The difference in GM-CSF-stimulated CD11bSurface between healthy people and autoimmune PAP patients was also observed at lower (1 ng/ml) and higher (100 ng/ml) GM-CSF stimulating concentrations, although there was a small increase (that remained significantly different from healthy controls) (Figure 8).
Fig. 8.
Detection of impaired GM-CSF signaling in human blood specimens. Fresh, heparinized blood was collected from healthy people (n = 22; gray bars) or patients with autoimmune PAP (n = 5, closed bars) and percent increase of CD11bSurface over baseline as described in the Methods except that different GM-CSF concentrations were used for stimulation (indicated). Comparisons were made by the Mann-Whitney rank sum test with post-hoc analysis using Dunnett's test. comparison of corresponding values for healthy people or PAP patients to the un-stimulated control (no GM-CSF) are indicated; * = P<0.05. Comparison of values for healthy people and PAP patients at each level of GM-CSF stimulation are also indicated; †† = P<0.01, ††† = P<0.001.
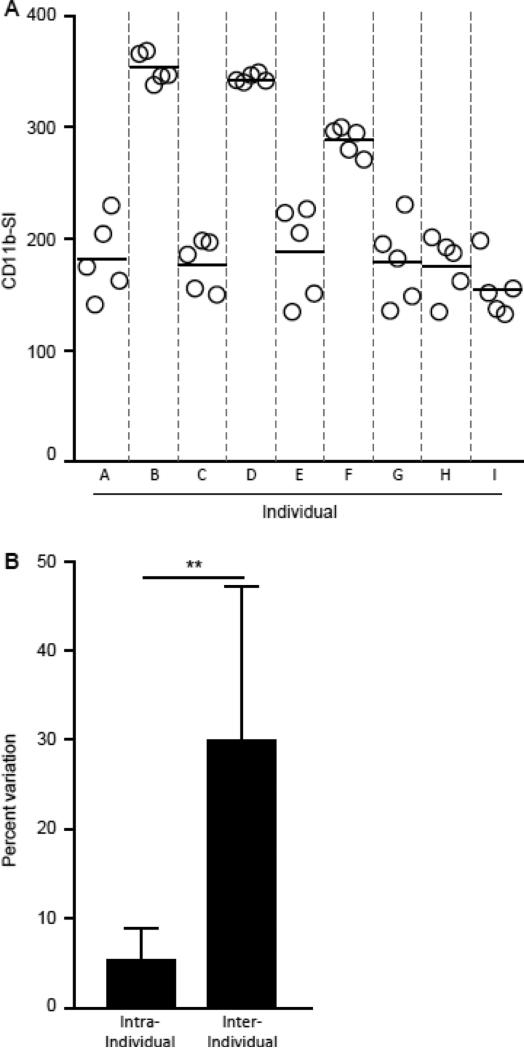
We determined CD11b stimulation index or CD11b-SI as change in CD11bSurface at 10 ng/mL of GM-CSF to maximize discrepancies between healthy controls and patients with PAP. The precision of the CD11b-SI test for measuring impaired GM-CSF signaling in clinical blood specimens was evaluated by measuring the inter-subject and intra-subject variation in fresh heparinized whole blood from nine healthy people (Figure 9A). The coefficient of variation for repeated measures in the same subject was 5.3 ± 3.5%, which is less than 15% in accordance with FDA guidance criteria for assay precision (Anonymous, 2001) (Figure 9B). Further, the coefficient of variation for measurements within subject measurements was less than that for measurements between subjects (Figure 9B). These data indicate the CD11b-SI test is reliable and can be used to measure impaired GM-CSF signaling in clinical blood samples.
Fig. 9.
Precision of the CD11b-SI test measurements in healthy people. A. Fresh, heparinized blood was collected from healthy people (n = 9, A-I) and evaluated repeatedly in independent CD11b-SI test procedures (n = 5 per person). Shown are the individual determinations and mean for each individual (separated by gray dashed lines). Each symbol represents an independent determination. B. Evaluation of the percent variation among repeated determinations of CD11b-SI within the same subject (intra-subject) or between different subjects (inter-subject), calculated as described in the Methods. Comparison was made using Student's t test; ** = P<0.01.
