Abstract
Background: Patient navigation (PN) can improve breast cancer care among disadvantaged women. We evaluated the impact of a PN program on follow-up after an abnormal mammogram.
Methods: Between 2007 and 2010, disadvantaged women with an abnormal mammogram (Breast Imaging-Reporting and Data System [BI-RADS] codes 0, 3, 4, 5) cared for in a community health center (CHC) with PN were compared to those receiving care in 11 network practices without PN. Multivariable logistic regression and Cox proportional hazards modeling were used to compare the percentages receiving appropriate follow-up and time to follow-up between the groups.
Results: Abnormal mammography findings were reported for 132 women in the CHC with PN and 168 from practices without PN. The percentage of women with appropriate follow-up care was higher in the practice with PN than in non-PN practices (90.4% vs. 75.3%, adjusted p=0.006). Results varied by BI-RADS score for women in PN and non-PN practices (BI-RADS 0, 93.7% vs. 90.2%, p=0.24; BI-RADS 3, 85.7% vs. 49.2%, p=0.003; BI-RADS 4/5, 95.1% vs. 82.8%, p=0.26). Time to follow-up was similar for BI-RADS 0 and occurred sooner for women in the PN practice than in non-PN practices for BI-RADS 3 and 4/5 (BI-RADS 3, adjusted hazard ratio [aHR], 95% confidence interval [CI]: 2.41 [1.36–4.27], BI-RADS 4/5, aHR [95% CI]: 1.41 [0.88–2.24]).
Conclusions: Disadvantaged women from a CHC with PN were more likely to receive appropriate follow-up after an abnormal mammogram than were those from practices without PN. Expanding PN to include all disadvantaged women within primary care networks could improve equity in cancer care.
Introduction
Breast cancer is the most commonly diagnosed cancer in women in the United States. It has been estimated that 232,670 women would be diagnosed and that 40,000 would die from the disease in 2014.1 Despite advances in screening, diagnosis, and treatment of breast cancer, patients continue to present with advanced disease. This is especially true for patients who are of racial and ethnic minorities, are underinsured, have low income, and have limited English proficiency.2–6
In 1990, Harold Freeman, MD, introduced patient navigation (PN) in New York City to improve equity in cancer care. By creating access through free screening, improving patient adherence, and decreasing delays to following up abnormal results, PN increased 5-year survival rates from 39% to 70% among black women diagnosed with breast cancer.7 Subsequently, PN has been implemented across the United States.8–10 Recently, the National Cancer Institute initiated the PN Research Program to measure PN effectiveness; large randomized controlled trials (RCTs) evaluated the timeliness of follow-up after an abnormal breast cancer screening in patients receiving care in community health centers (CHCs) and/or hospitals serving predominantly disadvantaged patients.11–19 Although reducing delays in breast cancer diagnosis, the impact of PN varied and was not always statistically significant.13,19 The objective of our study was to compare the follow-up of women with abnormal mammograms who received PN as part of their usual care at one CHC within a primary care practice network to the follow-up of disadvantaged women with abnormal mammograms who received care within the same primary care practice network but did not have access to PN.
Materials and Methods
Study setting
The study was performed in 12 primary care practices affiliated with the Massachusetts General Hospital (MGH) Practice-Based Research Network (PBRN). All practices shared an administrative and information technology infrastructure. One of four CHCs within this network, the MGH Chelsea HealthCare Center (MGH Chelsea) serves a low-income, predominantly Latino and immigrant population. Chelsea, MA, is located about 5 miles north of Boston. According to the 2010 census, 62% of the 35,000 Chelsea residents were Latino, and 66% did not speak English. The poverty level was twice the Massachusetts average.
