Abstract
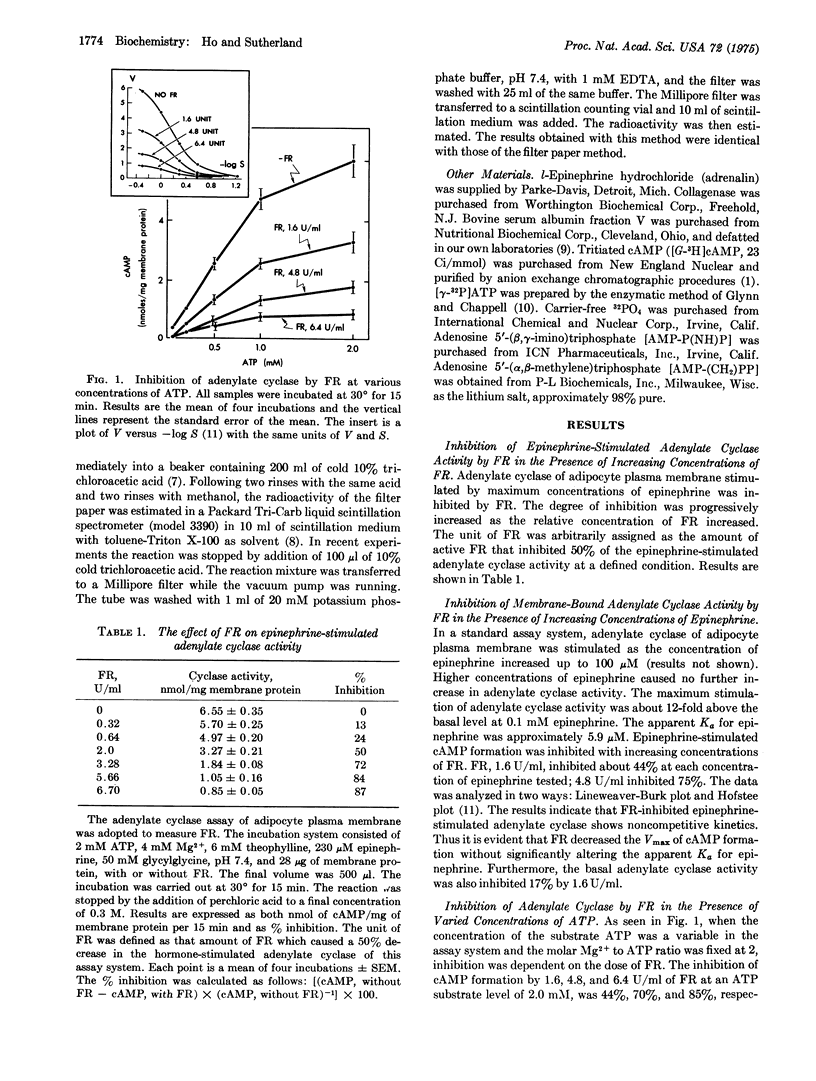
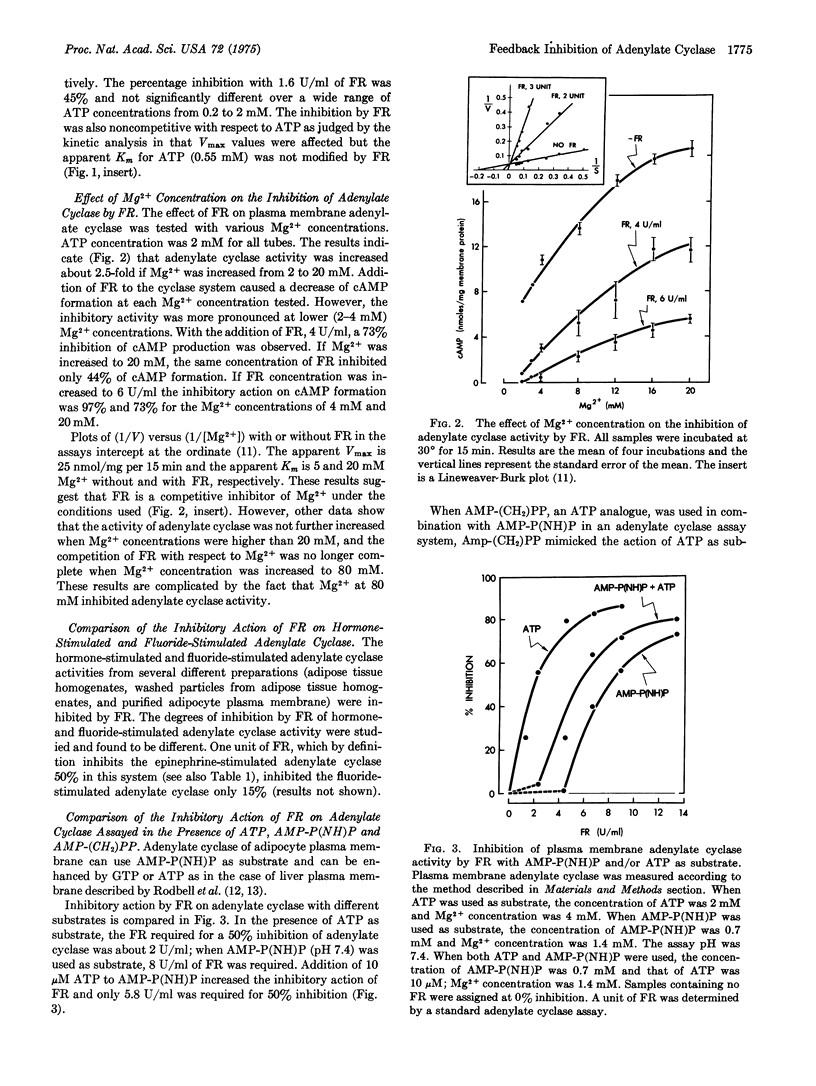
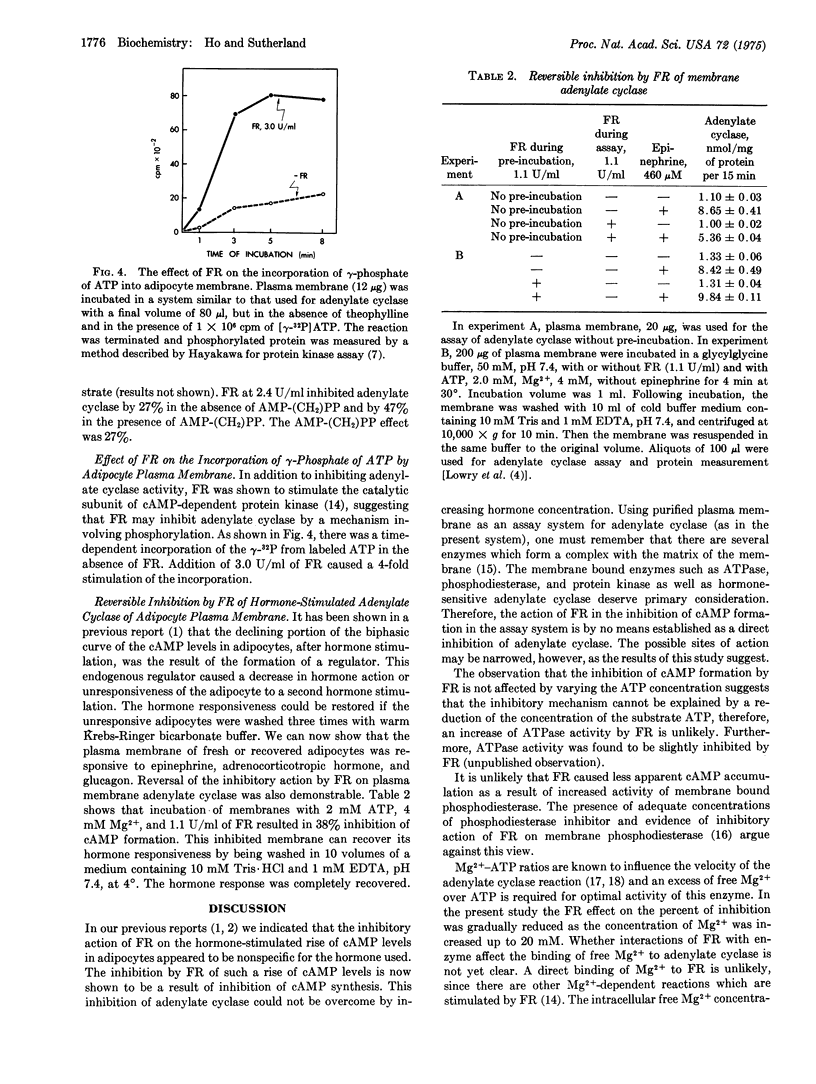
A factor [the feedback regulator (FR)] formed by adipocytes after the stimulation of a cAMP raising hormone has been found to be a potent inhibitor of membrane-bound adenylate cyclase [EC 4.6.1.1.; ATP pyrophosphate-lyase (cyclizing)]. In a standard assay system using rat adipocyte plasma membrane as the source of adenylate cyclase, the FR inhibited adenylate cyclase by lowering the Vmax without affecting the apparent Km for ATP (0.3-0.6 mM). The apparent Ka for epinephrine (5-6 muM) was also not affected by FR. The inhibitory action of FR was partially countered by Mg2+ ions. An increase in phosphorylation of plasma membrane was observed when FR was present in the incubation system. The concentration required for a 50% inhibition was four times higher when adenosine 5-(beta,gamma-imino) triphosphate [AMP-P(NH)P] replaced ATP as the substrate for adenylate cyclase, implying that adenylate cyclase was inactivated by phosphorylation caused by FR. Increase in FR inhibition obtained by adding low concentrations of adenosine 5-(alpha,beta-methylene) triphosphate or ATP to AMP-(NH)P as the substrate supports this view. The inhibitory action was reversible. These results are consistent with the previously reported phenomena that (1) the undue to the formation of FR, and (2) the recovery of responsiveness of the stimulated cells by washing the cells with regular buffer medium is a result of the removal of FR. The hormone-initiated biphasic curve of cAMP levels in adipocytes is believed to be due to the negative feedback action of FR on adenylate cyclase. The mechanism of action of FR on inhibition of adenylate cyclase appears to be related to the phosphorylation of certain membrane components.