3.3. Diagnostic cutoff of the CD11b-SI
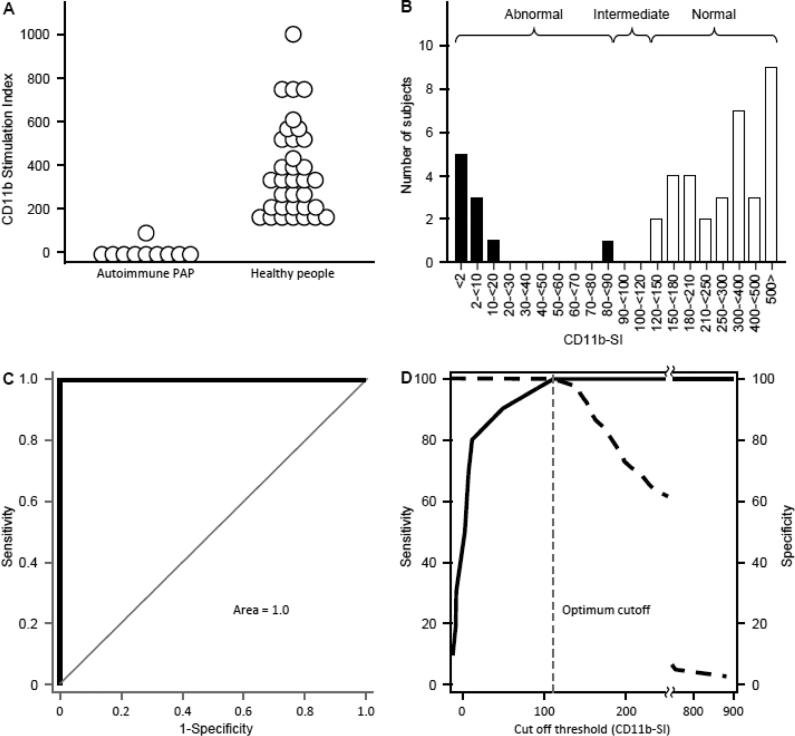
To further support the diagnostic use of the CD11b-SI test to diagnose impaired GM-CSF signaling in human clinical specimens, we measured the CD11b-SI in people previously diagnosed with autoimmune PAP and in healthy, asymptomatic people. The CD11b-SI in patients with autoimmune PAP (3.61 [-7.97 – 10.95]; n = 10) was markedly lower than in healthy people (321 [195 - 524]; n = 34) (Figure 10A). There was a clear separation between the high values in healthy people and the low values in autoimmune PAP patients (Figure 10B).
Fig. 10.
Measurement and ROC Analysis of CD11b-SI in autoimmune PAP patients and healthy people. A. CD11b-SI test values in autoimmune PAP patients (10) and healthy people (34) measured as described in the Methods. B. Histogram of the frequency distribution of CD11bSI test values in healthy people (open bars) and autoimmune PAP patients (filled bars). C-D. Receiver operating characteristic (ROC) curve analysis of CD11bSI results for 10 autoimmune PAP patients and 34 healthy people. Standard ROC analysis was performed to determine the sensitivity and specificity for the data shown in Panel A. The are under the curve was 1.0 (C), and, at a cut off value for CD11bSI of 112 determined by the software, the sensitivity and specificity of the CD11b-SI were both 100% (D).
Receiver operating characteristic (ROC) curve analysis for these results demonstrated the sensitivity and specificity to be 100% (Figure 10C) and identified an optimal cut off threshold value for the difference between normal and abnormal CD11b-SI values (i.e., in healthy people and patients with autoimmune PAP, respectively) to be 112 (Figure 10D).
4. Discussion
In this study, we optimized the experimental conditions of the CD11b-SI test (Uchida et al., 2007) and then evaluated the reliability of the optimized test for detecting impaired GM-CSF signaling in heparinized human blood specimens. In healthy people, GM-CSF rapidly stimulated a robust translocation of pre-formed CD11b to the cell surface of neutrophils while in patients with autoimmune PAP, this translocation response was blocked. The assay performed very well in distinguishing impaired GM-CSF signaling in autoimmune PAP patients from normal signaling in healthy people for which the sensitivity and specificity were both 100%.