Intervention
With Avon Foundation support, a PN program to improve breast cancer awareness, screening, and abnormal-result follow-up in women receiving care at MGH Chelsea was implemented in 2001. During the 13 years of the program, there were two patient navigators. The transition from one to the other occurred during the study period in 2009. Both were college-educated, bilingual (Spanish/English) Latinas. They were trained in breast cancer prevention and multiple aspects of patient navigation. The community health director and a practice nurse practitioner provided supervision. All women with an abnormal mammogram were referred to the PN program for help with obtaining appropriate follow-up care. The diagnostic mammograms and breast biopsies were performed only at the MGH main campus in Boston. The navigator explored each patient's specific barriers to receiving care and then developed and implemented an individualized plan to address these barriers. The PN interventions might include educating patients, scheduling appointments, making reminder calls, arranging transportation, resolving insurance issues, interpreting, accompanying patients to follow-up appointments, and making home visits.20
Study population
To evaluate the impact of the program, we retrospectively identified disadvantaged women from MGH Chelsea with abnormal screening mammograms who were referred for PN between 2007 and 2010. As a comparison group, we identified disadvantaged women with abnormal screening mammograms during the same time period from non-PN practices within the MGH primary care network. We defined “disadvantaged” women as nonwhite, non-English speakers, or with Medicaid, Free Care Program (provided by MGH to low-income patients), or no insurance. Abnormal mammograms were defined according to the Breast Imaging-Reporting and Data System (BI-RADS), with BI-RADS 0, 3, 4, and 5 considered abnormal.21 The intervention-group patients were identified using a MGH Chelsea PN database; the comparison-group patients, from electronic medical records.
Outcomes
The primary outcomes were the proportion of women who received appropriate follow-up after an abnormal mammogram and time to follow-up care. According to the American College of Radiology, follow-up recommendations for abnormal mammograms depend on the BI-RADS category. BI-RADS category 0 indicates that additional imaging is needed; BI-RADS 3, recommended return in 6 months for repeat imaging; and BI-RADS 4 and 5, recommended biopsy.21 Using these guidelines, we defined appropriate follow-up in this study as follows: BI-RADS 0, follow-up imaging within 3 months; BI-RADS 3, follow-up imaging within 9 months; BI-RADS 4/5, biopsy within 3 months. BI-RADS 0 women who received a follow-up mammogram with BI-RADS 3, 4, or 5 were reclassified into the higher BI-RADS category for analyses of these categories.
Analyses
Patient characteristics between navigated and comparison groups were evaluated using t-tests or chi-squared tests, as appropriate. For our primary outcome, we compared the proportion of patients who received appropriate follow-up of abnormal screening mammography between navigated and comparison groups. We used logistic regression and adjusted for patient age, race, primary language, number of clinic visits, and whether the patient was linked with a specific primary care physician (PCP).22 The time to appropriate follow-up was depicted with Kaplan-Meier survival plots and compared using the log-rank test. Cox proportional hazards models were used to compare groups while adjusting for potential confounders. Censoring occurred if women did not receive appropriate follow-up within 90 (BI-RADS 0, 4, 5) or 270 (BI-RADS 3) days. The Partners Institutional Review Board approved all study activities.
Results
Over the 4-year period 2007–2010, 146 women with abnormal mammograms were at the CHC with PN, and 223 women with abnormal mammograms were in the practices without PN. Women from the CHC with PN were more likely to be Latina, linked to a specific PCP, less likely to speak English, have more clinic visits over the prior 3 years, and more likely to have a mammogram with a BI-RADS of 4 or 5. There were no significant differences by age or insurance status between groups (Table 1). During the study period, 17 women receiving care at CHC with PN were diagnosed with invasive breast cancer compared to 9 diagnosed cancers in women seen in practices without PN.
Table 1.
Characteristics of Women in Navigated and Comparison Groups
| Characteristic, n (%) | Navigated (n=146) | Comparison (n=223) | p-value |
|---|---|---|---|
| Age, mean (SD) | 50.2 (9.3) | 51.7 (7.3) | 0.07 |
| Linkage status, PCP-linked | 115 (78.8%) | 149 (66.8%) | 0.04 |
| Race | <0.001 | ||
| Asian | 4 (2.7%) | 24 (10.8%) | |
| Black | 9 (6.2%) | 44 (19.7%) | |
| Hispanic | 84 (57.5%) | 39 (17.5%) | |
| Other/unknown | 4 (2.7%) | 17 (7.6%) | |
| White | 45 (30.8%) | 99 (44.4%) | |
| Language, English | 62 (42.5%) | 186 (83.4%) | <0.001 |
| Insurance | 0.40 | ||
| Commercial | 63 (43.2%) | 93 (41.7%) | |
| Free Care | 18 (12.3%) | 21 (9.4%) | |
| Medicaid | 41 (28.1%) | 65 (29.2%) | |
| Medicare | 20 (13.7%) | 28 (12.6%) | |
| Self-pay | 4 (2.7%) | 16 (7.2%) | |
| Practice visits in past 3 years, mean (SD) | 9.2 (7.3) | 7.2 (6.4) | 0.006 |
| BI-RADS (initial abnormal) | 0.02 | ||
| 0 | 111 (76.0%) | 193 (86.6%) | |
| 3 | 14 (9.6%) | 16 (7.2%) | |
| 4/5 | 21 (14.4%) | 14 (6.3%) | |
| BI-RADS (highest abnormal) | 0.001 | ||
| 0 | 43 (29.5%) | 106 (47.5%) | |
| 3 | 42 (28.8%) | 59 (26.5%) | |
| 4/5 | 61 (41.8%) | 58 (26.0%) |
BI-RADS, Breast Imaging-Reporting and Data System; PCP, primary care physician; SD, standard deviation.
