Abstract
Four neurotoxins that activate the action potential Na+ ionophore of electrically excitable neuroblastoma cells interact with two distinct classes of sites, one specific for the alkaloids veratridine, batrachotoxin, and aconitine, and the second specific for scorpion toxin. Positive heterotropic cooperativity is observed between toxins bound at these two classes of sites. Tetrodotoxin is a noncompetitive inhibitor of activation by each of these toxins (KI = 4-8 nM). These results suggest the existence of three functionally separable components of the action potential Na+ ionophore: two regulatroy components, which bind activating neurotoxins and interact allosterically in controlling the activity of a third ion-transport component, which binds tetrodotoxin.
Full text
PDF
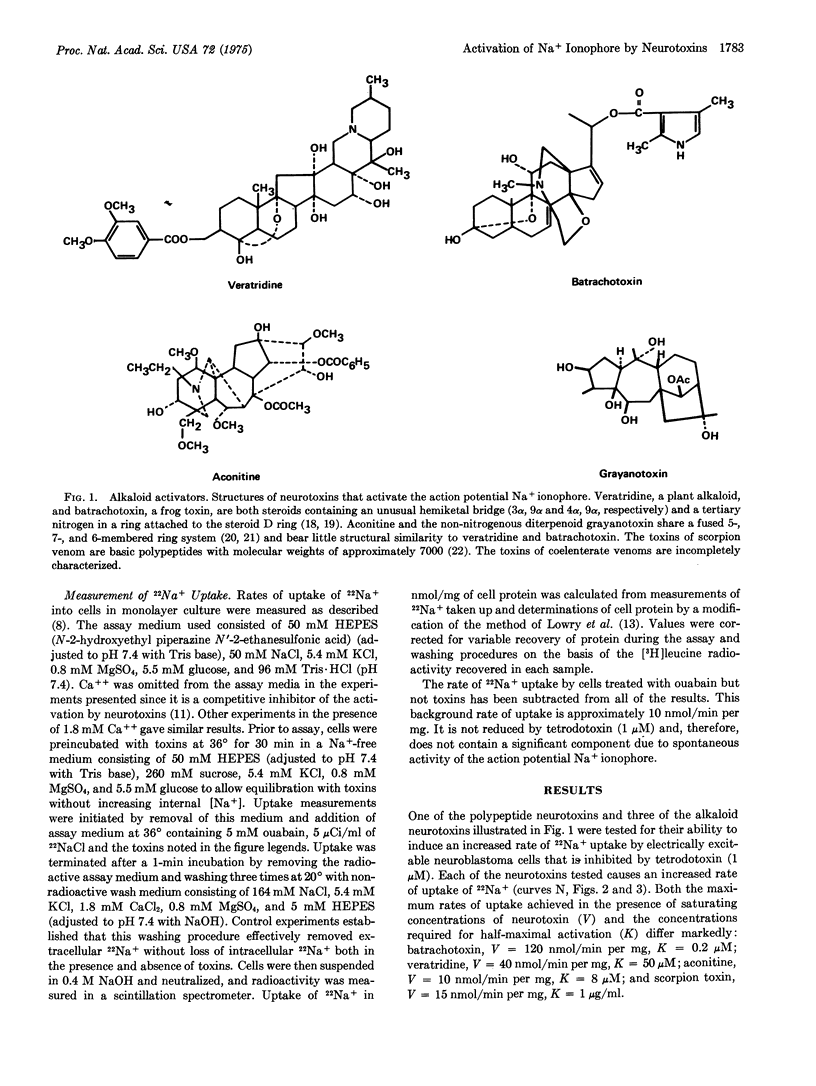
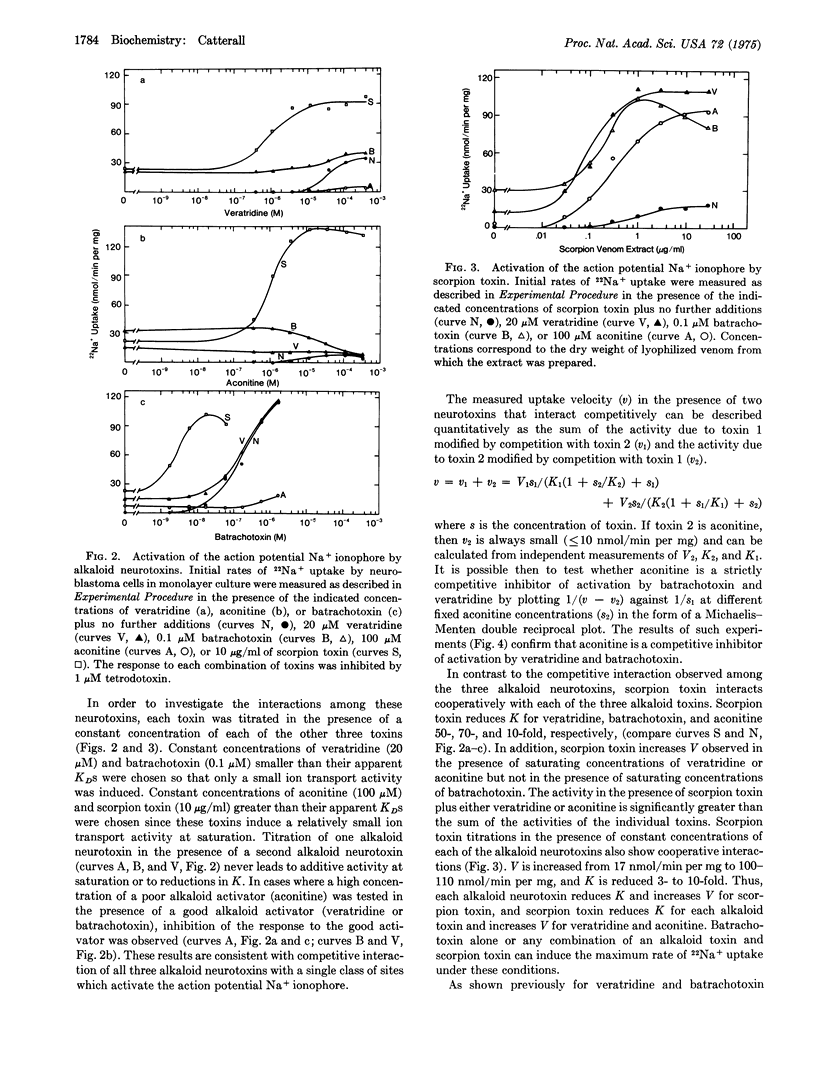
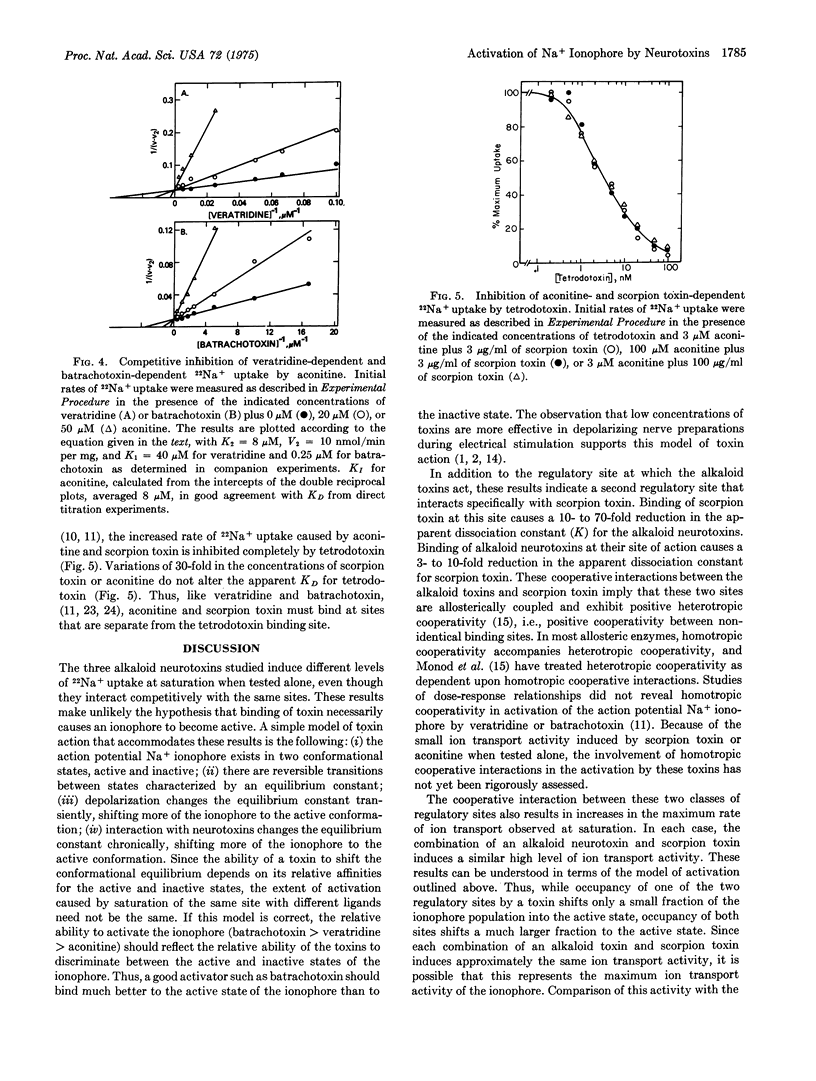

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Barnard E. A., Chiu T. H., Lapa A. J., Dolly J. O., Jansson S. E., Daly J., Witkop B. Acetylcholine receptor and ion conductance modulator sites at the murine neuromuscular junction: evidence from specific toxin reactions. Proc Natl Acad Sci U S A. 1973 Mar;70(3):949–953. doi: 10.1073/pnas.70.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Daly J. W., Witkop B. Batrachotoxin: chemistry and pharmacology. Science. 1971 Jun 4;172(3987):995–1002. doi: 10.1126/science.172.3987.995. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Sasa M., Avner B. P. Possible site of action of batrachotoxin. Nat New Biol. 1971 Nov 17;234(46):93–95. doi: 10.1038/newbio234093a0. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E. The pharmacology of batrachotoxin. IV. Interaction with tetrodotoxin on innervated and chronically denervated rat skeletal muscle. J Pharmacol Exp Ther. 1972 Mar;180(3):683–697. [PubMed] [Google Scholar]