These results help establish a basis for the routine clinical use of blood testing for the differential diagnosis of PAP. The serum GM-CSF autoantibody ELISA test (GMAb ELISA) used to measure GM-CSF autoantibodies (Schoch et al., 2002) was recently optimized and found to have a sensitivity and specificity of 100% for diagnosis of autoimmune PAP (Uchida et al., 2014). The diagnostic use of the GMAb ELISA was supported by the demonstration that GM-CSF autoantibodies are the cause and not an associated epiphenomenon of autoimmune PAP (Sakagami et al., 2009; Sakagami et al., 2010). Support was also provided by a critical threshold of GM-CSF autoantibodies (Bendtzen et al., 2007) above which the risk of PAP is increased in non-human primates injected with patient-derived GM-CSF autoantibodies (Sakagami et al., 2010) and in humans (Uchida et al., 2009) and by ROC curve analysis confirming the value of the critical threshold (~5 μg/ml) using the optimized GMAb ELISA (Uchida et al., 2014). Notwithstanding, while the latter demonstrated most healthy people and autoimmune PAP patients have GMAb ELISA test results below 3 μg/ml or above 9 μg/ml, respectively, it identified an intermediate concentration range (>3, <9 μg/ml) in which diagnostic utility may be reduced by proximity to the critical threshold. The CD11b-SI test, by measuring impaired GM-CSF signaling in whole blood, can be used to determine the functional significance of an increased GM-CSF autoantibody level when the GMAb ELISA test result is near the critical threshold. Results identified a CD11b-SI of 112 as the optimal cutoff value for distinguishing specimens known to have impaired GM-CSF signaling (e.g., autoimmune PAP) from those of healthy people. However, as a conservative approach to test interpretation, we recommend that test results between 90 and 120 be ‘read’ as intermediate since data from healthy people or PAP patients to confirm this cutoff were not available in this range. Additional studies may be useful in further evaluating the accuracy, precision, ruggedness of the CD11b-SI for routine clinical use as a diagnostic test. In seven patients with hereditary PAP caused by disruption of GM-CSF receptor function, we found no significant response to GM-CSF stimulation using the CD11b-SI test (Suzuki et al., 2010). Thus, the combination of compatible radiographic findings, a normal GMAb ELISA test result and an abnormal CD11b-SI test result suggest a diagnosis of hereditary PAP. Further, since stimulation at very high concentrations of GM-CSF can stimulate an increase in CD11bSurface in autoimmune PAP but likely not in hereditary PAP, the use of additional higher concentrations may be useful in the differential diagnosis of hereditary versus autoimmune PAP. However, further studies will be needed to explore and validate such an approach.
The CD11b-SI test may also be useful in other clinical or clinical research settings. For example, since CD11bSurface reflects neutrophil activation, it may be useful in patients with increased GM-CSF bioactivity such as serious infectious or inflammatory diseases (Shakoor and Hamblin, 1992; Palmer and Hamblin, 1993; Cook et al., 2013). Finally, it may be useful as an outcome measure in clinical trials aimed at reducing GM-CSF bioactivity, e.g., in autoimmune or inflammatory diseases (Cook et al., 2013), or the functional evaluation of cross-reacting anti-drug antibodies in individuals treated with recombinant human GM-CSF (Wadhwa et al., 1999). Finally, since this assay measures GM-CSF neutralizing capacity in the serum, it may be useful to monitor autoimmune PAP patients for spontaneous improvement or remission as expected if the GMAb were to fall below critical threshold (Uchida et al., 2009; Uchida et al., 2014).