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. P., Rodbell M. Inhibition by fluoride ion of hormonal activation of fat cell adenylate cyclase. J Biol Chem. 1973 Jul 25;248(14):4901–4904. [PubMed] [Google Scholar]
- Hayakawa T., Perkins J. P., Walsh D. A., Krebs E. G. Physiochemical properties of rabbit skeletal muscle phosphorylase kinase. Biochemistry. 1973 Feb;12(4):567–573. doi: 10.1021/bi00728a001. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Bomboy J. D., Wasner H. K., Sutherland E. W. Preparation and characterization of a hormone antagonist from adipocytes. Methods Enzymol. 1975;37:431–438. doi: 10.1016/s0076-6879(75)37039-0. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Jeanrenaud B. Insulin-like action of ouabain. I. Effect on carbohydrate metabolism. Biochim Biophys Acta. 1967 Aug 8;144(1):61–73. doi: 10.1016/0005-2760(67)90077-x. [DOI] [PubMed] [Google Scholar]
- Ho R. J., Sutherland E. W. Formation and release of a hormone antagonist by rat adipocytes. J Biol Chem. 1971 Nov 25;246(22):6822–6827. [PubMed] [Google Scholar]
- Johnson R. A., Sutherland E. W. Detergent-dispersed adenylate cyclase from rat brain. Effects of fluoride, cations, and chelators. J Biol Chem. 1973 Jul 25;248(14):5114–5121. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laudat M. H., Pairault J., Bayer P., Martin M., Laudat P. Preparation of fat cell membrane with high sensitivity to lipolytic hormones. Biochim Biophys Acta. 1972 Mar 17;255(3):1005–1008. doi: 10.1016/0005-2736(72)90413-0. [DOI] [PubMed] [Google Scholar]