- Catterall W. A., Nirenberg M. Sodium uptake associated with activation of action potential ionophores of cultured neuroblastoma and muscle cells. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3759–3763. doi: 10.1073/pnas.70.12.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Sodium transport by the acetylcholine receptor of cultured muscle cells. J Biol Chem. 1975 Mar 10;250(5):1776–1781. [PubMed] [Google Scholar]
- Cuervo L. A., Adelman W. J., Jr Equilibrium and kinetic properties of the interaction between tetrodotoxin and the excitable membrane of the squid giant axon. J Gen Physiol. 1970 Mar;55(3):309–335. doi: 10.1085/jgp.55.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZOG W. H., FEIBEL R. M., BRYANT S. H. THE EFFECT OF ACONITINE ON THE GIANT AXON OF THE SQUID. J Gen Physiol. 1964 Mar;47:719–733. doi: 10.1085/jgp.47.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Ritchie J. M., Strichartz G. R. Evidence that tetrodotoxin and saxitoxin act at a metal cation binding site in the sodium channels of nerve membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3936–3940. doi: 10.1073/pnas.71.10.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson S. E., Albuquerque E. X., Daly J. The pharmacology of batrachotoxin. VI. Effects on the mammalian motor nerve terminal. J Pharmacol Exp Ther. 1974 May;189(2):525–537. [PubMed] [Google Scholar]