One limitation of the CD11b-SI test is the short time period after phlebotomy during which the test must be conducted in order to obtain useful results. The GM-CSF-stimulated increase in CD11bSurface begins to diminish by 1 hour after phlebotomy, and is markedly blunted by 6 hours, and near zero by 24 hours. This effect is due to an increase in baseline CD11bSurface caused by generation of lysophosphatidylcholines during blood storage, which have potent priming effect on neutrophils (Silliman et al., 1994). Neutrophils undergo apoptosis spontaneously with time after phlebotomy, however, this is not significant at times less than three hours (Homburg et al., 1995, Uchida et al., 2007). These time-dependent effects can be overcome by conducting the GM-CSF stimulation component of the test followed by immunostaining and blood fixation within 1 hour of phlebotomy, after which flow cytometric analysis can be delayed for up to 6 days without compromising the results. Another limitation is the sensitivity of CD11bSurface to changes in the temperature at which blood is kept before testing – i.e., placement on ice followed by warming to either room temp. or 37°C, which has been reported previously (Shalekoff et al., 1998). In contrast, maintaining blood at 25 or 37°C gave acceptable results similar to a previous report (Youssef et al., 1995). Use of EDTA as the coagulant also reduced the GM-CSF- stimulated increase in CD11bSurface, consistent with the contribution of calcium ions to the translocation CD11b to the cell surface (Silliman et al., 2003). Variation in flow cytometer setting-dependent effects (e.g., variable laser output and machine settings) is a limitation (not evaluated here) that could significantly affect test results from day to day, especially if results are reported as a continuous variable corresponding to the numeric value of the CD11b-SI and interpreted based on normal and abnormal ranges of the test result. Finally, the magnitude of the GM-CSF-stimulated CD11bSurface (Figure 8) and the ability to detect impaired GM-CSF signaling in blood specimens from autoimmune PAP patients (Bendtzen et al., 2007) varied with the concentration of GM-CSF used for stimulation. Thus, CD11bSurface measurement is highly sensitive to pre-analytical conditions of blood preparation and storage as noted in prior efforts to standardize the measurement of neutrophil CD11bSurface (Latger-Cannard et al., 2004).
Our approach to these limitations has been to standardize/control the CD11b-SI assay with respect to each variable. In our standardized CD11b-SI test, blood is collected into sodium heparin- containing phlebotomy tubes, kept at room temperature (~25°C), stimulated with GM-CSF at a standard concentration (10 ng/ml) within 1 hour after phlebotomy, stained and immediately fixed. Blood from a healthy donor is collected and evaluated in parallel to control for day-to-day, machine, and inter-operator variability. Samples are then subjected to flow cytometric analysis as soon as possible usually in <24 hours, the data are expressed as a percent increase from baseline in CD11bSurface of the GM-CSF-stimulated sample. The test result is reported as a dichotomous variable - either “positive” or “negative” for GM-CSF signaling. These conditions have permitted the CD11b-SI test to be performed rapidly and reliably using whole blood specimens without any purification steps in the context of basic or clinical research. Because neutrophils in autoimmune PAP patients have normal ultrastructure, express expected phenotypic markers, and increase CD11bSurface after removal of GM-CSF autoantibodies, impaired CD11b-SI test results are interpreted as demonstrating the presence of functional GM-CSF autoantibodies (or the neutralizing capacity of whole blood) (Uchida et al., 2007; Uchida et al., 2009). Consequently, we have used the CD11b-SI to evaluate impaired GM-CSF signaling in autoimmune PAP patients (Uchida et al., 2007; Uchida et al., 2009), animal autoimmune PAP model with non-human primates induced by passive transfer of GM-CSF autoantibodies purified from patients (Sakagami et al., 2009; Sakagami et al., 2010), and other inflammatory diseases (Han et al., 2009).