- Layne P., Constantopoulos A., Judge J. F., Rauner R., Najjar V. A. The occurrence of fluoride stimulated membrane phosphoprotein phosphatase. Biochem Biophys Res Commun. 1973 Aug 6;53(3):800–805. doi: 10.1016/0006-291x(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Lemay A., Deschenes M., Lemaire S., Poirier G., Poulin L., Labrie F. Phosphorylation of adenohypophyseal plasma membranes and properties of associated protein kinase. J Biol Chem. 1974 Jan 10;249(1):323–328. [PubMed] [Google Scholar]
- Maguire M. E., Gilman A. G. Adenylate cyclase assay with adenylyl imidodiphosphate and product detection by competitive protein binding. Biochim Biophys Acta. 1974 Jul 17;358(1):154–163. doi: 10.1016/0005-2744(74)90267-8. [DOI] [PubMed] [Google Scholar]
- Najjar V. A., Constantopoulos A. The activation of adenylate cyclase. I. A postulated mechanism for fluoride and hormone activation of adenylate cyclase. Mol Cell Biochem. 1973 Nov 15;2(1):87–93. doi: 10.1007/BF01738682. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W. Adenyl cyclase. II. The enzymatically catalyzed formation of adenosine 3',5'-phosphate and inorganic pyrophosphate from adenosine triphosphate. J Biol Chem. 1962 Apr;237:1228–1232. [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Veloso D., Guynn R. W., Oskarsson M., Veech R. L. The concentrations of free and bound magnesium in rat tissues. Relative constancy of free Mg 2+ concentrations. J Biol Chem. 1973 Jul 10;248(13):4811–4819. [PubMed] [Google Scholar]
- Yount R. G., Babcock D., Ballantyne W., Ojala D. Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P--N--P linkage. Biochemistry. 1971 Jun 22;10(13):2484–2489. doi: 10.1021/bi00789a009. [DOI] [PubMed] [Google Scholar]
- Yount R. G., Ojala D., Babcock D. Interaction of P--N--P and P--C--P analogs of adenosine triphosphate with heavy meromyosin, myosin, and actomyosin. Biochemistry. 1971 Jun 22;10(13):2490–2496. doi: 10.1021/bi00789a010. [DOI] [PubMed] [Google Scholar]