- KUPCHAN S. M. Hypotensive veratrum ester alkaloids. J Pharm Sci. 1961 Apr;50:273–287. doi: 10.1002/jps.2600500402. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Incomplete sodium inactivation in nodes of Ranvier treated with scorpion venom. Experientia. 1968 Jan 15;24(1):41–42. doi: 10.1007/BF02136780. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Miranda F., Kupeyan C., Rochat H., Rochat C., Lissitzky S. Purification of animal neurotoxins. Isolation and characterization of eleven neurotoxins from the venoms of the scorpions Androctonus australis hector, Buthus occitanus tunetanus and Leiurus quinquestriatus quinquestriatus. Eur J Biochem. 1970 Nov;16(3):514–523. doi: 10.1111/j.1432-1033.1970.tb01111.x. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T., Moore J. W., Shapiro B. I. Condylactis toxin: interaction with nerve membrane ionic conductances. Science. 1969 Feb 14;163(3868):680–681. doi: 10.1126/science.163.3868.680. [DOI] [PubMed] [Google Scholar]
- Peper K., Trautwein W. The effect of aconitine on the membrane current in cardiac muscle. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;296(4):328–336. doi: 10.1007/BF00362532. [DOI] [PubMed] [Google Scholar]
- Seyama I., Narahashi T. Increase in sodium permeability of squid axon membranes by -dihydrograyanotoxin II. J Pharmacol Exp Ther. 1973 Feb;184(2):299–307. [PubMed] [Google Scholar]
- Tokuyama T., Daly J., Witkop B. The structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, Phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs. J Am Chem Soc. 1969 Jul 2;91(14):3931–3938. doi: 10.1021/ja01042a042. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]
- Young A. B., Snyder S. H. The glycine synaptic receptor: evidence that strychnine binding is associated with the ionic conductance mechanism. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4002–4005. doi: 10.1073/pnas.71.10.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]