Acknowledgements
We thank Carrie Stevens for help with collection of human blood specimens. This work was supported by grants from the National Heart Lung and Blood Institute (R01 HL085453; B.C.T.), National Center for Research Resources (U54 RR0198498, B.C.T. and K.U.), the National Institute for Health and Human Development, Japan Society for the Promotion of Science (A232490720001, B24390364, Y.Y. and K.U.), and Japan Ministry of Health Labor and Welfare (H24-Nanchitou(Nanchi)-Ippan-035, H24-Rinkensui-Ippan-003, K.U.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anonymous . Guidence Documents. Vol. 2012. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM); Bethesda: 2001. Guidance for Industry Bioanalytical Method Validation. [Google Scholar]
- Bendtzen K, Svenson M, Hansen MB, Busch T, Bercker S, Kaisers U, Uchida K, Beck DC, Trapnell BC. GM-CSF Autoantibodies in Pulmonary Alveolar Proteinosis. The New England journal of medicine. 2007;356:2001–2002. [PubMed] [Google Scholar]
- Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94:461–71. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- Cook AD, Pobjoy J, Sarros S, Steidl S, Durr M, Lacey DC, Hamilton JA. Granulocyte-macrophage colony-stimulating factor is a key mediator in inflammatory and arthritic pain. Annals of the rheumatic diseases. 2013;72:265–70. doi: 10.1136/annrheumdis-2012-201703. [DOI] [PubMed] [Google Scholar]
- Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–6. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- Graves V, Gabig T, McCarthy L, Strour EF, Leemhuis T, English D. Simultaneous mobilization of Mac-1 (CD11b/CD18) and formyl peptide chemoattractant receptors in human neutrophils. Blood. 1992;80:776–87. [PubMed] [Google Scholar]
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nature reviews. Immunology. 2008;8:533–44. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Han X, Uchida K, Jurickova I, Koch D, Willson T, Samson C, Bonkowski E, Trauernicht A, Kim MO, Tomer G, Dubinsky M, Plevy S, Kugathsan S, Trapnell BC, Denson LA. Granulocyte-macrophage colony-stimulating factor autoantibodies in murine ileitis and progressive ileal Crohn's disease. Gastroenterology. 2009;136:1261–71. e1–3. doi: 10.1053/j.gastro.2008.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D. Human Neutrophils Lose Their Surface FcyRIII and Acquire Annexin V Binding Sites During Apoptosis In Vitro. Blood. 1995;85:532–540. [PubMed] [Google Scholar]
- Ishii H, Trapnell BC, Tazawa R, Inoue Y, Akira M, Kogure Y, Tomii K, Takada T, Hojo M, Ichiwata T, Goto H, Nakata K. Comparative study of high-resolution CT findings between autoimmune and secondary pulmonary alveolar proteinosis. Chest. 2009;136:1348–55. doi: 10.1378/chest.09-0097. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. The Journal of experimental medicine. 1999;190:875–80. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, Kannourakis G, Williamson DJ, Klintworth GK, Gonda TJ, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–86. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Latger-Cannard V, Besson I, Doco-Lecompte T, Lecompte T. A standardized procedure for quantitation of CD11b on polymorphonuclear neutrophil by flow cytometry: potential application in infectious diseases. Clinical and laboratory haematology. 2004;26:177–86. doi: 10.1111/j.1365-2257.2004.00599.x. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). The New England journal of medicine. 1992;327:28–35. doi: 10.1056/NEJM199207023270106. [DOI] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT, Moore JP, Tavana G, Lewis LR, Zhu Y, Muzny DM, Gibbs RA, Huston DP. Pulmonary alveolar proteinosis caused by deletion of the GM-CSFRalpha gene in the X chromosome pseudoautosomal region 1. The Journal of experimental medicine. 2008;205:2711–6. doi: 10.1084/jem.20080759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogee LM. Genetic Basis of Children's Interstitial Lung Disease. Pediatric allergy, immunology, and pulmonology. 2010;23:15–24. doi: 10.1089/ped.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Hamblin AS. Increased CD11/CD18 expression on the peripheral blood leucocytes of patients with HIV disease: relationship to disease severity. Clinical and experimental immunology. 1993;93:344–9. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami T, Beck D, Uchida K, Suzuki T, Carey BC, Nakata K, Keller G, Wood RE, Wert SE, Ikegami M, Whitsett JA, Luisetti M, Davies S, Krischer JP, Brody A, Ryckman F, Trapnell BC. Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. American journal of respiratory and critical care medicine. 2010;182:49–61. doi: 10.1164/rccm.201001-0008OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, Whitsett JA, Trapnell BC, Luisetti M. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. The New England journal of medicine. 