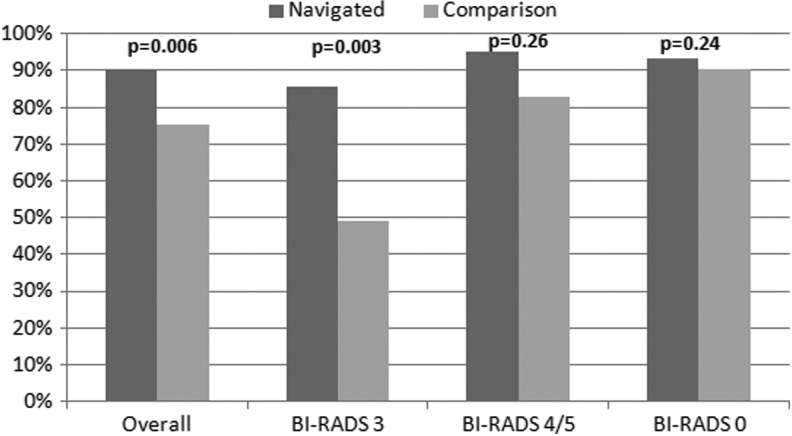
In the CHC with PN, 132 of 146 (90.4%) women with abnormal mammograms received appropriate and timely follow-up compared to 168 of 223 (75.3%) women in the other practices (adjusted p=0.006). Table 2 displays the proportions with appropriate follow-up by BI-RADS score in the navigated and comparison groups. Most women with BI-RADS 0 received appropriate follow-up in both groups (93.7% in the navigated group vs. 90.2% in the comparison group, p=0.24). In women with BI-RADS 3, a larger proportion of navigated women (85.7%) received appropriate follow-up than did women from practices without PN (49.2%, p=0.003). Among women with BI-RADS 4/5, 95.1% of women from the CHC with PN received appropriate follow-up compared with 82.8% of women in comparison practices (p=0.26) (Fig. 1).
Table 2.
Appropriate Follow-Up by BI-RADS Score in Navigated and Comparison Groups
| Navigated (n=146) | Comparison (n=223) | Unadjusted p-value | Adjusted ORa(95% CI) | Adjusted p-valuea | |
|---|---|---|---|---|---|
| Initial BI-RADS 0: follow-up within 3 monthsb | 104/111 (93.7%) | 174/193 (90.2%) | 0.29 | 1.89 (0.65–5.55) | 0.24 |
| BI-RADS 3: follow-up within 9 months | 36/42 (85.7%) | 29/59 (49.2%) | <0.001 | 5.39 (1.78–16.31) | 0.003 |
| BI-RADS 4/5: follow- up within 3 monthsc | 58/61 (95.1%) | 48/58 (82.8%) | 0.03 | 2.33 (0.54–10.18) | 0.26 |
| Appropriate follow-up based on highest BI-RADSc | 132/146 (90.4%) | 168/223 (75.3%) | <0.001 | 2.62 (1.31–5.22) | 0.006 |
Adjusted p-value from logistic regression model adjusting for age, number of clinic visits, linkage status, race, language.
Includes all patients with BI-RADS 0 for initial screening mammogram.
Includes patients initially BI-RADS 0 who had a follow-up mammogram that was BI-RADS 3 (n=71) or 4/5 (n=84).
CI, confidence interval; OR, odds ratio.
FIG. 1.
Proportion of patients who received appropriate follow-up after abnormal mammogram in navigated vs. comparison groups.
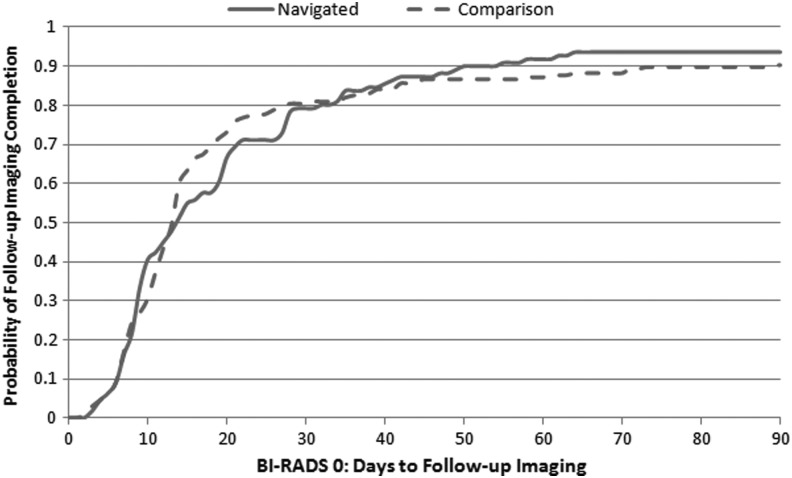
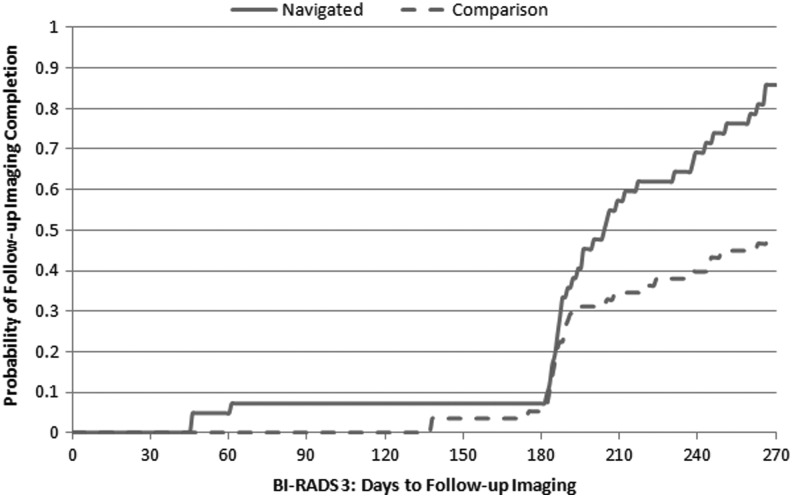
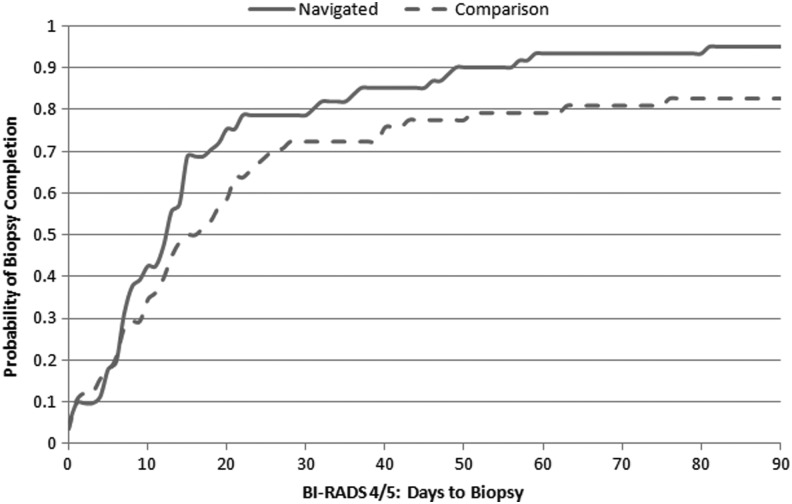
Figures 2, 3, and 4 show the Kaplan-Meier plots for time to follow-up in navigated and comparison groups, depending on the BI-RADS mammographic abnormality. Time to follow-up was similar between groups following BI-RADS 0 (Fig. 2), whereas the navigated group received follow-up sooner following BI-RADS 3 (Fig. 3) (p<0.001) and BI-RADS 4/5 (Fig. 4 (p=0.06). The hazard ratio comparing the navigated group to the comparison group was 1.17 (95% confidence interval [CI]: 0.88–1.56) following BI-RADS 0, 2.41 (95% CI: 1.36–4.27) following BI-RADS 3, and 1.41 (95% CI: 0.88–2.24) following BI-RADS 4/5 after adjusting for potential confounding factors (Table 3).