2009;361:2679–81. doi: 10.1056/NEJMc0904077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch OD, Schanz U, Koller M, Nakata K, Seymour JF, Russi EW, Boehler A. BAL findings in a patient with pulmonary alveolar proteinosis successfully treated with GM-CSF. Thorax. 2002;57:277–80. doi: 10.1136/thorax.57.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour JF, Doyle IR, Nakata K, Presneill JJ, Schoch OD, Hamano E, Uchida K, Fisher R, Dunn AR. Relationship of anti-GM-CSF antibody concentration, surfactant protein A and B levels, and serum LDH to pulmonary parameters and response to GM-CSF therapy in patients with idiopathic alveolar proteinosis. Thorax. 2003;58:252–7. doi: 10.1136/thorax.58.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor Z, Hamblin AS. Increased CD11/CD18 expression on peripheral blood leucocytes of patients with sarcoidosis. Clinical and experimental immunology. 1992;90:99–105. doi: 10.1111/j.1365-2249.1992.tb05839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalekoff S, Page-Shipp L, Tiemessen CT. Effects of anticoagulants and temperature on expression of activation markers CD11b and HLA-DR on human leukocytes. Clinical and diagnostic laboratory immunology. 1998;5:695–702. doi: 10.1128/cdli.5.5.695-702.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silliman CC, Clay KL, Thurman GW, Johnson CA, Ambruso DR. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. The Journal of laboratory and clinical medicine. 1994;124:684–94. [PMC free article] [PubMed] [Google Scholar]
- Silliman CC, Elzi DJ, Ambruso DR, Musters RJ, Hamiel C, Harbeck RJ, Paterson AJ, Bjornsen AJ, Wyman TH, Kelher M, England KM, McLaughlin-Malaxecheberria N, Barnett CC, Aiboshi J, Bannerjee A. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. Journal of leukocyte biology. 2003;73:511–24. doi: 10.1189/jlb.0402179. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL, Smolarek T, Dishop MK, Wert SE, Whitsett JA, Grabowski G, Carey BC, Stevens C, van der Loo JC, Trapnell BC. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. The Journal of experimental medicine. 2008;205:2703–10. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Sakagami T, Young LR, Carey BC, Wood RE, Luisetti M, Wert SE, Rubin BK, Kevill K, Chalk C, Whitsett JA, Stevens C, Nogee LM, Campo I, Trapnell BC. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. American journal of respiratory and critical care medicine. 2010;182:1292–304. doi: 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tazawa R, Trapnell BC, Inoue Y, Arai T, Takada T, Nasuhara Y, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ishii H, Yokoba M, Tanaka N, Yamaguchi E, Eda R, Tsuchihashi Y, Morimoto K, Akira M, Terada M, Otsuka J, Ebina M, Kaneko C, Nukiwa T, Krischer JP, Akazawa K, Nakata K. Inhaled granulocyte/macrophage-colony stimulating factor as therapy for pulmonary alveolar proteinosis. American journal of respiratory and critical care medicine. 2010;181:1345–54. doi: 10.1164/rccm.200906-0978OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annual review of physiology. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. The New England journal of medicine. 2003;349:2527–39. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, Carey BC, Filippi MD, Wert SE, Denson LA, Puchalski JT, Hauck DM, Trapnell BC. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. The New England journal of medicine. 2007;356:567–79. doi: 10.1056/NEJMoa062505. [DOI] [PubMed] [Google Scholar]
- Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, Koch DE, Stevens C, Inoue Y, Yamada Y, Trapnell BC. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. Journal of immunological methods. 2014;402:57–70. doi: 10.1016/j.jim.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Nakata K, Suzuki T, Luisetti M, Watanabe M, Koch DE, Stevens CA, Beck DC, Denson LA, Carey BC, Keicho N, Krischer JP, Yamada Y, Trapnell BC. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113:2547–56. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Nakata K, Trapnell BC, Terakawa T, Hamano E, Mikami A, Matsushita I, Seymour JF, Oh-Eda M, Ishige I, Eishi Y, Kitamura T, Yamada Y, Hanaoka K, Keicho N. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood. 2004;103:1089–98. doi: 10.1182/blood-2003-05-1565. [DOI] [PubMed] [Google Scholar]
- Wadhwa M, Skog AL, Bird C, Ragnhammar P, Lilljefors M, Gaines-Das R, Mellstedt H, Thorpe R. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clinical Cancer Research. 1999;5:1353–61. [PubMed] [Google Scholar]
- Whitsett JA, Wert SE, Trapnell BC. Genetic disorders influencing lung formation and function at birth. Human Molecular Genetics. 2004;13:R207–15. doi: 10.1093/hmg/ddh252. [DOI] [PubMed] [Google Scholar]
- Youssef PP, Mantzioris BX, Roberts-Thomson PJ, Ahern MJ, Smith MD. Effects of ex vivo manipulation on the expression of cell adhesion molecules on neutrophils. Journal of immunological methods. 1995;186:217–24. doi: 10.1016/0022-1759(95)00146-2. [DOI] [PubMed] [Google Scholar]