FIG. 2.
Kaplan-Meier curve for time to follow-up imaging following BI-RADS 0 in navigated vs. comparison groups. Log rank p-value=0.94.
FIG. 3.
Kaplan-Meier curve for time to follow-up imaging following BI-RADS 3 in navigated vs. comparison groups. Log rank p-value <0.001.
FIG. 4.
Kaplan-Meier curve for time to biopsy following BI-RADS 4/5 in navigated vs. comparison groups. Log rank p-value=0.06.
Table 3.
Time to Appropriate Follow-Up in Navigated and Comparison Groups
| Hazard ratio (95% CI) unadjusted | Hazard ratio (95% CI) adjusteda | p-value (adjusted)a | |
|---|---|---|---|
| Initial BI-RADS 0: follow-up within 3 monthsb | 1.01 (0.79–1.29) | 1.17 (0.88–1.56) | 0.27 |
| BI-RADS 3: follow-up within 9 months | 2.40 (1.45–3.95) | 2.41 (1.36–4.27) | 0.003 |
| BI-RADS 4/5: follow-up within 3 monthsc | 1.44 (0.98–2.12) | 1.41 (0.88–2.24) | 0.15 |
Adjusted p-value from Cox proportional hazards regression model adjusting for age, number of clinic visits, linkage status, race, language.
Includes all patients with BI-RADS 0 for initial screening mammogram (n=304).
Includes patients initially BI-RADS 0 who had a follow-up mammogram that was BI-RADS 3 (n=81) or 4/5 (n=84).
Discussion
Our study compared the follow-up of disadvantaged women with an abnormal screening mammogram according to whether they received PN. We showed that women with an abnormal mammogram receiving care in the CHC with PN as part of usual care more often received appropriate follow-up care than did disadvantaged women seen in practices without PN within the same primary care network. This finding was greatest for women with a BI-RADS 3 mammogram result, requiring a follow-up examination in 6 months.
Overall, PN improved appropriate follow-up of abnormal mammogram by 15% compared to the nonnavigated group. This result is similar to the improvements achieved in follow-up of mammographic abnormality published in two PN reviews.8,10 The recent large RCTs focused on time to resolution of the abnormal screening result. The study by Markossian et al. showed a positive effect of PN on follow-up of abnormal breast cancer screening during the whole navigated period, whereas other studies reported the significant difference between PN and control groups after 60 days,11,14 after 3 months,15 or only 6 months after the abnormal breast screening result.17
However, these studies did not always distinguish the type of mammogram abnormality and often included both women with abnormalities on screening mammogram and women with abnormal clinical findings. In our study, we assessed follow-up of abnormal screening mammogram results calling for immediate follow-up within 90 days and those calling for short-term follow-up within 6 months. We found that the magnitude of the benefit of PN depended on the type of abnormal mammogram. In contrast to the study by Raich et al.,18 our study showed high rates of appropriate follow-up in both PN and comparison patients following an abnormality, indicating the need for additional imaging (BI-RADS 0).
The lack of difference between the groups may have resulted from follow-up procedures by the radiology department. All patients with BI-RADS 0 mammogram assessment receive three phone calls and a letter to remind/help them schedule the follow-up mammogram. If the patient still does not complete a follow-up mammogram, the ordering provider is contacted. In our study, PN was most beneficial for women with BI-RADS 3 abnormality, similar to results reported by Raich et al. and Ramirez et al.18,23 Without navigation, less than half the disadvantaged women with BI-RADS 3 recommendations received appropriate follow-up care, compared to 86% in the navigated group. Currently, except for PN at MGH Chelsea, there are no hospital-wide systems to expedite follow-up post–BI-RADS 3 abnormality. Similarly as reported by Battaglia et al.,11 among women with BI-RADS 4/5 abnormalities, navigated women appeared to receive breast lesion biopsy earlier than women without navigation, although this result did not reach statistical significance in adjusted models. BI-RADS 4/5 recommends surgery or biopsy, which may require an additional scheduling step and, in our health system, requires patients to go to the main hospital rather than a local facility where they receive their screening mammogram. Navigators likely facilitated this transition, often accompanying patients to their biopsy appointments. In addition, fear may cause some patients to delay or refuse biopsy, and patient navigators may help allay patients' fears and communicate the importance of having a diagnostic biopsy.
MGH Chelsea, the CHC with PN, is part of a primary care network within a larger accountable care organization (ACO.) ACOs are an integral part of the restructuring of healthcare delivery with the implementation of the Affordable Care Act in the United States. An ACO is a provider-led organization whose mission is to manage the full continuum of care and to be accountable for the overall costs and quality of care for a defined population.24 The ACO goal is to provide integrated and efficient care by fostering local organizational accountability for quality and costs through performance measurement. An essential part of this mission is equity in care. Currently, most PN programs to improve follow-up after an abnormal breast cancer screening are located in health centers or primary care networks that serve predominately disadvantaged patients.11,12,14,16–19 Even in these settings, PN had the greatest benefit in populations at risk of being lost to follow-up.13 To be able to reach out to all disadvantaged patients within large primary care networks serving a diverse population, population management information technology systems need to identify high-risk patients in need of PN following an abnormal cancer screening result.25 Combining PN targeted to locations where disadvantaged patients are concentrated11,12,14–19 and individual targeting of high-risk, disadvantaged patients within any practice in an ACO may be complementary and could potentially reduce disparities more than either approach alone. Future studies should assess the clinical and cost effectiveness of these approaches.
Several important limitations of our study warrant consideration. PN was implemented as part of usual care, so we did not have an RTC group to assess the program's effectiveness. Because Chelsea has unique demographic characteristics within Massachusetts, finding a suitable comparison group was challenging. We chose as a comparison group disadvantaged women from other practices in our network at risk for disparities in cancer care. PN was offered in only one CHC within an academic primary care network and may not be generalizable to other clinical settings. If patients from other practices were more likely to have had follow-up of abnormal mammography outside of our network than women from MGH Chelsea, our comparison may overestimate the effect of PN. However, unlike MGH Chelsea, five practices within our primary care network are located near the main hospital, in the same geographic area where the diagnostic mammogram and biopsies are performed, making it more convenient for disadvantaged patients receiving care in those practices to receive timely follow-up. In this study, we did not assess the effect of distance from the practice/or patients' homes to the diagnostic facility in Boston on time to appropriate follow-up after an abnormal breast screening result. Although, according to MGH Chelsea protocol, every patient with an abnormal mammogram is referred to PN, not all eligible women seen in the PN clinic in fact engaged with a navigator. They might have moved, had care elsewhere, or refused services, or the navigator was not able to contact them. Since our analyses included patients regardless of whether the navigator was able to engage them in care, our results may underestimate the true impact of PN in those with abnormal mammograms.
Conclusions
The findings of this study suggest that among disadvantaged women, PN increases appropriate follow-up care after an abnormal screening mammogram. To improve equity and quality of cancer care, PN should be expanded to include high-risk disadvantaged patients within primary care networks.
Acknowledgments
We would like to thank the PNs and the Community Health Team at the MGH Chelsea HealthCare Center for their work on the program.
This program was funded by a grant from Avon Foundation. Sanja Percac-Lima, MD, is supported in part by the Control Career Development Award for Primary Care Physicians, CCCDAA-14-012-01-CCCDA, from the American Cancer Society and Lazarex Cancer Foundation. Anne Marie McCarthy, MD, is supported by a grant from the National Institutes of Health, National Cancer Institute: NCI U54 CA163313.
The study was presented at the Annual Meeting of the Society of General Medicine in San Diego, CA, in April 2014.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: A Cancer Journal for Clinicians 2014;64:9–29 [DOI] [PubMed] [Google Scholar]
- 2.Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer 2001;91:178–188 [DOI] [PubMed] [Google Scholar]
- 3.Cho YI, Johnson TP, Barrett RE, Campbell RT, Dolecek TA, Warnecke RB. Neighborhood changes in concentrated immigration and late stage breast cancer diagnosis. J Immigr Minor Health 2011;13:9–14 [DOI] [PubMed] [Google Scholar]
- 4.Dai D. Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health Place 2010;16:1038–1052 [DOI] [PubMed] [Google Scholar]
- 5.Flores G. Language barriers to health care in the United States. N Engl J Med 2006;355:229–231 [DOI] [PubMed] [Google Scholar]
- 6.Silber JH, Rosenbaum PR, Clark AS, et al. . Characteristics associated with differences in survival among black and white women with breast cancer. JAMA 2013;310:389–397 [DOI] [PubMed] [Google Scholar]
- 7.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract 1995;3:19–30 [PubMed] [Google Scholar]
- 8.Paskett ED, Harrop JP, Wells KJ. Patient navigation: An update on the state of the science. CA: A Cancer Journal for Clinicians 2011;61:237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson-White S, Conroy B, Slavish KH, Rosenzweig M. Patient navigation in breast cancer: A systematic review. Cancer Nursing 2010;33:127–140 [DOI] [PubMed] [Google Scholar]
- 10.Wells KJ, Battaglia TA, Dudley DJ, et al. . Patient navigation: State of the art or is it science? Cancer 2008;113:1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglia TA, Bak SM, Heeren T, et al. . Boston Patient Navigation Research Program: The impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev 2012;21:1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudley DJ, Drake J, Quinlan J, et al. . Beneficial effects of a combined navigator/promotora approach for Hispanic women diagnosed with breast abnormalities. Cancer Epidemiol Biomarkers Prev 2012;21:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freund KM, Battaglia TA, Calhoun E, et al. . Impact of patient navigation on timely cancer care: The Patient Navigation Research Program. J Natl Cancer Inst 2014;106:dju115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman HJ, LaVerda NL, Young HA, et al. . Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev 2012;21:1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Fulp W, Wells KJ, Meade CD, Calcano E, Roetzheim R. Patient navigation and time to diagnostic resolution: Results for a cluster randomized trial evaluating the efficacy of patient navigation among patients with breast cancer screening abnormalities, Tampa, FL. PloS One 2013;8:e74542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev 2012;21:1691–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paskett ED, Katz ML, Post DM, et al. . The Ohio Patient Navigation Research Program: Does the American Cancer Society patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiol Biomarkers Prev 2012;21:1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raich PC, Whitley EM, Thorland W, Valverde P, Fairclough D; Denver Patient Navigation Research Program. Patient navigation improves cancer diagnostic resolution: An individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev 2012;21:1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells KJ, Lee JH, Calcano ER, et al. . A cluster randomized trial evaluating the efficacy of patient navigation in improving quality of diagnostic care for patients with breast or colorectal cancer abnormalities. Cancer Epidemiol Biomarkers Prev 2012;21:1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Percac-Lima S, Milosavljevic B, Oo SA, Marable D, Bond B. Patient navigation to improve breast cancer screening in Bosnian refugees and immigrants. J Immigr Minor Health 2012;14:727–730 [DOI] [PubMed] [Google Scholar]
- 21.American College of Radiology. Breast imaging reporting and data system (BI_RADS): Breast imaging atlas, 4th ed. Reston, VA: American College of Radiology, 2003 [Google Scholar]
- 22.Atlas SJ, Chang Y, Lasko TA, Chueh HC, Grant RW, Barry MJ. Is this “my” patient? Development and validation of a predictive model to link patients to primary care providers. J Gen Intern Med 2006;21:973–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez AG, Perez-Stable EJ, Penedo FJ, et al. . Navigating Latinas with breast screen abnormalities to diagnosis: The Six Cities Study. Cancer 2013;119:1298–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rittenhouse DR, Shortell SM, Fisher ES. Primary care and accountable care—two essential elements of delivery-system reform. N Engl J Med 2009;361:2301–2303 [DOI] [PubMed] [Google Scholar]
- 25.Zai AH, Kim S, Kamis A, et al. . Applying operations research to optimize a novel population management system for cancer screening. J Am Med Inform Assoc 2014;21(e1):e129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]